Abstract
Comment on: Kolesnichenko M, et al. Cell Cycle 2012; 11:2391-401. and Pospelova TV, et al. Cell Cycle 2012; 11:2402-407.
Filling the Gaps
The Ser/Thr-kinase mTOR is a main metabolic sensor activated by the presence of nutrients, and whose activity orchestrates cell growth mainly through the inhibition of autophagy and the activation of protein synthesis.3 A large body of evidence indicates that reduced levels of mTOR activity favor organismal and cellular longevity, and the small drug compound rapamycin has been instrumental for the consolidation of this concept thanks to its ability to inhibit mTOR. In particular, chronic administration of rapamycin extends the lifespan of mice4 and, indeed, rapamycin is the only known compound able to increase longevity in mammals. Cellular senescence is a stress response that disables the proliferative capacity of cells while maintaining their viability,5 and there is evidence that cellular senescence and organismal aging share some mechanistic principles. Notably, contemporaneous to the description of the anti-aging activity of rapamycin in mice, it was also reported by Blagosklonny and collaborators that rapamycin delays cellular senescence induced by chemical stresses.6 Chemically induced senescence is a well-accepted model to study cellular senescence, but the question still remained whether replicative and oncogene-induced senescence are also delayed by rapamycin. Replicative senescence results after extensive proliferation of cells in vitro, and it is considered the most relevant type of cellular senescence in relation to organismal aging.5 On the other hand, oncogene-induced senescence is relevant for tumor suppression.5 These pending questions are now answered in the accompanying reports, where it is demonstrated that rapamycin impairs replicative senescence in human1 and rodent2 cells, as well as oncogene-induced senescence.1 This satisfactorily extends the longevity activity of rapamycin to in vitro cultured cells and validates cellular senescence as a model to study organismal aging in relation to the mTOR pathway.
Dissecting the mTOR Complexes
An important consideration regarding mTOR kinase derives from the fact that it assembles into two distinct multiprotein complexes named mTORC1 and mTORC2.3 To add further complexity, the inhibitory effect of rapamycin on these two complexes depends on the dose and duration of the treatment. In general, short treatments with rapamycin at low doses produce partial inhibition of mTORC1 (and activation of mTORC2 as a result of the attenuation of the negative feedback loop that connects mTORC1 with mTORC2), whereas chronic treatment or high doses can inhibit both mTORC1 and mTORC2.3 In this complex scenario, it is not obvious whether the effects of rapamycin on organismal and cellular longevity are mediated by mTORC1, mTORC2 or both. An initial clue came from the observation that female mice lacking S6K1, a key substrate of mTORC1 (but not of mTORC2), have an increased longevity.7 This has been further supported by the report this year that female mice doubly heterozygous in mTOR and mLST8 are partially deficient in mTORC1 activity (but not in mTORC2 activity) and also present an extended lifespan.8 The molecular basis for the selective deficit in mTORC1 activity is not fully understood, because mLST8 is common to both mTORC1 and mTORC2 complexes, although a simple explanation could be their differential affinity for mLST8. The emerging central role of mTORC1 inhibition in longevity is now complete thanks to the accompanying report by Sun and collaborators.1 In this paper, the authors demonstrate that selective inhibition of mTORC1, but not of mTORC2, impairs replicative and oncogene-induced senescence (Fig. 1).1
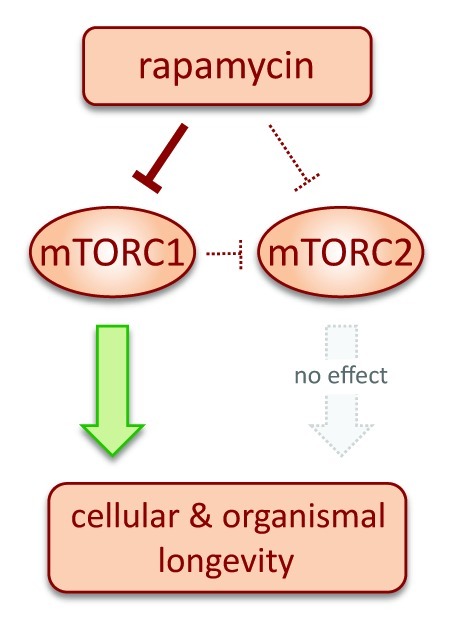
Figure 1. Inhibition of mTORC1, but not of mTORC2, promotes cellular and organismal longevity.
Implications
These two new papers extend and solidify the anti-aging activity of rapamycin through inhibition of mTORC1.1,2 A point of worry is the inhibition of oncogene-induced senescence by rapamycin, which could suggest a pro-tumorigenic effect of rapamycin. This concern, however, is mitigated by the fact that chronic treatment of mice with rapamycin does not produce an increase in spontaneous tumors.4 Nonetheless, rapamycin is not without undesirable effects, due to its pro-diabetic activity.9 The pro-diabetic activity of rapamycin is now known to be due to the inhibition of mTORC2 (and not to the inhibition of mTORC1).8 In this state of affairs, the identification of rapamycin analogs highly selective for mTORC1 inhibition holds the promise of a more potent anti-aging activity.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21065
References
- 1.Kolesnichenko M, et al. Cell Cycle. 2012;11 doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pospelova TV, et al. Cell Cycle. 2012;11 doi: 10.4161/cc.20882. [DOI] [PubMed] [Google Scholar]
- 3.Zoncu R, et al. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison DE, et al. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collado M, et al. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Demidenko ZN, et al. Cell Cycle. 2009;8:1888–95. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- 7.Selman C, et al. Science. 2009;326:140–4. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamming DW, et al. Science. 2012;335:1638–43. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham JT, et al. Nature. 2007;450:736–40. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]


