Abstract
The recent introduction of effective therapies in psoriatic arthritis (PsA) has increased the demand for efficient tools for diagnosis, monitoring and prognostication of PsA, and has caused an increased research effort within imaging in this disease. The clinical appearance of PsA is very diverse, involving the spine, sacroiliac joints, peripheral joints and/or entheses, and accordingly imaging findings vary. In the present paper, we present a review of the recent advances in imaging in PsA, focusing primarily on ultrasonography and magnetic resonance imaging of peripheral disease manifestations.
Keywords: magnetic resonance imaging, psoriatic arthritis, ultrasonography
Introduction
Psoriatic arthritis (PsA) is an inflammatory arthritis, which is associated with psoriasis, and characterized by inflammation in axial and peripheral joints and entheses. Furthermore joint damage, i.e. bone erosion, and new bone formation are frequently seen. Reliable tools for diagnosing, monitoring and prognosticating PsA have become more important in recent years due to the appearance of potent treatment options. The specific pathologies of PsA as well as the extent of disease can be visualized by different imaging modalities [Ory et al. 2005].
Conventional radiography has been the mainstay in imaging in inflammatory joint diseases, but is not able to detect the inflammatory changes, i.e. the earliest disease manifestations in PsA and other inflammatory joint diseases. In contrast, magnetic resonance imaging (MRI) and ultrasonography (US) allow direct visualization of early inflammatory and destructive joint changes [Østergaard et al. 2008], and the technical and scientific developments within MRI and US in inflammatory joint diseases have been tremendous in the last decade. Consequently, in this review we primarily focus on recent advances in MRI and US in peripheral PsA, particularly within approximately the last 5 years. Other imaging modalities, such as bone scintigraphy, computed tomography and single positron emission computed tomography, are also available, but their role in the diagnosis and management of PsA is very limited [Tan and McGonagle, 2008], and they will not be described further.
Conventional radiography
Conventional radiography is the most widely used imaging method in PsA. Radiographs comprise a record of the cumulative joint damage caused by the disease [van der Heijde and Østergaard, 2009], but do not visualize inflammatory changes. Although there are similarities with rheumatoid arthritis (RA) there are also major differences in, for example, the type and site of lesions as well as the joints involved. While RA is characterized by mainly osteodestructive lesions, in PsA there are both osteodestructive and osteoproliferative manifestations, which may even coexist not only in the same patient, but also in the same joint [van der Heijde and Østergaard, 2009]. In particular, the osteoproliferative lesions on radiography are characteristic, and are included in the new classification criteria (CASPAR) for PsA [Taylor et al. 2006].
Structural joint damage on conventional radiography is an important outcome measure in PsA. Different radiographic scoring methods have been developed, e.g. the Sharp—van der Heijde modified scoring method for PsA, which is a detailed scoring system for evaluating erosions and joint space narrowing, while osteolysis and pencil in cup phenomena are assessed separately [van der Heijde et al. 2005]. Scoring systems are primarily used in clinical trials [van der Heijde et al. 2005]. No major recent advances have been observed in this field.
Ultrasonography
Ultrasonography in psoriatic arthritis
In recent years, it has been highlighted that US is a highly sensitive tool for both clinical and research purposes in rheumatology [Brown et al. 2007; Grassi et al. 2005]. US is able to evaluate both structural changes and changes in perfusion in joints, tendons and other soft tissues. The main focus has been on RA, but in recent years an increasing attention has been given the use of US in spondyloarthritis (SpA), including PsA. US examinations have mainly been performed using greyscale (B-mode) US, but newer US techniques include the use of colour or power Doppler US. In Doppler US colour information is superimposed on the greyscale image displaying the reflection of the ultrasound by the moving erythrocytes thereby providing information about perfusion. Doppler US is used in the assessment of changes in tissue vascularization that may occur in inflammatory conditions.
Consensus definitions for ultrasound-related pathologies independent of disease were published in the Journal of Rheumatology in 2005 including definitions for synovitis, erosions and enthesopathy [Wakefield et al. 2005]. For PsA, peripheral joint involvement has received little attention and focus has mainly been on entheseal involvement.
What can be visualized with ultrasonography?
The US findings in peripheral joints are nonspecific for PsA as they may occur also in patients with other inflammatory conditions. These findings include synovitis, effusion, bone erosions and osteophytes (see Figure 1) and extra-articular pathologies such as bursitis, tenosynovitis and enthesitis. The distributions of joint involvement in patients with PsA are different from those in RA, and there is a predominance of synovitis in distal interphalangeal (DIP) joints compared with patients with RA [Wiell et al. 2007].
Figure 1.
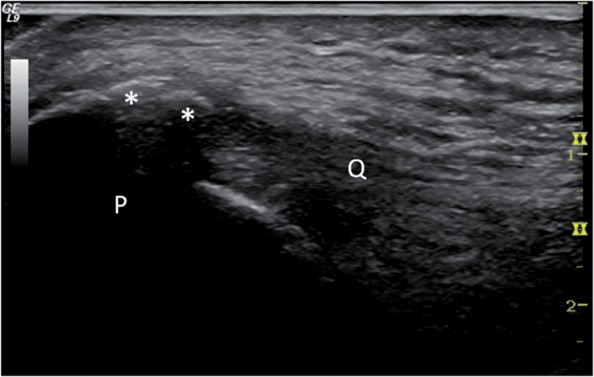
Ultrasonography of calcification and bone spur (*) in the quadriceps tendon (Q) at insertion at the patella (P).
There are diverging reports regarding the sensitivity of US compared with MRI in diagnosing synovitis. One study with greyscale US reported that MRI is more sensitive in diagnosing synovitis when evaluating metatarsophalangeal (MTP) joints [Weiner et al. 2008], but in a study using Doppler US, MRI and Doppler US was found to be equally sensitive in diagnosing inflammatory changes in MTP, metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints and Doppler US even more sensitive than MRI in DIP joints [Wiell et al. 2007].
US contrast agents in the form of microbubbles are used to enhance the scattering reflection of erythrocytes by amplifying the Doppler signal in order to increase the sensitivity of the Doppler examination to low-velocity flow. A single study found that contrast enhancement improved the correlation to MRI synovitis of peripheral joint affection compared with Doppler US alone [Solivetti et al. 2010].
Concerning structural bone changes, i.e. bone erosions and osteophytes, results from different studies are ambiguous. In one study, conventional radiography was superior to both MRI and US in the detection of bony changes [Weiner et al. 2008], whereas another study found that both US and MRI detect more erosions in MCP joints than radiography, and US detects more osteophytes in MCP and PIP joints than conventional radiography. Furthermore, US was even more sensitive than MRI in detecting bony changes in PIP joints [Wiell et al. 2007]. Bone changes in the DIP joints have been reported to be found only in PsA patients, not in RA patients [Wiell et al. 2007; Fournie et al. 2006]. However, they are difficult to differentiate from osteoarthritis changes, and further studies are warranted in this area.
Even though tenosynovitis in PsA is reported with a lower frequency than in RA patients [Wiell et al. 2007], flexor tenosynovitis partly explains dactylitis [Gutierrez et al. 2010a; Kane et al. 1999] and subclinical tenosynovitis may be seen in relation to the extensor tendons of the hand [De Filippis et al. 2005]. Entheses are the insertion sites of ligaments, tendons and capsules into the bone. Enthesitis is a characteristic feature of SpA patients. Although enthesitis is not only found in PsA entheses [Frediani et al. 2002], sonographic signs of enthesitis are found more frequently in patients with SpA than in controls or in RA patients [D'Agostino et al. 2003].
There is some diversity in the description of elementary lesions in enthesitis and the following features are described: presence of increasing thickness and hypoechogenicity of the entheses, presence of enthesophytes, calcifications and erosions at the insertion site and finally bursitis or cortical irregularities [Gutierrez et al. 2010a; Alcalde et al. 2007; Falsetti et al. 2003; Balint et al. 2002].
In recent years, Doppler US has been applied to evaluate signs of hyperaemia at the insertion site [de Miguel et al. 2009; D'Agostino et al. 2003]. Despite an increasing number of studies there is a lack of consensus regarding the US definitions for enthesitis both relating to the structural/degenerative changes seen in greyscale, and the inflammatory changes seen by Doppler ultrasound [D'Agostino et al. 2009, 2003; de Miguel et al. 2009; Alcalde et al. 2007; Balint et al. 2002].
Diagnosis by ultrasonography
Like in the RA studies US also appears to be more sensitive than clinical examination for the detection of synovitis, tenosynovitis and enthesitis in patients with PsA [Delle et al. 2010; Milosavljevic et al. 2005; Galluzzo et al. 2000]. There are diverging reports on the occurrence of synovitis in PsA patients compared with RA patients in PIP and MCP joints. Some report an increased frequency of synovitis findings in MCP and PIP joints in PsA patients compared with RA patients [Wiell et al. 2007] while others state that synovitis occur with equally frequency in the two diseases [Fournie et al. 2006]. There are no reports on the differences in synovitis appearance and distribution in PsA compared with RA.
Since enthesitis is a prominent feature in patients with SpA and may precede joint symptoms, it has been of interest to evaluate the entheses by use of US, as a means of diagnosing SpA and therefore also PsA. Studies have found that enthesitis changes may be found in patients with psoriasis and no joint involvements [Gutierrez et al. 2010b; Gisondi et al. 2008]. Likewise, Doppler activity in greyscale enthesitis changes is reported in patients with SpA, but not in RA patients and patients with mechanical low back pain [D'Agostino et al. 2003]. Despite promising reports it is currently not possible to diagnose PsA solely on the sonographic appearances. Further work is needed to clarify the role of US in the diagnosis of PsA.
What joint and entheses to assess with ultrasonography?
There is no general agreement on which joints or entheses to include in the evaluation of patients with PsA. The main focus on entheseal involvement has primarily been on the lower limb [Alcalde et al. 2007; D'Agostino et al. 2003; Balint et al. 2002].
Monitoring with ultrasonography
Most studies which aim to monitor treatment response have applied semi-quantitative scoring systems for grey-scale and/or Doppler changes. The scoring systems used are either those used in monitoring RA treatment [Szkudlarek et al. 2003], or similarly semi-quantitative scores developed by the respective authors [Solivetti et al. 2010; Fiocco et al. 2005]. In a study with TNF-alpha blocker treatment, it was determined that disease-modifying antirheumatic drug (DMARD)-resistant knee arthritis, in both RA and PsA, responded well to treatment with a significant reduction in both greyscale and Doppler semiquantitative scores at 12 months follow up. There was no significant difference in treatment response between the RA and the PsA patients [Fiocco et al. 2005]. We are not aware of a scoring system developed only for PsA.
More attention has been given to the scoring of entheseal involvement. The first to propose a greyscale scoring system of lower limb enthesitis were Balint and colleagues [Balint et al. 2002], and since then several other scoring systems have been proposed also including Doppler US [de Miguel et al. 2009; D'Agostino et al. 2003]. None of the scoring systems have been validated for monitoring PsA or other spondyloarthritides, and the sensitivity to change has not been assessed. More work to develop standardized and responsive US outcome measures in PsA is needed.
Prognostication with ultrasonography
There are no studies evaluating the role of US for prognosticating PsA. Entheseal involvement in patients with psoriasis, but without clinical PsA indicate that enthesitis may be a predictor of development of PsA [Gisondi et al. 2008]. However, longitudinal studies are required to clarify this [Szkudlarek et al. 2003].
Axial ultrasonography
There are no studies involving US in the evaluation of spinal inflammation in patients with PsA. The sacroiliac (SI) joints are often involved in PsA, but US can only visualize the superficial part of the joint and the surrounding soft tissue structures. Furthermore, the image quality may be considerably impaired in obese patients.
Very few studies involve US in the evaluation of the SI joints in PsA. A recent study evaluated SI joints in SpA patients compared to healthy controls and found that US detected a significantly higher degree of effusion in patients with SpA compared with controls, and that US detected effusion was associated with low back pain in SpA patients [Spadaro et al. 2009]. There are no US scoring systems for inflammation of the spine and SI joints.
New techniques in ultrasonography
Within the last 5 years there has been a great technological development with high-frequency probes and high-frequency Doppler, which have made it possible to improve the resolution of the greyscale image and the sensitivity to low-velocity flow. Some of the new probes allow three-dimensional (3D) US, a new ultrasound modality which seems promising in the assessment of joint pathology in inflammatory diseases. One of the advantages may be related to the virtual operator independence due to image acquisition of infinite 3D data sets obtained by transducer automated sweeping.
3D US has been shown to demonstrate enthesitis pathology well [Iagnocco et al. 2009], and appears to improve interobserver reliability in the assessment of synovitis and bony erosions [Naredo et al. 2010]. Further studies are needed to validate this technique in PsA.
Magnetic resonance imaging
MRI in psoriatic arthritis
The clinical appearance of PsA is very diverse, involving the spine, SI joints, peripheral joints and/or entheses, and accordingly MRI findings vary. MRI in PsA has received less research scrutiny than in RA and ankylosing spondylitis, but this is likely to change, as MRI outcome measures are increasingly being used in clinical trials of new therapeutic agents. Only a few studies have been published where the focus was specifically on PsA, and most of the current knowledge is from studies of groups of patients with different spondyloarthritides [McQueen et al. 2006].
Relevant reviews of the status of MRI in PsA in recent years have been published [Cimmino et al. 2009; McQueen et al. 2008, 2006]. MRI findings, e.g. synovitis, tenosynovitis, periosteal post-contrast enhancement and bone oedema are very frequent in symptomatic PsA [Ghanem et al. 2007; Wiell et al. 2007].
What can be visualized with MRI?
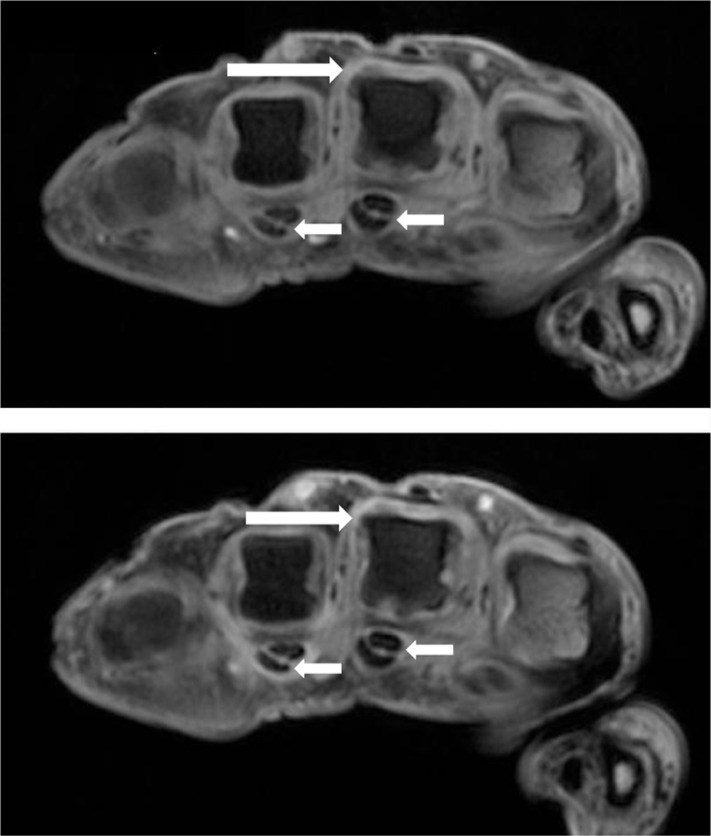
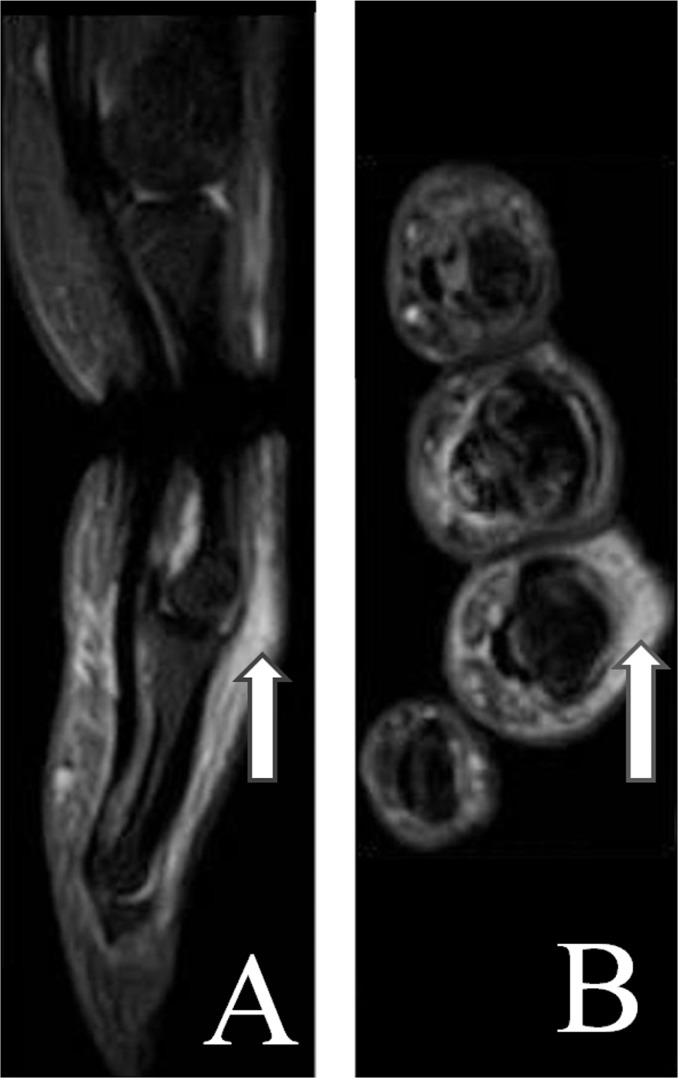
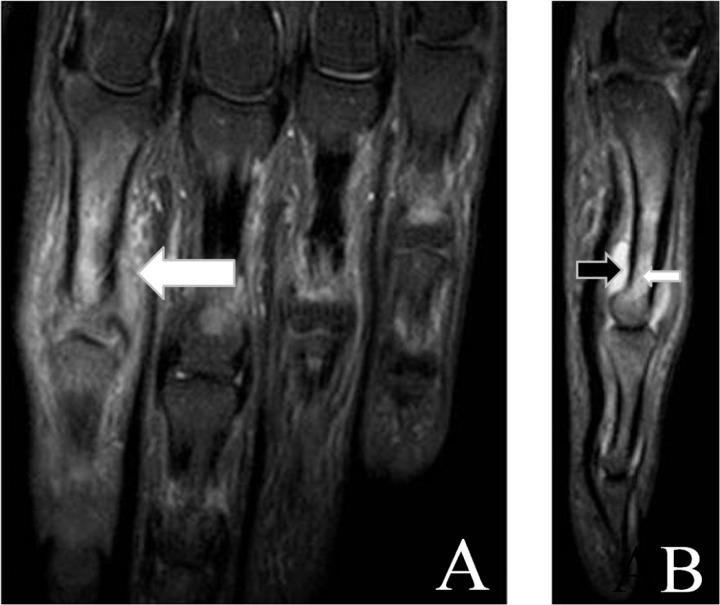
MRI can visualize both peripheral and axial musculoskeletal anatomy and PsA disease manifestations. Findings include synovitis, tenosynovitis (Figure 2), periarticular inflammation (Figure 3), enthesitis, bone oedema (Figure 4), bone erosion and bone proliferation [østergaard et al. 2009].
Figure 2.
Axial T1-weighted magnetic resonance images of the metacarpophalangeal joints, obtained before (upper image) and after (lower image) intravenous contrast injection in a patient with PsA. Synovitis is seen in the third MCP joint (long arrows). Flexor tenosynovitis in the third and fourth flexor tendons are also seen (short arrows).
Figure 3.
Sagittal (A) and axial (B) short tau inversion recovery (STIR) magnetic resonance images of the 4th finger. Arrows mark periarticular inflammation.
Figure 4.
Coronal (A) and sagittal (B) short tau inversion recovery (STIR) magnetic resonance images showing synovitis (black arrow), bone marrow oedema (small white arrow) and periarticular oedema (big white arrow) of the second proximal interphalangeal joint region.
In a retrospective study of 10 patients with the clinical diagnosis of PsA, MRI of 13 hands and 10 wrists were analysed for involvement of bones, joints, tendons and other soft tissues [Tehranzadeh et al. 2008]. Synovitis was found in all wrists and hands. Bone marrow oedema, erosions and soft tissue involvement were more prevalent in the wrist than in the hand. No hypertrophic bone changes were detected, which may be due to the fact that only patients with normal radiographs were included.
MRI is highly sensitive for active enthesitis [Eshed et al. 2007]. It has been reported that the bone marrow oedema in PsA is often located close to the entheses, in contrast to the situation in RA, where bone marrow oedema often is located close to the capsular attachments, and to osteoarthritis where it is mainly located in sub-chondral areas [Totterman, 2004].
The entheses has attracted attention, as a possible primary location of disease. McGonagle has reported that perientheseal bone oedema is an integral feature of enthesitis, and suggest that synovitis is a secondary feature [McGonagle, 2005]. Another MRI study of 6 PsA patients with dactylitic fingers concluded that dactylitis is mainly due to flexor tenosynovitis, whereas no bone oedema was found at the insertions of extensor or flexor tendons, or at other sites in the phalanges, suggesting that enthesitis has no key role in PsA dactylitis [Olivieri et al. 2002].
Nail disease is common in PsA. The relationship between extensor tendon enthesitis in the PsA DIP joint and the nail bed has been studied by Tan, McGonagle and coworkers, e.g. in a study of 10 patients, in which diffuse DIP joint inflammation on MRI was found to extend to the nail bed [Tan et al. 2007]. However, as it was a cross-sectional observational study, it was not possible to draw any conclusions on the sequence of events. A later MRI study of nails in patients with skin psoriasis found characteristic nail involvement in all patients, and the authors suggested that ony-chopathy is preceding DIP joint damage in PsA, and that MRI of nails is of diagnostic value in undifferentiated SpA [Soscia et al. 2009].
PsA can be clinically silent, which was shown in a study of 25 patients with active nummular and/or plaque psoriasis, where clinical arthropathy was ruled out by a rheumatologist. It was found that 68% was positive for at least one arthritic sign [periarticular oedema, tendon sheath effusion, intra-articular effusion, synovial pannus, bone erosion, bone cysts, subchondral changes or joint (sub)luxation] using MRI of the hand, and 32% using a conventional X-ray of the hand [Offidani et al. 1998]. Only in one of 12 examined healthy controls significant pathology (a bone cyst) was found.
Another study of 26 patients with skin psoriasis without arthritic signs or symptoms and 10 healthy controls, describes that pathologic changes were identified by MRI of the feet in 24 patients (92%), whereas no abnormalities were found in the control group [Erdem et al. 2008]. Similarly, MRI of the knees detected enthesitis at the patellar tendon insertion in five out of six skin psoriasis patients, without clinical joint involvement, and were absent in 20 healthy controls [Emad et al. 2010]. The result described above illustrates the potential of MRI for assessing subclinical disease. The fact that subclinical joint inflammation may occur in psoriasis is supported by a bone scintigraphy study of 50 patients with psoriasis without clinical arthropathy. The study found 35 (70%) patients with axial and/or peripheral joint involvement, compared with 4 (16%) of 25 controls referred to scintigraphy for unrelated problems [Raza et al. 2008].
Which joints to assess with MRI?
PsA affects both axial and peripheral joints and entheses, and a general agreement on which joints to image to assess PsA activity and damage is not established, and possibly needs to be individualized, based on the disease pattern. It is generally suggested that T1-weighted (T1w) sequences are in two planes, supplemented by a T2-weighted (T2w) fat-suppressed or short tau inversion recovery (STIR) sequence, preferable also in two planes. Intravenous contrast injection is optimal for assessment of synovitis and tenosynovitis, but can be omitted if the aim is purely to detect bone erosion, bone oedema and bone proliferation [Østergaard et al. 2009].
Diagnosis with MRI
As described above, MRI can detect the different pathologies involved in PsA. However, there are no studies that have documented that MRI in an early undifferentiated arthritis cohort can be used to differentiate PsA from other arthritides. Most studies have compared patients with known diagnoses: in a study by McQueen and colleagues, MRI could not distinguish between peripheral PsA and RA when synovitis and erosions were evaluated [McQueen et al. 2006].
In another study, MRI of the hand and wrist was reported to be able to differentiate between established disease in PsA and RA [Schoellnast et al. 2006]. This was a retrospective analysis of 18 PsA and 21 RA patients with arthralgia. Significant differences were found in the frequency of bone erosions (86% of RA and only 17% of PsA patients) and periostitis (0% of RA and 78% of PsA patients), and they also found that PIP joints were more frequently affected in PsA patients. A limitation of the study was that the DIP joints were not scanned [McGonagle et al. 1999].
Monitoring with MRI
Most studies only report qualitative MRI assessments of the different pathologies of PsA (see McQueen et al. [2006] for a summary up to 2005). Quantitative assessment of contrast enhancement has been reported [Cimmino et al. 2005; Antoni et al. 2002]. In an early study of anti-TNF agents in PsA, treatment response to infliximab was monitored with MRI at baseline, and after 10 weeks [Antoni et al. 2002]. Eight patients were imaged with a dynamic MRI sequence, and time-dependent signal intensity increase, reflecting synovitis, was substantially and significantly reduced after 10 weeks.
Some authors have described scoring systems for bone marrow oedema, erosions and/or synovitis [e.g. Anandarajah et al. 2010; Tehranzadeh et al. 2008], but these have only been used in a few patients and not outside the introducing centre. In one of these studies, a study of 11 PsA patients treated with the anti-TNF agent adalimumab for 24 weeks, MRI of a wrist or knee at baseline and to have a standardized system for scoring follow up showed significant improvements at 24 and in MRI bone marrow oedema and effusion, but not synovitis [Anandarajah et al. 2010)]. Furthermore, Healy and colleagues have found therapy-induced decreases in both clinical and MRI assessments of dactylitis [Healy et al. 2008].
To be able to compare the results of different (Table 1) has very good reliability for assessment to have a standardized system for scoring the pathologies. The international OMERACT MRI in inflammatory arthritis group has developed the OMERACT Psoriatic Arthritis Magnetic Resonance Image Scoring System (PsAMRIS) for the evaluation of inflammatory and destructive change in PsA hands [Østergaard et al. 2009; McQueen et al. 2007]. The last version of the PsAMRIS system (Table 1) has very good reliability for assessment of inflammatory change (synovitis, tenosynovitis, periarticular inflammation), as the interobserver intraclass correlation coefficients for both status and change scores were all >0.85 [Bøyesen et al. 2010a]. Furthermore, the reliability was good for damage (bone erosion and bone proliferation) status scores (0.77—0.97), while only moderate for changes in bone erosions (0.44). The sensitivity to change was good for inflammatory parameters (standardized response means all >0.80) [Bøyesen et al. 2010b]. The usefulness of the OMERACT PsAMRIS in clinical trials and practice needs further testing.
Table 1.
The OMERACT MRI in inflammatory arthritis task force recommendations for MRI definitions of important pathologies in peripheral psoriatic arthritis (PsA) and a PsA MRI scoring system [Østergaard et al. 2009].
A. Definitions of important PsA joint pathologies
|
B. Scoring system (OMERACT PsAMRIS) for hands
|
PsAMRIS, Psoriatic Arthritis Magnetic Resonance Image Scoring System.
Prognosticating with MRI
In RA, bone marrow oedema is an important predictor of subsequent progressive radiographic joint damage [Hetland et al. 2009; Haavardsholm et al. 2008; McQueen et al. 1999]. No formal studies of the prognostic value of MRI findings in PsA are available. Based on a cross-sectional MRI study of 11 patients with the aggressive arthritis mutilans (AM) form of PsA, and 17 non-AM patients (erosive PsA without bony lysis), in which there was close relation between presence of erosion and bone oedema, the authors suggest that MRI bone oedema is also in PsA a ‘forerunner’ of structural joint damage [Tan et al. 2009]. Further longitudinal studies are needed to clarify this.
Axial MRI in psoriatic arthritis
The knowledge about MRI of axial involvement in PsA mainly originates from studies including various spondyloarthritides, and axial disease is not the topic of this paper. However, MRI is very sensitive for detection of sacroiliitis and spondylitis in SpA [Østergaard et al. 2010; Weber et al. 2009].
New MRI techniques
Whole-body MRI [Weckbach et al. 2009; Althoff et al. 2007; Appel et al. 2007] potentially allows simultaneous assessment of both peripheral and axial disease manifestations in PsA, and if the methodology is further refined and proves successful in future studies, it may constitute a major step forward for the monitoring of the overall disease status in clinical trials and practice in PsA.
With a dynamic, gadolinium-enhanced MRI technique it is possible to evaluate the inflammatory activity, as assessed by histology, in RA joints [Østergaard et al. 1998; Gaffney et al. 1995]. By dynamic MRI it may be possible to differentiate active disease from remission in RA [Cimmino et al. 2003]. There are very limited data from PsA patients, but in a small study of wrists in PsA and RA, no difference in dynamic contrast enhancement in the synovium was found between the PsA and RA patients, when matched for disease activity, but all PsA patients had significant higher enhancement than healthy controls [Cimmino et al. 2005].
In another comparative study of 10 PsA and 10 RA patients with at least one swollen MCP joint, conventional and dynamic contrast-enhanced MRI was not able to differentiate between the diagnoses, but more diffuse extracapsular enhancement/entheseal-based pathology was found in patients with PsA [Marzo-Ortega et al. 2009]. Thus, the diagnostic utility of dynamic MRI has so far proved limited, but this technique may constitute an improved method for monitoring inflammatory activity in PsA. However, further studies are needed.
Conclusions
MRI and ultrasonography are excellent tools for the evaluation of patients with PsA, because both peripheral and axial (only MRI) pathology can be visualized. However, a lot of scientific work to determine their potential value for diagnosis, monitoring and prognostication is still to be done.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None declared.
References
- Alcalde M., Acebes J., Gonzalez-Hombrado L., Herrero-Beaumont G., Sanchez-Pernaute O. (2007) A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis. Ann Rheum Dis 66: 1015–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff C.E., Appel H., Rudwaleit M., Sieper J., Eshed I., Hamm B., et al. (2007) Whole-body MRI as a new screening tool for detecting axial and peripheral manifestations of spondyloarthritis. Ann Rheum Dis 66: 983–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandarajah A.P., Ory P., Salonen D., Feng C, Wong R.L., Ritchlin C.T. (2010) Effect of adalimumab on joint disease: Features of patients with psoriatic arthritis detected by magnetic resonance imaging. Ann Rheum Dis 69: 206–209 [DOI] [PubMed] [Google Scholar]
- Antoni C, Dechant C, Lorenz P.D. Hanns-Martin, Wendler J., Ogilvie A., Lueftl M., et al. (2002) Open-label study of infliximab treatment for psoriatic arthritis: Clinical and magnetic resonance imaging measurements of reduction of inflammation. Arthritis Rheum 47: 506–512 [DOI] [PubMed] [Google Scholar]
- Appel H., Hermann K.G., Althoff C.E., Rudwaleit M., Sieper J. (2007) Whole-body magnetic resonance imaging evaluation of widespread inflammatory lesions in a patient with ankylosing spondylitis before and after 1 year of treatment with infliximab. J Rheumatol 34: 2497–2498 [PubMed] [Google Scholar]
- Balint P.V., Kane D., Wilson H., Mclnnes LB, Sturrock R.D. (2002) Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis 61: 905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyesen P., Gandjbakh E, McQueen F., Lillegraven S., Coates L., Wiell C., et al. (2010a) Reader reliability of the OMERACT psoriatic arthritis magnetic resonance score (PsAMRIS): Results from an OMERACT workshop. Arthritis Rheum 60(Suppl): 290 [Google Scholar]
- Boyesen P., Gandjbakhch F., McQueen F., Lillegraven S., Coates L., Wiell C., et al. (2010b) Change and responsiveness of the OMERACT psoriatic arthritis magnetic resonance imaging score (PsAMRIS): Results from an OMERACT workshop. Ann Rheum Dis 69(Suppl. 3): 50 [Google Scholar]
- Brown A.K., Roberts T.E., Wakefield R.J., Karim Z., Hensor E., O'Connor P.J., et al. (2007) The challenges of integrating ultrasonography into routine rheumatology practice: Addressing the needs of clinical rheumatologists. Rheumatology (Oxford) 46: 821–829 [DOI] [PubMed] [Google Scholar]
- Cimmino M.A., Innocenti S., Livrone F., Magnaguagno F., Silvestri E., Garlaschi G. (2003) Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum 48: 1207–1213 [DOI] [PubMed] [Google Scholar]
- Cimmino M.A., Parodi M., Innocenti S., Succio G., Banderali S., Silvestri E., et al. (2005) Dynamic magnetic resonance of the wrist in psoriatic arthritis reveals imaging patterns similar to those of rheumatoid arthritis. Arthritis Res Ther 7: R725–R731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino M.A., Parodi M., Zampogna G., Paparo F., Silvestri E., Garlaschi G., et al. (2009) Magnetic resonance imaging of the hand in psoriatic arthritis. J Rheumatol Suppl 83: 39–41 [DOI] [PubMed] [Google Scholar]
- D'Agostino M.A., Aegerter P., Jousse-Joulin S., Chary-Valckenaere I., Lecoq B., Gaudin P., et al. (2009) How to evaluate and improve the reliability of power Doppler ultrasonography for assessing enthesitis in spondylarthritis. Arthritis Rheum 61: 61–69 [DOI] [PubMed] [Google Scholar]
- D'Agostino M.A., Said-Nahal R., Hacquard-Bouder C.j, Brasseur J.L., Dougados M., Breban M. (2003) Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: A cross-sectional study. Arthritis Rheum 48: 523–533 [DOI] [PubMed] [Google Scholar]
- De Filippis L., Caliri A., Lo Gullo R., Bartolone S., Miceli G., Cabbavo S., et al. (2005) Ultrasonography in the early diagnosis of psoriasis-associated enthesopathy. Int J Tissue React 27: 159–162 [PubMed] [Google Scholar]
- de Miguel E., Cobo T, Munoz-Fernandez S., Naredo E., Uson J., Acebes J., et al. (2009) Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis 68: 169–174 [DOI] [PubMed] [Google Scholar]
- Delle S.A., Riente L., Filippucci E., Scire C.A., Iagnocco A., Gutierrez M., et al. (2010) Ultrasound imaging for the rheumatologist XXVI. Sonographic assessment of the knee in patients with psoriatic arthritis. Clin Exp Rheumatol 28: 147–152 [PubMed] [Google Scholar]
- Emad Y., Ragab Y., Bassyouni I., Moawayh O., Fawzy M., Saad A., et al. (2010) Enthesitis and related changes in the knees in seronegative spondy-loarthropathies and skin psoriasis: Magnetic resonance imaging case–control study. F Rheumatol 37: 1709–1717 [DOI] [PubMed] [Google Scholar]
- Erdem C.Z., Tekin N.S., Sarikaya S., Erdem L.O., Gulec S. (2008) MR imaging features of foot involvement in patients with psoriasis. Eur J Radiol 67: 521–525 [DOI] [PubMed] [Google Scholar]
- Eshed I., Bollow M., McGonagle D.G., Tan A.L., Althoff C.E., Asbach P., et al. (2007) MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann Rheum Dis 66: 1553–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti P., Frediani B., Fioravanti A., Acciai C, Baldi F., Filippou G., et al. (2003) Sonographic study of calcaneal entheses in erosive osteoarthritis, nodal osteoarthritis, rheumatoid arthritis and psoriatic arthritis. Scand J Rheumatol 32: 229–234 [DOI] [PubMed] [Google Scholar]
- Fiocco U, Ferro F., Vezzu M., Cozzi L., Checchetto C, Sfriso P., et al. (2005) Rheumatoid and psoriatic knee synovitis: Clinical, grey scale, and power Doppler ultrasound assessment of the response to etanercept. Ann Rheum Dis 64: 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie B., Margarit-Coll N., de Ribes T.L. Champetier, Zabraniecki L., Jouan A., Vincent V, et al. (2006) Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power-Doppler study versus rheumatoid arthritis. Joint Bone Spine 73: 527–531 [DOI] [PubMed] [Google Scholar]
- Frediani B., Falsetti P., Storri L., Allegri A., Bisogno S., Baldi F., et al. (2002) Ultrasound and clinical evaluation of quadricipital tendon enthesitis in patients with psoriatic arthritis and rheumatoid arthritis. Clin Rheumatol 21: 294–298 [DOI] [PubMed] [Google Scholar]
- Gaffhey K., Cookson J., Blake D., Coumbe A., Blades S. (1995) Quantification of rheumatoid synovitis by magnetic resonance imaging. Arthritis Rheum 38: 1610–1617 [DOI] [PubMed] [Google Scholar]
- Galluzzo E., Lischi D.M., Taglione E., Lombardini F., Pasero G., Perri G., et al. (2000) Sonographic analysis of the ankle in patients with psoriatic arthritis. Scand J Rheumatol 29: 52–55 [DOI] [PubMed] [Google Scholar]
- Ghanem N., Uhl M., Pache G., Bley T., Walker U.A., Langer M. (2007) MRI in psoriatic arthritis with hand and foot involvement. Rheumatol Int 27: 387–393 [DOI] [PubMed] [Google Scholar]
- Gisondi P., Tinazzi I., El-Dalati G., Gallo M., Biasi D., Barbara L., et al. (2008) Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: A hospital-based case–control study. Ann Rheum Dis 67: 26–30 [DOI] [PubMed] [Google Scholar]
- Grassi W., Salaffi F., Filippucci E. (2005) Ultrasound in rheumatology. Best Pract Res Clin Rheumatol 19: 467–485 [DOI] [PubMed] [Google Scholar]
- Gutierrez M., Filippucci E., De Angelis R., Filosa G., Kane D., Grassi W. (2010a) A sonographic spectrum of psoriatic arthritis: “the five targets”. Clin Rheumatol 29(2): 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M., Filippucci E., De A. R., Salaffi F., Filosa G., Ruta S., et al. (2010b) Subclinical entheseal involvement in patients with psoriasis: An ultrasound study. Semin Arthritis Rheum, in press. [DOI] [PubMed] [Google Scholar]
- Haavardsholm E.A., Boyesen P., Ostergaard M., Schildvold A., Kvien T.K. (2008) Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: Bone marrow oedema predicts erosive progression. Ann Rheum Dis 67: 794–800 [DOI] [PubMed] [Google Scholar]
- Healy P.J., Groves C., Chandramohan M., Helliwell P.S. (2008) MRI changes in psoriatic dac-tylitis–extent of pathology, relationship to tenderness and correlation with clinical indices. Rheumatology (Oxford) 47: 92–95 [DOI] [PubMed] [Google Scholar]
- Hetland M.L., Ejbjerg B., Horslev-Petersen K., Jacobsen S., Vestergaard A., Jurik A.G., et al. (2009) MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis 68: 384–390 [DOI] [PubMed] [Google Scholar]
- Iagnocco A., Riente L., Delle S.A., Filippucci E., Salaffi F., Meenagh G., et al. (2009) Ultrasound imaging for the rheumatologist. XXII. Achilles tendon involvement in spondyloarfhritis. A multi-centre study using high frequency volumetric probe. Clin Exp Rheumatol 27: 547–551 [PubMed] [Google Scholar]
- Kane D., Greaney T., Bresnihan B., Gibney R., FitzGerald O. (1999) Ultrasonography in the diagnosis and management of psoriatic dactylitis. F Rheumatol 26: 1746–1751 [PubMed] [Google Scholar]
- Marzo-Ortega H., Tanner S.F., Rhodes L.A., Tan A.L., Conaghan P.G., Hensor E.M., et al. (2009) Magnetic resonance imaging in the assessment of metacarpophalangeal joint disease in early psoriatic and rheumatoid arthritis. Scand J Rheumatol 38: 79–83 [DOI] [PubMed] [Google Scholar]
- McGonagle D. (2005) Imaging the joint and enthesis: Insights into pathogenesis of psoriatic arthritis. Ann Rheum Dis 64(Suppl. 2): ii58–ii60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Conaghan P.G., Emery P. (1999) Psoriatic arthritis: A unified concept twenty years on. Arthritis Rheum 42: 1080–1086 [DOI] [PubMed] [Google Scholar]
- McQueen F., Lassere M., Bird P., Haavardsholm E.A., Peterfy C., Conaghan P.G., et al. (2007) Developing a magnetic resonance imaging scoring system for peripheral psoriatic arthritis. F Rheumatol 34: 859–861 [PubMed] [Google Scholar]
- McQueen F., Lassere M., Ostergaard M. (2006) Magnetic resonance imaging in psoriatic arthritis: A review of the literature. Arthritis Res Ther 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen F.M., Dalbeth N., Doyle A. (2008) MRI in psoriatic arthritis: Insights into pathogenesis and treatment response. Curr Rheumatol Rep 10: 303–310 [DOI] [PubMed] [Google Scholar]
- McQueen F.M., Stewart N., Crabbe J., Robinson E., Yeoman S., Tan P.L., et al. (1999) Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis 58: 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic J., Lindqvist U., Elvin A. (2005) Ultrasound and power Doppler evaluation of the hand and wrist in patients with psoriatic arthritis. Acta Radiol 46: 374–385 [DOI] [PubMed] [Google Scholar]
- Naredo E., Moller I., Acebes C, Batlle-Gualda E., Brito E., de Agustin J.J., et al. (2010) Three-dimensional volumetric ultrasonography Does it improve reliability of musculoskeletal ultrasound?. Clin Exp Rheumatol 28: 79–82 [PubMed] [Google Scholar]
- Offidani A., Cellini A., Valeri G., Giovagnoni A. (1998) Subclinical joint involvement in psoriasis: Magnetic resonance imaging and X-ray findings. Acta Derm Venereol 78: 463–65 [DOI] [PubMed] [Google Scholar]
- Olivieri I., Salvarani C, Cantini F., Scarano E., Padula A., Niccoli L., et al. (2002) Fast spin echo-T2-weighted sequences with fat saturation in dactylitis of spondylarfhritis. No evidence of entheseal involvement of the flexor digitorum tendons. Arthritis Rheum 46: 2964–2967 [DOI] [PubMed] [Google Scholar]
- Ory P.A., Gladman D.D., Mease P.J. (2005) Psoriatic arthritis and imaging. Ann Rheum Dis 64(Suppl. 2): ii55–ii57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- østergaard M., McQueen F., Wiell C, Bird P., Boyesen P., Ejbjerg B., et al. (2009) The OMERACT psoriatic arthritis magnetic resonance imaging scoring system (PsAMRIS): Definitions of key pathologies, suggested MRI sequences, and preliminary scoring system for PsA Hands. F Rheumatol 36: 1816–1824 [DOI] [PubMed] [Google Scholar]
- østergaard M., Pedersen S.J., Dohn U.M. (2008) Imaging in rheumatoid arthritis–status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and Rheumatol 22: 1019–1044 [DOI] [PubMed] [Google Scholar]
- østergaard M., Poggenborg R., Pedersen S.J. (2010) Magnetic resonance imaging in spondyloar-thritis–how to quantify findings and measure response. Best Pract Res Clin Rheumatol 24: 631–651 [DOI] [PubMed] [Google Scholar]
- østergaard M., Stoltenberg M., Lovgreen-Nielsen P., Volck B., Sonne-Holm S., Lorenzen I. (1998) Quantification of synovitis by MRI: Correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging 16: 743–754 [DOI] [PubMed] [Google Scholar]
- Raza N., Hameed A., Ali M.K. (2008) Detection of subclinical joint involvement in psoriasis with bone scintigraphy and its response to oral methotrexate. Clin Exp Dermatol 33: 70–73 [DOI] [PubMed] [Google Scholar]
- Schoellnast H., Deutschmann H.A., Hermann J., Schaffler G.J., Reittner P., Kammerhuber F., et al. (2006) Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol 187: 351–357 [DOI] [PubMed] [Google Scholar]
- Solivetti F.M., Elia R, Teoli M., De Mutiis C, Chimenti S., Berardesca E., et al. (2010) Role of contrast-enhanced ultrasound in early diagnosis of psoriatic arthritis. Dermatology 220: 25–31 [DOI] [PubMed] [Google Scholar]
- Soscia E., Scarpa R., Cimmino M.A., Atteno M., Peluso R., Sirignano C., et al. (2009) Magnetic resonance imaging of nail unit in psoriatic arthritis. F Rheumatol Suppl 83: 42–45 [DOI] [PubMed] [Google Scholar]
- Spadaro A., Iagnocco A., Baccano G., Ceccarelli F., Sabatini E., Valesini G. (2009) Sonographic-detected joint effusion compared with physical examination in the assessment of sacroiliac joints in spondyloarfhritis. Ann Rheum Dis 68: 1559–1563 [DOI] [PubMed] [Google Scholar]
- Szkudlarek M., Court-Payen, Jacobsen S., Klarlund M., Thomsen H.S., Ostergaard M. (2003) Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum 48: 955–962 [DOI] [PubMed] [Google Scholar]
- Tan A.L., Benjamin M., Toumi H., Grainger A.J., Tanner S.F., Emery P., et al. (2007) The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis–a high-resolution MRI and histological study. Rheumatology (Oxford) 46: 253–256 [DOI] [PubMed] [Google Scholar]
- Tan A.L., McGonagle D. (2008) Imaging of seronegative spondyloarfhritis. Best Pract Res Clin Rheumatol 22: 1045–1059 [DOI] [PubMed] [Google Scholar]
- Tan Y.M., Ostergaard M., Doyle A., Dalbeth N., Lobo M., Reeves Q., et al. (2009) MRI bone oedema scores are higher in the arthritis mutilans form of psoriatic arthritis and correlate with high radiographic scores for joint damage. Arthritis Res Ther 11: R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W., Gladman D., Helliwell P., Marchesoni A., Mease P., Mielants H. (2006) Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum 54: 2665–2673 [DOI] [PubMed] [Google Scholar]
- Tehranzadeh J., Ashikyan O., Anavim A., Shin J. (2008) Detailed analysis of contrast-enhanced MRI of hands and wrists in patients with psoriatic arthritis. Skeletal Radial 37: 433–442 [DOI] [PubMed] [Google Scholar]
- Totterman S.M. (2004) Magnetic resonance imaging of psoriatic arthritis: Insight from traditional and three-dimensional analysis. Curr Rheumatol Rep 6: 317–321 [DOI] [PubMed] [Google Scholar]
- van der Heijde D., Ostergaard M. (2009) Assessment of disease activity and damage in inflammatory arthritis, In: Bijlsma J. J. (ed.). The EULAR Compendium on Rheumatic Diseases, London: BMJ Publishing Group, pp. 182–201 [Google Scholar]
- van der Heijde D., Sharp J., Wassenberg S., Gladman D.D. (2005) Psoriatic arthritis imaging: A review of scoring methods. Ann Rheum Dis 64(Suppl. 2): ii61–ii64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield R.J., Balint P.V., Szkudlarek M., Filippucci E., Backhaus M., D'Agostino M.A., et al. (2005) Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 32: 2485–2487 [PubMed] [Google Scholar]
- Weber U., Hodler J., Kubik R.A., Rufibach K., Lambert R.G., Kissling R.O., et al. (2009) Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum 61: 900–908 [DOI] [PubMed] [Google Scholar]
- Weckbach S., Schewe S., Michaely H.J., Steffinger D., Reiser M.F., Glaser C. (2009) Whole-body MR imaging in psoriatic arthritis: Additional value for therapeutic decision making. Eur J Radial, 23 July 2009 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Weiner S.M., Jurenz S., Uhl M., Lange-Nolde A., Warnatz K., Peter H.H., et al. (2008) Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: A comparison with radiography, MRI and scintigraphy. Clin Rheumatol 27: 983–989 [DOI] [PubMed] [Google Scholar]
- Wiell C.j, Szkudlarek M., Hasselquist M., Moller J.M., Vestergaard A., Norregaard J., et al. (2007) Ultrasonography, magnetic resonance imaging, radiography, and clinical assessment of inflammatory and destructive changes in fingers and toes of patients with psoriatic arthritis. Arthritis Res Ther 9: R119. [DOI] [PMC free article] [PubMed] [Google Scholar]