Abstract
Comment on: Shchebet A, et al. Cell Cycle 2012; 2122-7
Keywords: H2B, P-TEFb, PCNA, UBE2A, cdk9, monoubiquitination
Preserving genome stability is critically important for cell survival. DNA is under continuous attacks from damaging agents that threaten proper replication and gene expression. The DNA damage response (DDR) coordinates repair throughout the cell cycle.
Cyclin-dependent kinase-9 (CDK9) has been implicated in double-strand break (DSB) repair and suppression of DNA:RNA hybrid (R-loop) formation. Moreover, recently it has been shown that it is important in the response to replication stress induced by HU and aphidicolin.1,2
CDK9, together with cyclins T1/T2, forms the positive transcription elongation factor-b (P-TEFb). CDK9 acts mainly through phosphorylation of Ser2 within the C-terminal repeat domain (CTD-S2ph) on RNA polymerase II and subsequent co-transcriptional histone modifications and mRNA processing. CTD-S2 phosphorylation leads to histone H2B monoubiquitination (H2Bub1) and histone H3 trimethylations.1
UBE2A (yeast Rad6 homolog) serves as the E2 and complexes with E3 ubiquitin ligase formed by RNF20/40 to monoubiquitinate H2B. CDK9-induced CTD-S2 phosphorylation recruits the RNF20/40 through interaction with WAC protein.3 Interestingly, in the absence of CTD-S2ph, some H2Bub1 still persists compared with CDK9 knockdown, suggesting additional modes of regulation.4
This report investigates the interaction between CDK9 and UBE2A. The authors show that CDK9 interacts with UBE2A and phosphorylates it in vitro and in vivo, similar to what has been found in yeast. Moreover, they explore the mechanisms for modulation of UBE2A activity and demonstrate that UBE2A phosphorylation affects the enzymatic activity but not its interaction with binding partners. Knockdown of CDK9 affects not only H2B but also PCNA monoubiquitination in the UV damage response, potentially affecting the DNA damage tolerance (DDT) and/or the Fanconi anemia (FA) pathways. Interestingly, cyclin T1 seems to contribute to this activity. Collectively, this report shows that the role of P-TEFb-mediated phosphorylation is more complex that initially realized and contributes to understanding of CDK9 in the response to the DNA damage.5
Yeast cells have two CTD-S2 kinases, Bur1 and Ctk1.6 CDK9 was considered the only mammalian homolog fulfilling both roles. Cyclin K has been implied to be the important player for the different pathways. However, recent studies have characterized a new kinase complex, CDK12/CyclinK.7 More and more evidence points at CDK12 as the functional homolog of Ctk1. Both play a role in DDR; however, their contributions are quite distinct (Fig. 1). CDK12 maintains genomic stability by direct regulation of the expression of ATR, BRCA1 and FANCI.7 However, CDK9 can act through both direct and indirect mechanisms. First it co-immunoprecipitates with the ATR-ATRIP-claspin complex, acting in a common pathway in response to HU.2 On the other hand, it is a potential global chromatin modulator due to its induction of H2Bub1 modification.1 H2Bub1 could orchestrate chromatin changes to allow repair mechanisms onto the DNA in response to specific DNA changes.
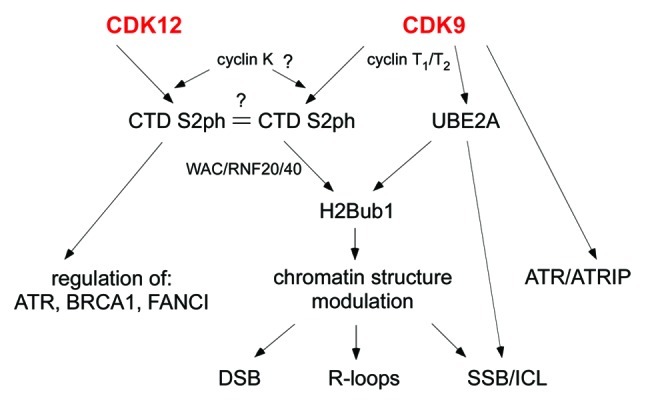
Figure 1. Schematic representation of CDK9/CDK12 influence on DNA Damage Response. CDK9 interacts with cyclins T1/T2 and possibly also cyclin K. CDK12 binds cyclin K. Both kinase/cyclin complexes can phosphorylate RNA polymerase II C-terminal on Serine 2 (CTD S2ph). There seem to be at least 2 forms of Ser2 marks, possibly distinct in response to the action of the two kinases. CDK12 affects genomic stability through the regulation of gene expression of DNA damage responsive genes (ATR, BRCA1, FANCI). CDK9 can have direct and indirect effects on genomic stability. CDK9 can directly form a complex with ATR/ATRIP. Moreover, CDK9 phosphorylates CTD (which recruits E3 ligases -RNF20/40) and E2 (UBE2A) both required for H2B monoubiquitination (H2Bub1). This modification plays a critical role in chromatin structure modulation, potentially affecting the response to different damaging stresses: double strand breaks (DSB), DNA-RNA hybrid structures (R-loops), single strand breaks (SSB) and interstrand DNA crosslinks (ICLs). UBE2A phosphorylation can also directly affect SSB and ICLs.
It is still not clear whether both CDK9 and CDK12 act in parallel or are activated in response to different stress signals. Interestingly, it seems that there are at least two forms of Ser2 marks, and they respond distinctly to depletion of the two kinases.7 Moreover, whether cyclin K is bound exclusively to CDK12 or if it also interacts with CDK9 under specific conditions remains to be verified. This study provides the first evidence that cyclin T1 contributes to DDR. It will be interesting to test whether the other cyclins (K and T2) can also play a role in the UV damage response. This is particularly interesting in the light of the recent study showing importance of cyclin K in response to camptothecin and ionizing radiation but not MMS (a UV mimetic).8
Both CDK9 and CDK12 can potentially contribute to cancer. This could be especially important in the response to inter-strand DNA cross-links in the FA pathway, where CDK9 can contribute by PCNA modification and CDK12 can directly affect the FANCI and FANCD2 expression. Encouragingly, studies in gastric and breast cancers have shown that the CDK9 inhibitor flavopiridol can increase the efficiency of Mitomycin C in the induction of apoptosis.9 Combining the CTD-S2ph inhibitors with radiotherapy or chemotherapy could refine the present strategies to fight cancer.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20963
References
- 1.Johnsen SA. FEBS Lett. 2012;586:1592–601. doi: 10.1016/j.febslet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Yu DS, et al. EMBO Rep. 2010;11:876–82. doi: 10.1038/embor.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F, et al. Mol Cell. 2011;41:384–97. doi: 10.1016/j.molcel.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirngruber J, et al. Cell Cycle. 2009;8:3636–42. doi: 10.4161/cc.8.22.9890. [DOI] [PubMed] [Google Scholar]
- 5.Shchebet A, et al. Cell Cycle. 2012;11 doi: 10.4161/cc.20548. [DOI] [PubMed] [Google Scholar]
- 6.Wood A, et al. Cell Cycle. 2006;5:1066–8. doi: 10.4161/cc.5.10.2769. [DOI] [PubMed] [Google Scholar]
- 7.Blazek D, et al. Genes Dev. 2011;25:2158–72. doi: 10.1101/gad.16962311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Haaften G, et al. Curr Biol. 2006;16:1344–50. doi: 10.1016/j.cub.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GK, et al. Clin Cancer Res. 1997;3:1467–72. [PubMed] [Google Scholar]


