Abstract
Comment on: van Leeuwen IMM, et al. Cell Cycle 2012; 11:1851-61
Keywords: aurora kinase inhibitors, chemoprotection, chemotherapeutics, cyclotherapy, drug selectivity, inhibitors, nutlin, p53
The occurrence of intolerable side effects in patients undergoing chemotherapy is still a major clinical hurdle. Finding tumor-specific therapy and exploiting the differences between normal cells and tumor cells has led to strategies targeting oncogenic mutations or the deficiency of tumor suppressor pathways in cancers. Alternatively, focusing efforts to protect normal cells from the side effects of chemotherapy has gained much interest, and harnessing cell cycle checkpoints to target tumor cells while sparing normal cells is an attractive strategy.
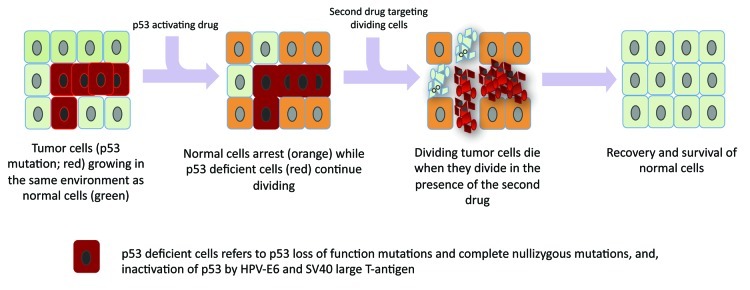
Contrary to achieving drug synergism when targeting tumor cells, one hopes to achieve drug “antagonism” on normal cells in a proposed strategy, termed “cyclotherapy.”1 The concept requires the use of two drugs, the first to arrest the normal cells and the second, to kill only cycling (tumor) cells (Fig. 1). The high frequency of p53 mutations in human cancers presents an opportunity for selectively targeting the p53-deficient tumors using S- or M-phase poisons, by activating a checkpoint arrest only in the normal cells. Indeed, several recent studies demonstrate that p53 activation protects normal cells from the toxicity of drugs acting in the S-phase or M-phase, including Ara-C,2 taxol,3 Aurora kinase inhibitors4 and Polo-like kinase inhibitors.5 The therapeutic window, in this case, is defined by (1) the toxicity of p53 activation in normal tissues, (2) the specificity of the “first” drug for p53 activity and (3) the effectiveness of the “second” drug in inducing p53-independent apoptosis. Exploring various combinations of p53-activating drugs and cell cycle-specific poisons may help to clarify the importance of each criteria. Ingeborg et al.6 recently performed a systematic screening of 16 drug combinations of p53 activators and S- or M-phase poisons for the protection of wild-type p53 cells while rendering selective toxicity against p53-deficient cells. Remarkably, all of the tested p53-activating drugs (Nutlin-3, a small-molecule antagonist of MDM2, Actinomycin D, Tenovin-6, Leptomycin B) antagonize the activity of microtuble-targeting compounds (vinblastine, vinorelbine) and antimetabolites (Ara-C and gemcitabine) in normal primary human fibroblasts, thus protecting them. They further explored the specificity of the observed drug antagonism for wild-type p53 cells by testing the same combinations on cell lines deficient for p53 functions. Interestingly, different classes of p53-activating drugs have varied outcomes in mutant p53 cells. While Nutlin and Actinomycin D, as expected, do not induce any arrest in mutant p53 cells, Tenovin-6 and Leptomycin B activated a cell cycle arrest that protected the mutant p53 cells against vinca alkaloids. Curiously, the cell cycle arrest only partially protected the mutant p53 cells from Gemcitabine and Ara-C. The study revealed that the specificity of the p53-activating drug for wild-type p53 is critical for cyclotherapy against p53-mutant tumors. Understanding what invokes a p53-independent cell cycle arrest in response to Tenovin-6 and Leptomycin B may help to prescribe the use of these drugs as chemoprotectants in another context.
Figure 1. Harnessing p53-dependent cell cycle arrest to protect normal cells and render selective drug toxicity to p53 mutant cells. Sequential drug therapy using a p53-activating drug that results in cell cycle arrest in normal cells and a second drug that targets only proliferating cells (any S- or M-phase specific poisons), results in selective killing of p53-deficient tumor cells while sparing normal cells.
Before p53-based cyclotherapy can be used in the clinic, one would like to know (1) the consequences of p53 activation in various normal tissues, (2) the outcome of p53 activation by different drugs, which may differentially effect p53-dependent gene transcription, either favoring cell cycle arrest, apoptosis or senescence, and (3) the right dosage of drugs to use for chemoprotection. High drug doses may induce other p53-independent cytotoxic effects that trigger DNA damage, synergizing with the activity of p53 to induce apoptosis instead. The observation that Nutlin can suppress neutropenia,5 a common side effect in chemotherapy, in mice treated with Polo-like kinase inhibitors provides great optimism.
Should the selection of new small molecules activating p53 for chemoprotection, be based on their selectivity for cell cycle arrest (over apoptosis)? Nutlin, for example, has low dose potency; however, because of its specificity for p53 activity and its low toxicity on normal tissues, it seems like a good candidate as a chemoprotectant. Apart from the p53 pathway, perhaps other oncogenic pathways may be considered for the cyclotherapy approach? For example, inhibition of the EGF, MEK or PI3-K prevents proliferation in normal cells but not in some tumor cells. UCN-01, a CHK kinase inhibitor, also prevents cell cycle progression and suppresses the toxicity of Topotecan in bone marrow.7 Finally, establishing in vivo models to test for potential drug combinations in cyclotherapy would be exciting in the future.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20961
References
- 1.Blagosklonny MV, et al. Cancer Res. 2001;61:4301–5. [PubMed] [Google Scholar]
- 2.Kranz D, et al. Cancer Res. 2006;66:10274–80. doi: 10.1158/0008-5472.CAN-06-1527. [DOI] [PubMed] [Google Scholar]
- 3.Carvajal D, et al. Cancer Res. 2005;65:1918–24. doi: 10.1158/0008-5472.CAN-04-3576. [DOI] [PubMed] [Google Scholar]
- 4.Cheok CF, et al. Cell Death Differ. 2010;17:1486–500. doi: 10.1038/cdd.2010.18. [DOI] [PubMed] [Google Scholar]
- 5.Sur S, et al. Proc Natl Acad Sci USA. 2009;106:3964–9. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Leeuwen IMM, et al. Cell Cycle. 2012;11:1851–61. doi: 10.4161/cc.20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redkar AA, et al. Int J Oncol. 2001;19:193–9. [PubMed] [Google Scholar]