Abstract
Comment on: Gärtner A, et al. EMBO J 2012; <span class="b">31</span>:1893-903
Keywords: N-cadherin, axon, centrosome, cortex, migration, neuronal polarity
The very first step in the establishment of neuronal polarity consists of the generation of a neurite from a spherical cell. Later, other neurites form, and typically one differentiates into the axon, while the remaining neurites become dendrites. The spatial selection of the site from which the first neurite emerges is a crucial developmental event, since (1) it determines the location from which the second neurite grows—at the diametrically opposite pole—creating a bipolar phenotype and (2) it confers to cultured hippocampal neurons some “preferred axon growth characteristics.”1 The bipolar phenotype is responsible for directed migration and thus, final positioning of cortical neurons, implying that any molecular defect in this first step of neuronal polarity will severely affect brain development, altering the final orientation and integration of neurons in the cortex.
An essential question is to what extent the selection of the site where the first neurite forms is extrinsically or intrinsically determined. The idea that neuronal polarity is established cell-autonomously derives from the observation that most isolated neurons in vitro can establish their first axon/dendritic polarity in the absence of asymmetric extracellular cues.2 However, it is also known that the asymmetric presence of external cues can influence the position of the axon.3,4 We believe that two recent publications from our laboratory5,6 help to clarify this question. The main findings and conclusions are summarized below.
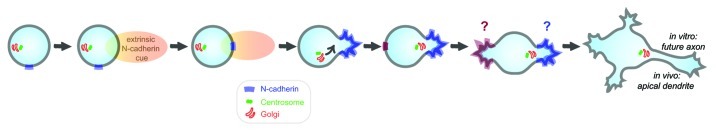
A number of extrinsic cues such as laminin, tenascin and N-cadherin are found in situ in the cortex and are likely to contribute to early neuronal development. Therefore, we tested in hippocampal neurons in vitro the effect of locally restricted stimulation of one cell pole with the abovementioned cues. This study revealed that N-cadherin specifically has the capacity to assign the site of first neurite outgrowth.5 Moreover, this experiment revealed that the centrosome and Golgi moved to the pole induced by N-cadherin only after the first neurite started to grow at the N-cadherin crescent.5 These results indicate that intracellular organelle positioning has, if any, a secondary, non-instructive role in the hierarchy of polarity triggering mechanisms. A similar sequence of polarization events was observed in sensory neurons in Drosophila, where a primary cadherin landmark was seen shortly after neuron generation, followed by the recruitment of the centrosome.6 These results are consistent with the view that neuronal polarization is triggered by extrinsic cues. However, as previously mentioned, neurons can polarize in the absence of asymmetric cues, suggesting that newly generated neurons may have already a polarized adhesive machinery, and then not the triggering but the instruction of polarity might be intrinsic. In agreement with this, N-cadherin is already polarized in dissociated neurons in the absence of extrinsic N-cadherin signals before cell attachment and neurite formation, and N-cadherin-coated beads cluster at a single pole of dissociated cells.5 Also, newly generated neurons in the cortex in situ accumulate N-cadherin at one pole.5 One question for future research is to determine the mechanism by which N-cadherin is concentrated to one pole. Although our recent work in Drosophila sensory neurons suggests that this is a consequence of remnants from the last mitosis,6 direct evidence is still missing.
N-cadherin has important roles in the development of the cortex, since we observed that in utero electroporation-mediated expression of a dominant-negative form of N-cadherin in progenitors and nascent neurons caused a deviation from the typical radial cell axis of neurons exiting the intermediate zone. This subsequently led to defects in the final cortical positioning.5 Two important questions arise from our study: (1) what is the role of the centrosome at the first pole after morphological polarization, and (2) what are the mechanisms by which the accumulated N-cadherin leads to neurite growth and/or neuronal migration? The fact that the centrosome is consistently relocated to the N-cadherin crescent suggests that its positioning is important in subsequent stages of brain development. However, recent work based on the physical or genetic ablation of the centrioles demonstrated that a compact centrosome is not essential for neuronal polarity, per se.7,8 Regarding the fine mechanisms by which N-cadherin defines growth, our data show that N-cadherin is important immediately after a neuron is generated from radial glia in the VZ or from basal progenitors in the SVZ,5 possibly indicating that the migratory alignment cues might be provided by interaction with other newly generated neurons and/or progenitor cells. Cell-specific downregulation of N-cadherin might clarify where the cell-cell contacts that trigger polarized growth exactly arise. We5 and Jossin and Cooper9 demonstrated a defective alignment of neurons right after their exit from the multipolar stage in the intermediate zone. Neurons in the intermediate zone lose their contact to radial glia cells, and their re-entry into radial migration could be facilitated by N-cadherin mediated interactions with radial glia cells and/or already radially oriented neurons. Furthermore, another study allocates N-cadherin in the process of glia-independent somal translocation.10
It is now reasonable to conclude that the early polarization of the adhesion molecule N-cadherin is a key determinant of neuronal polarity: newly generated neurons present polarized N-cadherin, which, later, upon interaction with N-cadherin from other cells, neuron or glia, triggers directed growth and migration. (Fig. 1)
Figure 1. Model for N-cadherin involvement in neuronal polarization.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20797
References
- 1.Calderon de Anda F, et al. J Cell Sci. 2008;121:178–85. doi: 10.1242/jcs.023143. [DOI] [PubMed] [Google Scholar]
- 2.Dotti CG, et al. J Neurosci. 1988;8:1454–68. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esch T, et al. J Neurosci. 1999;19:6417–26. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polleux F, et al. Nature. 2000;404:567–73. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 5.Gärtner A, et al. EMBO J. 2012;31:1893–903. doi: 10.1038/emboj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollarolo G, et al. Nat Neurosci. 2011;14:1525–33. doi: 10.1038/nn.2976. [DOI] [PubMed] [Google Scholar]
- 7.Basto R, et al. Cell. 2006;125:1375–86. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Stiess M, et al. Science. 2010;327:704–7. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- 9.Jossin Y, et al. Nat Neurosci. 2011;14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco SJ, et al. Neuron. 2011;69:482–97. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]