Abstract
Comment on: Thaunat O, et al. Science 2012; 335:475-9.
Keywords: B cell differentiation, B lymphocyte, antigen distribution, asymmetric division, cell polarisation, germinal centre
Following the binding of B cell receptors (BCR) to cognate antigens exposed on presenting cells, B cells rapidly spread over antigen-containing surface.1 During B cell spreading, antigen–BCR microclusters are continually assembled at the periphery of the contact area between the cells. The initial spreading response is followed by a contraction phase, which results in the accumulation of antigen–BCR complexes in the center of the mature immunological synapse.1 Recent evidence suggests that the centripetal movement of antigen-BCR complexes is mediated by the recruitment of the microtubule motor dynein to the signaling BCR microcluster.2 Trafficking of antigen–BCR complexes to the minus end of the underlying microtubule network may also explain how antigen–BCR complexes are extracted from mature immunological synapse and targeted to endosomes. Antigen internalisation in B cells indeed correlates with a dramatic reorganization of endosomal compartment,3 during which discrete scattered peripheral endosomes fuse to form a cluster of few large MHC II-enriched vesicles containing antigens (MIIC) that are concentrated in the vicinity of the microtubule organization center.4 Once established, antigen polarization is maintained in antigen-experienced B cells when they migrate to the B zone-T zone boundary.4 There, endosomal antigen stock allows for presentation of processed antigenic peptide in MHC II molecules to cognate CD4+ T cells, which, in turn, provide costimulatory signals that trigger B cell division and differentiation. Optimal protection against pathogens indeed requires that following encounter of antigen, specific B cell clones expand, and that B cell progeny diversifies into either short-lived plasmablasts that generate an early burst of germline-encoded low affinity antibodies, or germinal center B cells responsible for delayed effector functions but long-term protection.
The factors that govern the allocation of a proportion of activated B cells to each cell fate remain elusive. A recent in vitro study has postulated that internal stochastic mechanisms could contribute to this process.5 On the other hand, instructive elements, such as the strength of the interaction between BCR and antigen,6 have been implicated in influencing activated B cell fate in vivo.
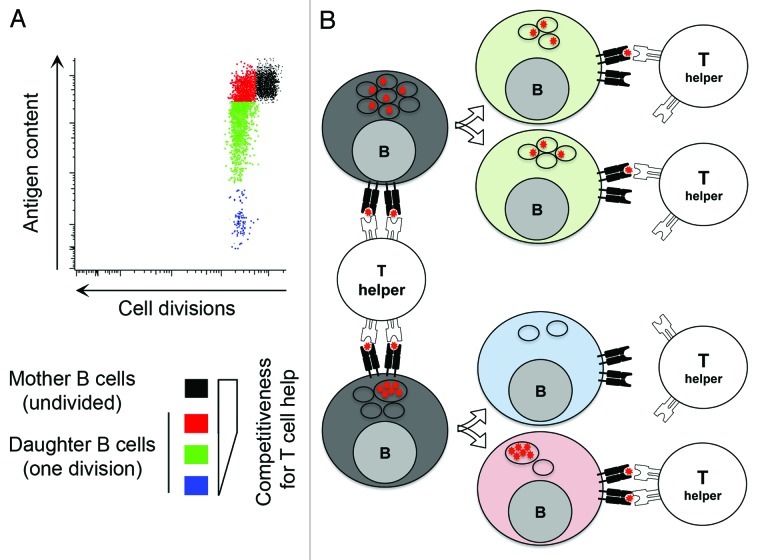
Our group has demonstrated that antigen polarization is maintained in activated B cells across the successive stages of mitosis, promoting asymmetric antigen segregation among progeny4 (Fig. 1). Antigen inheritance correlates with the ability to interact with T cells. Daughter cells receiving larger antigen stocks exhibit a prolonged capacity to present antigen,4 which renders them more effective at competing for limited T cell help (Fig. 1), a key regulator for the entry in germinal center.7 Asymmetric antigen segregation could also regulate the balance of proliferation vs. differentiation during germinal center reaction. Daughter B cells retaining antigen could undergo prolonged interaction with T follicular helper cells (TFH) and extended CD40L-dependent signaling, which have been shown to promote the transition from a germinal center gene-expression program to that of a plasma cell. Moreover, MIIC may also directly signal through Erk and Akt kinases for differentiation to plasma cells without further interaction with TFH cells.8 Alternatively, asymmetric antigen segregation might be functionally significant during affinity maturation through the rapid “cleansing” progeny from antigen. In this scenario, the survival of antigen-negative B cells is entirely dependent on the accumulation of new antigen, allowing proofreading of the newly mutated BCR and the preferential selection of higher affinity variants.
Figure 1. (A) Identical BCR transgenic B cells were stained with a division tracking dye and loaded with cognate fluorescent antigen. B cells containing high levels of antigen were sorted and cultured. Flow cytometry profile shows undivided B cells (black, mother cells) and B cells that have undergone one division (daughter B cells). Asymmetric segregation of antigen during B cell division induces a wide variation of antigen content for daughter B cells (color coded as: red > green > blue), which correlates with competitiveness for T cell help. (B) Schematic representation of the impact of antigen distribution in the endosomal compartment of mother cell on the level of asymmetry of antigen segregation during B cell division. The influence of this process on competitiveness of daughter B cells for T cell help is shown on the right hand side.
Regardless, it is tempting to speculate that asymmetric segregation of organelles during B cell division contributes to diversification of B cell clonal progeny, a role analogous to what proposed for asymmetric division of proteins in dividing T cells.9 Reiner’s group was indeed the first to demonstrate unequal partitioning of membrane and cytoplasmic proteins in dividing T lymphocytes, a process that leads to the generation of two phenotypically distinct populations of daughter cells. Unlike this division in T cells, asymmetric antigen segregation is not dependent on contact with an antigen-presenting cell.
So, what are the mechanisms involve in unequal inheritance of antigen in daughter B cells then? Using high-resolution imaging analysis we observed an unsuspected heterogeneity in antigen polarization within activated B cells. Identical BCR transgenic B cells that have internalised the same amount of antigen have it distributed in a different number of endosomal vesicles4 (Fig. 1). Interestingly, endosomes do not fragment during mitosis,10 they colocalize with microtubules and display directional movement leading to clustering at the mitotic spindle poles. Because endosome association with either spindle is a random process, the partitioning is ultimately not more precise than expected from a stochastic mechanism.10 Finally, the number of endosomes loaded with antigen in the mother cell, and the association of these with either spindle (which is independent of their antigen content) represent two separate processes that combine to define the level of asymmetry of antigen segregation between the two daughter cells (Fig. 1).
Thus, asymmetric antigen segregation during B cell division may represent a deterministic element in B cell differentiation the importance of which need to be further assessed.
Acknowledgments
We thank Naomi E. Harwood for critical reading of the manuscript. This work was funded by Cancer Research UK. F.D.B. also receives support from the Royal Society Wolfson Merit Award and O.T. receives financial support from Société Française de Transplantation, Société de Nephrologie, and Fondation pour la Recherche Medicale.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20634
References
- 1.Fleire SJ, et al. Science. 2006;312:738–41. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 2.Schnyder T, et al. Immunity. 2011;34:905–18. doi: 10.1016/j.immuni.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Siemasko K, et al. J Immunol. 1998;160:5203–8. [PubMed] [Google Scholar]
- 4.Thaunat O, et al. Science. 2012;335:475–9. doi: 10.1126/science.1214100. [DOI] [PubMed] [Google Scholar]
- 5.Duffy KR, et al. Science. 2012;335:338–41. doi: 10.1126/science.1213230. [DOI] [PubMed] [Google Scholar]
- 6.Paus D, et al. J Exp Med. 2006;203:1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Victora GD, et al. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dustin ML, et al. Science. 2012;335:408–9. doi: 10.1126/science.1218165. [DOI] [PubMed] [Google Scholar]
- 9.Chang JT, et al. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 10.Bergeland T, et al. Curr Biol. 2001;11:644–51. doi: 10.1016/S0960-9822(01)00177-4. [DOI] [PubMed] [Google Scholar]