Abstract
Introduction
Folate receptor alpha (FRα) and reduced folate carrier-1 (RFC1) regulate uptake of folate molecules inside the cell. FRα is a potential biomarker of tumors response to antifolate chemotherapy and a target for therapy using humanized monocloncal antibody. Information on the protein expression of these receptors in non–small cell lung carcinoma (NSCLC) is limited.
Material and Methods
Expressions of FRα and RFC1 were examined by IHC in 320 surgically resected NSCLC (202 adenocarcinomas and 118 squamous cell carcinomas) tissue specimens and correlated with patients’ clinicopathologic characteristics. FOLR1 mRNA expression was examined using publicly available microarray datasets. FRα expression was correlated with thymidylate synthase (TS) and p53 expression in NSCLCs, and with EGFR and KRAS mutations in adenocarcinomas.
Results
NSCLC overexpressed FRα and RFC1. In a multivariate analysis, lung adenocarcinomas were more likely to express FRα in the cytoplasm (odds ratio [OR] = 4.39; P<0.0001) and membrane (OR = 5.34; P<0.0001) of malignant cells than squamous cell carcinomas. Tumors from never-smokers were more likely to express cytoplasmic (OR = 3.35; P<0.03) and membrane (OR = 3.60; P=0.0005) FRα than those from smokers. In adenocarcinoma, EGFR mutations correlated with higher expression of membrane FRα and FOLR1 gene expressions. High levels of FRα expression was detected in 42 NSCLC advanced metastatic tumor tissues.
Conclusions
FRα and RFC1 proteins are overexpressed in NSCLC tumor tissues. The high levels of FRα in lung adenocarcinomas may be associated to these tumors’ better responses to antifolate chemotherapy and represents a potential novel target for this tumor type.
Keywords: non–small cell lung carcinoma, EGFR, membrane transporter, FRα, FRC1
INTRODUCTION
Lung cancer represents the first cause of death for cancer worldwide 1. Most patients with lung cancer are diagnosed at advanced metastatic stage (IV), requiring systemic treatment.1 Two types of non-small cell carcinoma (NSCLC), adenocarcinoma and squamous cell carcinoma (SCC), are the most frequent (~80%) histological types of lung cancer.2 Despite intensive research on molecular targeted therapy, chemotherapy still represents the main treatment option for patients with advanced NSCLC.3 In addition, over recent years chemotherapy after surgical resection has become the standard of care for treatment of selected patients with early stage (i.e., stage IB, II, or IIIA) NSCLC.4 However, a subset of tumors does not respond to chemotherapy, and most tumors develop drug resistance, leading to chemotherapy failure.2 The factors associated with chemotherapy resistance are not well understood, but some phenomena have been associated with this resistance, including, among others, decreases or alterations in the membrane transporters involved in drug uptake systems or increase in drug efflux pumps.5
Folate receptor alpha (FRα) and reduced folate carrier-1 (RFC1) regulate cellular uptake of folate molecules inside the cell.5–7 Folates are required in the synthesis of nucleotide bases, amino acids, and other methylated compounds, and consequently, they are required in larger quantities by proliferating cells.5 FRα is a glycoprotein that is anchored to the apical cell membrane of normal epithelial cells,8 and binds folate at a high affinity to mediate transport into the cytoplasm of cells.5 RFC1 is more ubiquitously expressed in normal cells, binds folate at low affinity, and represents the sole folate uptake pathway for most normal cells 7.
FRα expression is upregulated in a range of human tumors, including ovarian, mesothelioma, lung and colorectal cancer.9–13 However, the level of expression of RFC1 in tumors is less known. FRα has emerged as a potential marker for response to treatment of human carcinomas with the drug pemetrexed,14 a potent inhibitor of thymidylate synthase (TS) and other folate-dependent enzymes.15–17 Interestingly, FRα has been also investigated as a potential novel molecular target for human tumors.18–20 Recently, a humanized monoclonal antibody against FRα has been tested in a Phase I clinical trial in patients with advanced chemorefractory ovarian carcinomas.19
In this study, we aimed to characterize the expression of FRα and RFC1 proteins in a large series (n=320) of surgically resected NSCLC tissue specimens with annotated clinicopathologic features. In addition, we investigated the expression of FRα in a small series (n=42) of advanced metastatic NSCLC tumor tissues. In surgically resected tumors we correlated the expression of FRα with the expression of TS. Our findings of higher expression of FRα expression in lung tumors with adenocarcinoma histology and tumors obtained from never-smokers prompted us to correlate the expression of FRα with tumors’ epidermal growth factor receptor (EGFR) and KRAS mutation status in adenocarcinomas, and with tumors’ p53 protein expression in all NSCLCs.
METHODS
Case selection and tissue microarray (TMA) construction
We obtained archived formalin-fixed and paraffin-embedded (FFPE) NSCLC tissues from the Lung Cancer Tissue Bank at The University of Texas M. D. Anderson Cancer Center (Houston, TX). We selected lung cancer tissue specimens from surgically resected NSCLCs with curative intent between 1997 and 2001, and constructed TMAs using three 1-mm diameter cores. Detailed clinico-pathologic information was available for most cases (Table 1). In addition, we selected FFPE NSCLC tumor tissues from diagnostic tissue specimens from 42 advanced metastatic NSCLCs. The tissue specimens were histologically classified according to the 2004 World Health Organization classification.2 The institutional review board at M. D. Anderson Cancer Center approved our study.
Table 1.
Summary of clinicopathologic features of patients with NSCLC examined for membrane transporter and thymidylate synthase expression.
| Feature | NSCLC Histologic Type
|
||
|---|---|---|---|
| Squamous Cell Carcinoma (n = 118) | Adenocarcinoma (n = 202) | Total (n = 320) | |
| Mean age, years (SD), (range) | 68.4 (9.20), (43–90) | 64.9 (11.5), (33–88) | 66.2 (10.85), (33–90) |
| Sex | |||
| Male | 73 | 77 | 150 |
| Female | 45 | 125 | 170 |
| Smoking status† | |||
| Never | 4 | 52 | 56 |
| Ever | 113 | 150 | 263 |
| TNM stage | |||
| I | 62 | 134 | 196 |
| II | 36 | 25 | 61 |
| III | 18 | 36 | 54 |
| IV | 2 | 7 | 9 |
Smoking status and history were not available for one patient with squamous cell carcinoma.
Immunohistochemical staining and evaluation
To test the expression of the membrane transporters we used a monoclonal homemade antibody against FRα (clone Mb343, IgG), dilution 1:500, 13 and a polyclonal antibody against RFC1 (Abcam, Cambridge, MA), dilution 1:100. To assess the expression of TS, we used a monoclonal antibody (Zymed Carlsbad, CA, USA), dilution 1:100. For p53 analysis, we used mouse monoclonal antihuman p53, clone DO7 (Dako, Carpinteria, CA), dilution 1:400.
For FRα we used a previously published immunohistochemistry protocol.13 For RFC1 and TS, immunohistochemical staining was performed as follows: 5-μM FFPE tissue sections were deparaffinized and hydrated, and underwent heat-induced epitope retrieval in a DAKO antigen retrieval bath at 121°C for 30 seconds and 90°C for 10 seconds in a decloaking chamber (Biocare, Concord, CA), followed by a 30-min cool down. Prior to antibody immunostaining, endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 30 min. To block nonspecific antibody binding, tissue sections were incubated with 10% fetal bovine serum in Tris-buffered saline solution with Tween 20 for 30 min. The slides were incubated with primary antibody at ambient temperature for 60 min for all antibodies. This was followed by incubation with biotin-labeled secondary antibody (Envision Dual Link +, DAKO) for 30 min. Staining was developed with 0.05% 3′,3-diaminobenzidine tetrahydrochloride, which had been freshly prepared in 0.05 mol/L Tris buffer at pH 7.6 containing 0.024% H2O2, and then the slides were counterstained with hematoxylin, dehydrated, and mounted.
Two observers (M.N. and I.W.) jointly quantified the immunohistochemical expression of the membrane transporters (magnification 20×) in normal bronchial epithelium and lung tumor malignant epithelial cells. For each membrane transporter and TS, we defined 3 categories of intensity of immunostaining (0 to 3+). Next, an expression score (range, 0–300) was obtained by multiplying the intensity of staining by the percent of cells (0–100%) staining. p53 expression was categorized by percentage of tumor cells expressing nuclear p53 as positive (≥5%) or negative (0–5%).
EGFR and KRAS mutation analysis
Exons 18 through 21 of EGFR and exon 1 of KRAS were amplified by polymerase chain reaction (PCR) using intron-based primers as previously described.21, 22
Assessment of membrane transporter expression in microarray datasets
The cancer microarray database and integrated data-mining platform Oncomine23 was utilized to analyze the expression of FOLR1 (FRα) and SLC19A1 (RFC1), and in microarray databases of NSCLC available online.24–27 The statistical significances of differences in expression of the genes were provided by Oncomine and confirmed by a two-tailed t-test with random variance. Gene expression data of lung adenocarcinomas with annotated mutation data of EGFR and KRAS were obtained from the Ladanyi and Gerald laboratories at the Memorial Sloan-Kettering Cancer Center (MSKCC) (http://cbio.mskcc.org/Public/lung_array_data/).28 Available Affymetrix® raw data files of the transcriptomes of 190 adenocarcinomas (set I, n=88; set II, n=102) were analyzed using the BRB-ArrayTools version 3.7.0 software developed by using the BRB-ArrayTools v.3.7.0 developed by Dr. Richard Simon and BRB-ArrayTools Development Team.29 Robust multi-array analysis (RMA) was used for normalization of gene expression data using the R language environment.30 FOLR1 mRNA expression levels in both MSKCC datasets were median-centered by the Cluster v.2.11 software. Differences in normalized median-centered FOLR1 expression levels were assessed for statistical significance by the two-tailed test and P < 0.05 were considered statistically significant.
Statistical methods
Associations between biomarker expression scores and patient clinico-pathologic data were assessed using the Wilcoxon’s rank sum test or Kruskal-Wallis test, as appropriate, for continuous variables and the chi square test for categorical variables. The immunohistochemical expression of the markers was dichotomized in negative (score=0) and positive (score>0) expressions based on the graphical distribution of the scores. For recurrence free survival (RFS) and overall survival (OS) analyses, we tested binary cutoff points of biomarkers using the median expression score for each marker. Univariate and multivariate Cox proportional hazards regression models were used to assess the effects of covariates on survival. All statistical tests were two-sided, and P values <0.05 were considered statistically significant.
RESULTS
Expression of FRα and RFC1 in surgically resected tumors
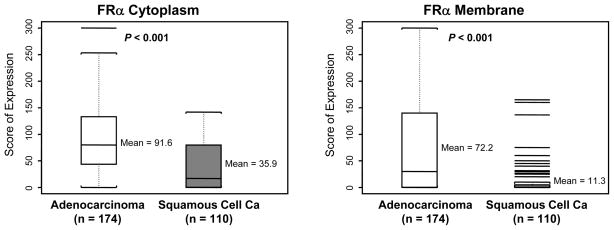
Both adenocarcinoma and SCC expressed relatively high levels of FRα and RFC1 in the malignant cells (Fig. 1 and Table 2). For FRα, the average expression scores and frequency of any expression (score >0) were significantly higher in adenocarcinomas than in SCCs at membrane (P<0.001) and cytoplasmic (P<0.001) localizations (Fig. 2). Both NSCLC histologies demonstrated similar levels of cytoplasmic and membrane RFC1 expression. For both markers the tumor cells exhibited stronger immunohistochemical expression than the 11 samples of normal bronchial epithelia adjacent to tumors (data not shown).
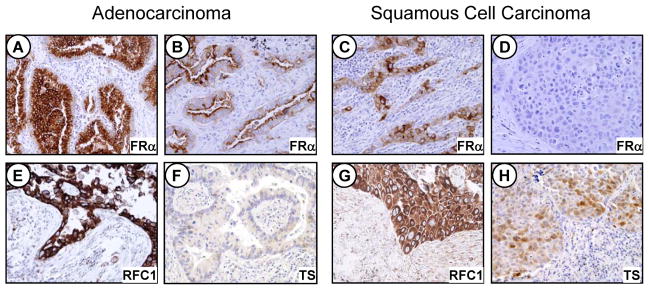
Figure 1.
Photomicrographs showing immunohistochemical expression of FRα, FRC1 and TS in NSCLC tissue specimens by histologic type. FRα: A, strong cytoplasmic and membrane expression in tumor cells; B and C, moderate expression in tumor cells; D, lack of expression in malignant cells. RFC1: E and G, strong cytoplasmic expression in malignant cells. TS: F and H, negative and moderate cytoplasmic and nuclear expression in tumor cells, respectively. Original magnification, ×200.
Table 2.
Frequency of membrane transporters and thymidylate synthase (TS) immmunohistochemical expression in NSCLC by tumor histology
| Marker | Any Expression (Score > 0) | Average Score | ||||
|---|---|---|---|---|---|---|
| Adenocarcinoma Positive/Total (%) | SCC¥ Positive/Total (%) | P Value* | Adenocarcinoma Score (SD) | SCC¥ Score (SD) | P Value† | |
| FRα | ||||||
| Cytoplasm | 152/174 (87%) | 63/110 (57%) | < 0.001 | 91.6 (66.4) | 35.9 (40.3) | < 0.001 |
| Membrane | 107/174 (61%) | 29/110 (26%) | < 0.001 | 72.2 (89.0) | 11.29 (28.8) | < 0.001 |
| RFC1 | ||||||
| Cytoplasm | 181/182 (99%) | 110/112 (98%) | 0.56 | 162.7 (83.2) | 153.2 (72.0) | 0.34 |
| Membrane | 164/182 (90%) | 103/112 (92%) | 0.68 | 128.1 (95.9) | 119.2 (86.1) | 0.59 |
| TS | ||||||
| Cytoplasm | 130/165 (79%) | 82/102 (80%) | 0.75 | 52.2 (40.1) | 55.6 (42.0) | 0.565 |
| Nuclear | 58/165 (35%) | 59/102 (58%) | 0.0003 | 9.3 (21.1) | 13.8 (27.7) | 0.0043 |
SCC = Squamous cell carcinoma
Fisher’s exact test
Wilcoxon rank-sum test
Figure 2.
FRα expression scores by tumor histology. In the box-plots, black bar indicates median scores.
Correlation of FRα and RFC1 expression with clinicopathologic features in surgically resected tumors
The multivariate analysis of the immunohistochemical expression of the two membrane transporters as a dichotomized variable (positive, score >0, vs. negative, score =0), after adjustment for patient’s tumor histology, smoking history, sex, and disease stage, revealed that adenocarcinomas were more likely than SCCs to express cytoplasmic (odds ratio [OR] = 4.39; P<0.0001) and membrane (OR = 5.34; P<0.0001) FRα. In addition, tumors from never-smokers were significantly more likely to express cytoplasmic (OR = 3.35; P < 0.03) and membrane (OR = 3.60; P=0.0005) FRα than those of smokers. In the multivariate analysis, the patient’s sex was not an independent significant factor influencing tumor expression of FRα. No correlation was found between expression of both membrane transporters and RFS or OS in 230 patients with stage I or II NSCLCs (median follow up, 7.2 years).
Correlation between FRα expression and tumors’ p53 expression and EGFR and KRAS mutation status
Our findings of higher expression of FRα expression in lung tumors with adenocarcinoma histology and tumors obtained from never-smokers prompted us to correlate the expression of FRα with tumors’ EGFR and KRAS mutation status in adenocarcinomas, and with tumors’ p53 protein expression in all NSCLCs.
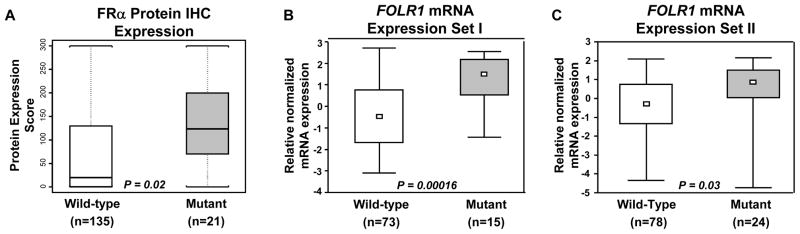
In lung adenocarcinomas, EGFR mutant tumors demonstrated significantly higher expression scores for membrane FRα (mean scores: mutant 134.8 vs. wild-type 67.1; P=0.002) than wild-type tumors (Figure 3, A). No correlation between FRα expression and adenocarcinoma tumors’ KRAS mutation status was detected.
Figure 3.
FRα protein (A) and FOLR1 mRNA (B and C) expression scores in lung adenocarcinoma by EGFR mutation status. A, FRα protein expression was determined by immunohistochemistry (IHC). B and C, FOLR1 expression is based in 2 publicly microarray datasets available.28 In the box-plots, small open box indicates median mRNA expression scores.
Of all NSCLCs tested, 38% (75/195) of adenocarcinomas and 69% (80/116) of SCCs had a positive p53 level (≥5%). Interestingly, we found that the scores for FRα expression in both membrane (P=0.001) and cytoplasm (P<0.001) were significantly lower in malignant cells from NSCLC tumors with positive p53 expression (mean score: membrane 33.4, SD 59.9, and cytoplasm 58.3, SD 60.0) than in tumors with negative p53 expression (mean score: membrane 65.3, SD 90.6, and cytoplasm 83.55.3, SD 65.3).
Expression of FRα in advanced metastatic tumors
To determine the levels of FRα expression in the entire spectrum of NSCLC, we examined FRα expression in 42 tumor tissues obtained from advanced NSCLCs (27 from lung/pleural tumors and 15 from metastatic sites). The tumor histologies corresponded to 23 adenocarcinomas, 5 SCCs, and 14 tumors classified as NSCLCs without features of specific histology (NSCLC-no otherwise specified [NOS]). We found that advanced tumors demonstrated similar levels of FRα expression than surgically resected tumors by examining the average expression scores and frequency of any expression (score >0). Although small numbers, in the advanced tumors the FRα average expression scores were higher in adenocarcinomas than SCCs at membrane (mean score: adenocarcinoma 62.2. SD 81.2; SCC 20.0, SD 44.7; P=0.042) and cytoplasmic (mean score: adenocarcinoma 104.1, SD 88.5; SCC 22.0, SD 39.0; P=0.319) locations. NSCLC-NOS showed intermediate levels of FRα expression (mean score: membrane 20.7, SD 56.9, and cytoplasm 64.6, SD 96.5). In advanced tumors, any membrane expression (score > 0) of FRα was detected in 48% (11/23) of adenocarcinomas and 20% (1/5) of SCCs, whereas cytoplasmic expression was observed in 78% (18/23) and 40% (2/5) SCCs. No significant difference in the expression of FRα was detected when comparing lung/pleural tumors with metastatic sites (data not shown).
FOLR1 mRNA expression in tumor tissues
Our findings that protein levels of FRα was greater in adenocarcinomas than in SCCs incited us to analyze expression levels of the mRNA of the FOLR1 in published microarray datasets of surgically resected (satges I–III) NSCLC tumor specimens and compare them by histologic type.24–27 In accordance with our immunohistochemistry data, FOLR1 mRNA expression levels were significantly higher in adenocarcinomas (n=197) than in SCCs (n=210) in all four datasets available: 1.8 vs. 1.0 (P<0.0001),25 0.81 vs. 0.73 (P=0.03),27 2.61 vs. 0.98 (P<0.0001),26 and 0.93 vs. 0.31 (P<0.0001). 24
To confirm our findings on the increased FRα immunoreactivity in tumors obtained from EGFR mutant lung adenocarcinomas compared to wild type tumors, we probed this association using the mRNA expression levels of FOLR1 in publicly available microarray datasets with information on EGFR and KRAS mutation status.28 Notably, the analysis of the microarray data further revealed the statistically significant up-regulation of FOLR1 mRNA levels in EGFR mutant lung adenocarcinomas compared to wild-type tumors in both available datasets (P=0.00016 and P =0.003) (Fig. 3, B and C). In addition, no statistically significant differences were found in FOLR1 expression levels between KRAS mutant lung adenocarcinomas and wild-type tumors (data not shown). These findings confirm the close positive association between FOLR1 expression and EGFR mutation status which we had found at the protein level by assessment of FRα immunoreactivity.
Correlation of immunohistochemical expression of TS and FRα
TS was expressed frequently in the nucleus and cytoplasm of malignant NSCLC cells. However, the frequency of any TS expression (score >0) was higher in the cytoplasm (212/267, 79%) than in the nucleus (117/267, 44%) of these cells. Although cytoplasmic expression of TS was similar in both NSCLC histologic types (Table 2), nuclear expression was significantly higher (P=0.003) in SCCs (mean score: 13.8, SD 27.7) than in adenocarcinomas (mean score: 9.3, SD 27.1). The level of TS expression did not correlate with clinicopathologic characteristics, including RFS and OS. In all NSCLC, significantly (P=0.02) higher expression of nuclear TS immunostaining was detected in tumors with positive p53 expression (67/114, 58%) than in those with negative p53 staining (65/147, 44%). In adenocarcinomas, there was no correlation between TS expression and EGFR or KRAS mutation status.
We correlated the expression of TS and FRα in NSCLC tissue specimens. The score for nuclear TS expression correlated negatively with the score for cytoplasmic FRα expression in SCCs (r = −0.20; P=0.04), and showed marginally significant negative correlation with membrane FRα expression in adenocarcinomas (r = −0.16; P=0.05). When we examined the correlation of any expression (score >0) of both markers in tumors, we found that in SCCs expression of nuclear TS was significantly inversely correlated (P=0.03) with membrane expression of FRα, and that most tumors positive for TS (62/79, 79%) lacked membrane FRα. This correlation was not detected in adenocarcinomas.
DISCUSSION
Membrane transporters FRα and RFC1 are considered potential biomarkers of tumor response to antifolate chemotherapy.14 Additionally, FRα represents a novel targets for therapy in human carcinomas utilizing monoclonal antibodies.19, 20 Information on the protein expression of these receptors in NSCLC is limited. Here, we report for the first time that NSCLC frequently overexpress both FRα and RFC1 proteins by studying a large series of cases with annotated clinico-pathologic information. Importantly, we report that tumor cells from lung adenocarcinoma histology expressed significantly higher levels of cytoplasmic and membrane FRα than SCC, and tumors from never-smokers were significantly more likely to express cytoplasmic FRα than those from smokers. In lung adenocarcinomas, the presence of EGFR mutations correlated with higher expression of membrane FRα and FOLR1 gene expression. NSCLC tissue specimens from advanced metastatic tumors showed similar levels of FRα expression than surgically resected tumors. We postulate that this information may be useful in selecting which patients with NSCLC may benefit from receiving treatment with antifolate inhibiting agents and monoclonal antibodies against FRα.
Our study showed that RFC1 is expressed frequently in the membrane and cytoplasm of malignant cells of NSCLC tumor tissues. While RFC1 performs its important biological functions at the cell membrane, the cytoplasmic expression can be explained as part of synthesis of the protein.31 The only report available on the expression of RFC1 in human tumors showed relatively high levels of mRNA gene expression in NSCLC, with similar expression in adenocarcinomas and SCCs.11 These data are consistent with our protein expression data showing that levels of expression of RFC1 were similar in the two histologic types.
Interestingly, in our study the expression of membrane and cytoplasmic FRα was significantly higher in surgically resected lung adenocarcinomas compared with SCCs. FRα is bound to the cell membrane where binds to folate and internalize it in the cytoplasm via endocytosis.6 Similar trend was detected in a small set of advanced metastatic NSCLC tumor tissues. FRα has been shown by immunohistochemical studies to be overexpressedpreviously in small sets of NSCLC tissue specimens.11, 12 However, to the best of our knowledge, there is not published report of FRα protein expression in NSCLC tumors and correlation with clinical and pathological features. Our protein expression findings agree with the significantly higher levels of expression of FOLR1 (FRα gene) mRNA in adenocarcinomas than in SCCs in all four public microarray datasets available.24–27 Similar findings have been reported in a quantitative (q)PCR study of mRNA expression of 119 NSCLC tissue specimens.11
The findings of higher levels of FRα protein and FOLR1 mRNA expression in adenocarcinomas than in SCCs of the lung may have important clinical implications. The higher level of FRα protein expression in adenocarcinoma cells may explain the better response of advanced NSCLC of non-squamous histology when treated with the combination of cisplatin and the multitargeted antifolate agent pemetrexed.32 However, this needs to be further tested in NSCLC tumor tissue specimens obtained from patients treated with pemetrexed. In addition, FRα is currently considered an attractive target for biologic therapy in tumors in which it is overexpressed compared to corresponding normal epithelium such as ovarian cancer by using the humanized monoclonal antibody against FRα Farletuzumab.19, 20 Our findings of high expression of FRα in NSCLC compared with normal bronchial epithelium suggest that this protein could be considered a novel potential target for NSCLC, particularly in lung adenocarcinomas.
Our finding that NSCLCs of never-smokers have a higher expression of FRα than those of smokers is of interest. Our data showing significantly higher cytoplasmic and membrane FRα expression in NSCLCs obtained from never-smokers are in agreement with the previous report of higher levels of mRNA FOLR1 by qPCR in adenocarcinomas from non-smokers and light smokers than in those from heavy smokers.11 These differences in the expression of FRα by smoking status are consistent with our findings of higher FRα expression in NSCLCs lacking p53 expression and in adenocarcinomas harbouring EGFR mutation, two features associated with the pathogenesis of non–smoking-related lung cancer.33 Of interest, the analysis of the publicly available microarray data confirmed at mRNA gene expression level our observation that EGFR mutant adenocarcinoma tumors expressed higher levels of FRα protein. There are not data available on the response to antifolate chemotherapy agents in lung adenocarcinomas based on EGFR mutation status. However, it has been shown that advanced stage adenocarcinoma harbouring this mutation showed improved response to other type of (carboplatin and paclitaxel) chemotherapy.34
Because of their roles in metabolism of the chemotherapy agent pemetrexed,14, 35 we correlated the expressions of TS and FRα in NSCLC tissue specimens by histologic type. As previously reported,36, 37 TS protein was expressed frequently in the nucleus (44%) and cytoplasm (79%) of malignant NSCLC cells. In our analysis we determined that nuclear expression was significantly higher in SCCs than in adenocarcinomas. Ceppi et al36 previously reported that immunohistochemical expression of TS mRNA and protein was significantly higher in SCCs of the lung than in adenocarcinomas. In this previously reported immunohistochemical analysis, however, expression of TS in the malignant cells was not distinguished as nuclear or cytoplasmic. It has been shown that low levels of TS mRNA expression significantly correlated with in vitro chemosensitivity of freshly explanted human tumor specimens to pemetrexed.38 It has been hypothesized that the higher mRNA and protein expressions of TS observed in SCCs explains the lower rate of response to pemetrexed in this NSCLC type.32 Recently, Sun et al39 reported that low immunohistochemical TS protein expression in tumors correlated with worse progression-free survival in stage IIIB and IV patients with non-squamous cell lung carcinomas treated with pemetrexed,
When we correlated FRα and TS protein expression in NSCLC tumors, we found that in SCCs the expression of nuclear TS had a significant inverse correlation with expression of membrane FRα, and most TS-positive SCCs (79%) lacked membrane FRα immunostaining. Furthermore, we speculate that the more frequent occurrence of the FRα membrane-negative/TS nuclear-positive expression pattern in lung SCCs than in adenocarcinomas could be associated with the lower response rate to pemetrexed in this tumor type. While FRα is most biologically active at the cell membrane,6 there is strong evidence of the important role of TS as translational regulation in the nucleus of cells.40
In summary, our findings indicate that membrane transporter FRα and RFC1 proteins are frequently overexpressed in NSCLC tissues. The higher level of FRα in adenocarcinomas than in SCCs may help explain differences in efficacy of antifolate chemotherapy between these tumor types. We postulate that this information may be useful in selecting which patients with NSCLC may benefit from and should receive treatment with antifolate inhibiting agents and with monoclonal antibodies against FRα.
Acknowledgments
Acknowledgments of research support: This study was supported in part by grants from the Department of Defense (W81XWH-07-1-0306 to D.J.S., and I.I.W.), the Specialized Program of Research Excellence in Lung Cancer (P50CA70907 to I.I.W.), and the National Cancer Institute (Cancer Center Support Grant CA-16672).
Abbreviations
- EGFR
epidermal growth factor receptor
- FFPE
formalin-fixed paraffin-embedded
- FRα
folate receptor alpha
- RFC1
reduced folate carrier 1
- TS
thymidylate synthase
- NSCLC
non–small cell lung carcinoma
- OR
odds ratio
Footnotes
Financial disclosure: there are no financial disclosures from any authors
References
- 1.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Muller-Hermelink HK, et al. Tumours of the lung. In: Travis WD, Brambilla E, Muller-Hermelink HK, et al., editors. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer (IARC); 2004. pp. 9–124. [Google Scholar]
- 3.Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y. Pharmacogenetics/genomics of membrane transporters in cancer chemotherapy. Cancer Metastasis Rev. 2007;26:183–201. doi: 10.1007/s10555-007-9050-6. [DOI] [PubMed] [Google Scholar]
- 6.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119:243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 7.Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26:111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- 8.Weitman SD, Weinberg AG, Coney LR, et al. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res. 1992;52:6708–6711. [PubMed] [Google Scholar]
- 9.Hartmann LC, Keeney GL, Lingle WL, et al. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–942. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 10.Bueno R, Appasani K, Mercer H, et al. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–233. doi: 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- 11.Iwakiri S, Sonobe M, Nagai S, et al. Expression status of folate receptor alpha is significantly correlated with prognosis in non-small-cell lung cancers. Annals of surgical oncology. 2008;15:889–899. doi: 10.1245/s10434-007-9755-3. [DOI] [PubMed] [Google Scholar]
- 12.Jin M, Kawakami K, Fukui Y, et al. Different histological types of non-small cell lung cancer have distinct folate and DNA methylation levels. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shia J, Klimstra DS, Nitzkorski JR, et al. Immunohistochemical expression of folate receptor alpha in colorectal carcinoma: patterns and biological significance. Hum Pathol. 2008;39:498–505. doi: 10.1016/j.humpath.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti GV, Ceppi P, Capelletto E, et al. Updated clinical information on multitargeted antifolates in lung cancer. Clin Lung Cancer. 2009;10 (Suppl 1):S35–40. doi: 10.3816/CLC.2009.s.006. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EC, Kuhnt D, Shih C, et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5- yl)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem. 1992;35:4450–4454. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 16.Schultz RM, Patel VF, Worzalla JF, et al. Role of thymidylate synthase in the antitumor activity of the multitargeted antifolate, LY231514. Anticancer Res. 1999;19:437–443. [PubMed] [Google Scholar]
- 17.Shih C, Habeck LL, Mendelsohn LG, et al. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA) Adv Enzyme Regul. 1998;38:135–152. doi: 10.1016/s0065-2571(97)00017-4. [DOI] [PubMed] [Google Scholar]
- 18.Ebel W, Routhier EL, Foley B, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- 19.Konner JA, Bell-McGuinn KM, Sabbatini P, et al. Farletuzumab, a Humanized Monoclonal Antibody against Folate Receptor {alpha}, in Epithelial Ovarian Cancer: a Phase I Study. Clin Cancer Res. 2010;16:5288–5295. doi: 10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 20.Spannuth WA, Sood AK, Coleman RL. Farletuzumab in epithelial ovarian carcinoma. Expert Opin Biol Ther. 10:431–437. doi: 10.1517/14712591003592069. [DOI] [PubMed] [Google Scholar]
- 21.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Shigematsu H, Bekele BN, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65:7568–7572. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomida S, Koshikawa K, Yatabe Y, et al. Gene expression-based, individualized outcome prediction for surgically treated lung cancer patients. Oncogene. 2004;23:5360–5370. doi: 10.1038/sj.onc.1207697. [DOI] [PubMed] [Google Scholar]
- 25.Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 26.Kim J. [Accessed August 29, 2007];Prediction of Recurrence-Free Survival in Postoperative NSCLC Patients—a Useful Prospective Clinical Practice. Available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE8894.
- 27.Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 28.Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene. 2009;28:2773–2783. doi: 10.1038/onc.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon R, Lam A, Li MC, et al. Analysis of Gene Expression Data Using BRB-Array Tools. Cancer Inform. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- 30.Irizarry RA, Bolstad BM, Collin F, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadlish H, Williams FM, Flintoff WF. Cytoplasmic domains of the reduced folate carrier are essential for trafficking, but not function. Biochem J. 2002;364:777–786. doi: 10.1042/BJ20011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 33.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 34.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 35.Adjei AA. Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res. 2004;10:4276s–4280s. doi: 10.1158/1078-0432.CCR-040010. [DOI] [PubMed] [Google Scholar]
- 36.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Z, Li X, Schell MJ, et al. Thymidylate synthase in situ protein expression and survival in stage I nonsmall-cell lung cancer. Cancer. 2008;112:2765–2773. doi: 10.1002/cncr.23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanauske AR, Eismann U, Oberschmidt O, et al. In vitro chemosensitivity of freshly explanted tumor cells to pemetrexed is correlated with target gene expression. Invest New Drugs. 2007;25:417–423. doi: 10.1007/s10637-007-9060-9. [DOI] [PubMed] [Google Scholar]
- 39.Sun JM, Han J, Ahn JS, et al. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol. 2011;6:1392–1399. doi: 10.1097/JTO.0b013e3182208ea8. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Schmitz JC, Lin X, et al. Thymidylate synthase as a translational regulator of cellular gene expression. Biochim Biophys Acta. 2002;1587:174–182. doi: 10.1016/s0925-4439(02)00080-7. [DOI] [PubMed] [Google Scholar]