Summary
Cytokine-induced beta-cell apoptosis is important to the etiology of type-1 diabetes. Although previous reports have shown that general inhibitors of histone deacetylase (HDAC) activity, such as suberoylanilide hydroxamic acid and trichostatin A, can partially prevent beta-cell death, they do not fully restore beta-cell function. To understand HDAC isoform selectivity in beta cells, we measured the cellular effects of eleven structurally diverse HDAC inhibitors on cytokine-induced apoptosis in the rat INS-1E cell line. All eleven compounds restored ATP levels and reduced nitrite secretion. However, caspase-3 activity was reduced only by MS-275 and CI-994, both of which target HDAC1, 2, and 3. Importantly, both MS-275 and genetic knock-down of Hdac3 alone were sufficient to restore glucose-stimulated insulin secretion in the presence of cytokines. These results suggest that HDAC3-selective inhibitors may be effective in preventing cytokine-induced beta-cell apoptosis.
Introduction
Type-1 diabetes involves autoimmune attack of insulin-producing beta cells in the pancreas. The inflammatory cytokines interleukin-1β (IL-1β), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNFα) are important mediators in the impaired function and destruction of pancreatic beta cells (Cnop et al., 2005; Fornoni et al., 2008; Soldevila et al., 1991) and are commonly used in vitro to mimic the events leading to beta-cell apoptosis. IL-1β and TNF-α induce NFκB expression, and downstream gene expression is thought to be regulated by nitric oxide (NO) signaling, which increases endoplasmic reticulum stress-response pathways and decreases beta cell-specific functions (Darville and Eizirik, 1998). NO is a highly reactive molecule that inhibits the electron-transport chain, decreasing glucose oxidation rates, ATP generation, and insulin production; cellular nitrite is more stable and serves as a surrogate marker for NO. NFκB activation and IFN-γ-induced STAT-1 signaling work together to induce beta-cell death by the intrinsic apoptotic pathway (Grunnet et al., 2009), including the loss of glucose-stimulated insulin secretion (GSIS), one of the most important physiological function of beta cells. Small molecules capable of preventing cytokine-induced beta-cell apoptosis could lead to new avenues for therapeutic intervention.
Recently, an important role for histone deacetylases (HDACs) in suppressing beta-cell apoptosis was suggested, perhaps by decreasing NFκB transactivation (reviewed in (Christensen et al., 2011))). Larsen et al. demonstrated that the HDAC inhibitors SAHA and trichostatin A (TsA) partially prevented cytokine-induced beta-cell toxicity (Larsen et al., 2007). However, neither SAHA nor TsA could restore GSIS. ITF2357, another broad-spectrum HDAC inhibitor, had in vivo activity, protecting mouse islets from cytokines and preventing hyperglycemia in streptozocin-treated mice (Lewis et al., 2010).
Histone deacetylases (HDACs) regulate gene transcription by modulating the lysine acetylation of histones. A number of HDAC inhibitors have been developed to study chromatin biology and are clinical candidates for cancer therapy (Minucci and Pelicci, 2006). Recently, a study demonstrated that ostensibly broad-spectrum HDAC inhibitors actually display different biochemical isoform selectivities (Bradner et al., 2010). For example, suberoylanilide hydroxamic acid (SAHA) inhibits the activities of HDAC1, 2, 3, 6, and 8, while MS-275 only inhibits the activities of HDAC1, 2, and 3. Based on these results, we reasoned that a more selective HDAC inhibitor could be more beneficial to beta-cell survival. To that end, we examined a structurally diverse panel of HDAC inhibitors, and genetically knocked down individual isoforms in the rat cell line INS-1E. Our results demonstrate that inhibition of HDAC3 appears to be sufficient to suppress beta-cell apoptosis in this system, and point to this enzyme as a potential target for preventing beta-cell apoptosis.
Results and Discussion
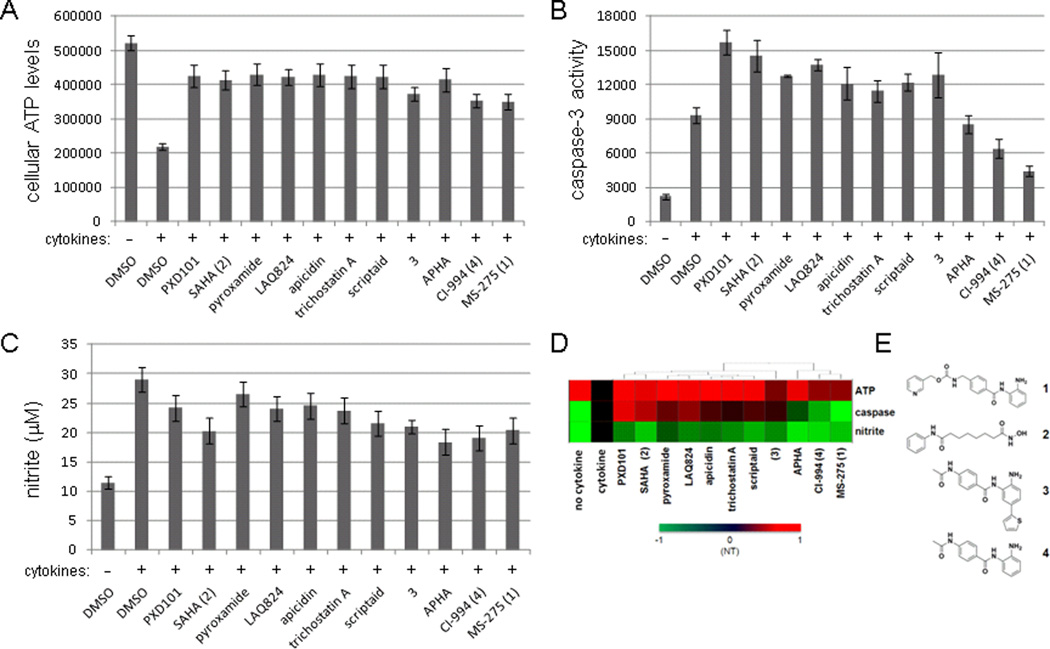
Using cellular ATP levels as a surrogate for cell viability (Chou et al., 2010), we tested the effects of eleven structurally diverse HDAC inhibitors (Figure S1) in protecting the rat INS-1E insulinoma cell line (Merglen et al., 2004) from cytokine-induced apoptosis. Most of the compounds tested suppressed the inhibitory effects of cytokines on ATP levels to varying degrees (Figure 1A). However, we observed very different effects on caspase-3 activity after compound treatment. MS-275 and CI-994 were the only two compounds that reduced caspase-3 activity significantly, while other HDAC inhibitors either were inactive or, like PXD101 and SAHA, even increased caspase-3 activity (Figure 1B). Most compounds only slightly decreased nitrite secretion, a surrogate for nitric oxide production, which was induced by cytokine treatment (Figure 1C). When we performed hierarchical clustering on the data, the compounds with the most beneficial effects on cytokine-treated beta cells clustered together, and in particular were those that selectively inhibited the activities of HDAC1, 2, and 3 (MS-275 and CI-994) (Figure 1D, E). These results suggest that only certain HDAC isoforms are important to cytokine-induced beta-cell apoptosis, and that the selectivity of HDAC inhibitors is an important consideration in assessing this activity.
Figure 1. Phenotypic profiling of eleven HDAC inhibitors in beta cells.
INS-1E cells were treated for 48 hours with a cocktail of pro-inflammatory cytokines (see Experimental Procedures) in the absence or presence of eleven HDAC inhibitors, and were assessed for (A) cellular ATP levels, (B) caspase-3 activity, and (C) nitrite production. Concentrations used were: PXD101, 0.2 µM; SAHA, 1 µM; pyroxamide, 5 µM; LAQ824, 1 µM; apicidin, 0.05 µM; trichostatin A, 0.05 µM; scriptaid, 1 µM; 3, 5 µM; APHA, 5 µM; CI-994, 10 µM; MS-275, 5 µM. Data represent the mean ± standard deviation of 24 independent wells. (D) Data in (A), (B), and (C) represented as a heat map. Activity for each compound treatment was scaled to a range of -1 (green), indicating a decrease in signal relative to the cytokine-treated state (“NT”), to 1 (red), indicating an increase in signal relative to the cytokine-treated state. The profile for each compound indicates the extent to which each assay was restored to the untreated (“no cytokine”) state. (E) The chemical structures of HDAC inhibitors studied in more detail: 1 (MS-275), 2 (SAHA), 3 (Methot et al., 2008), and 4 (CI-994). See also Figures S1 and S2.
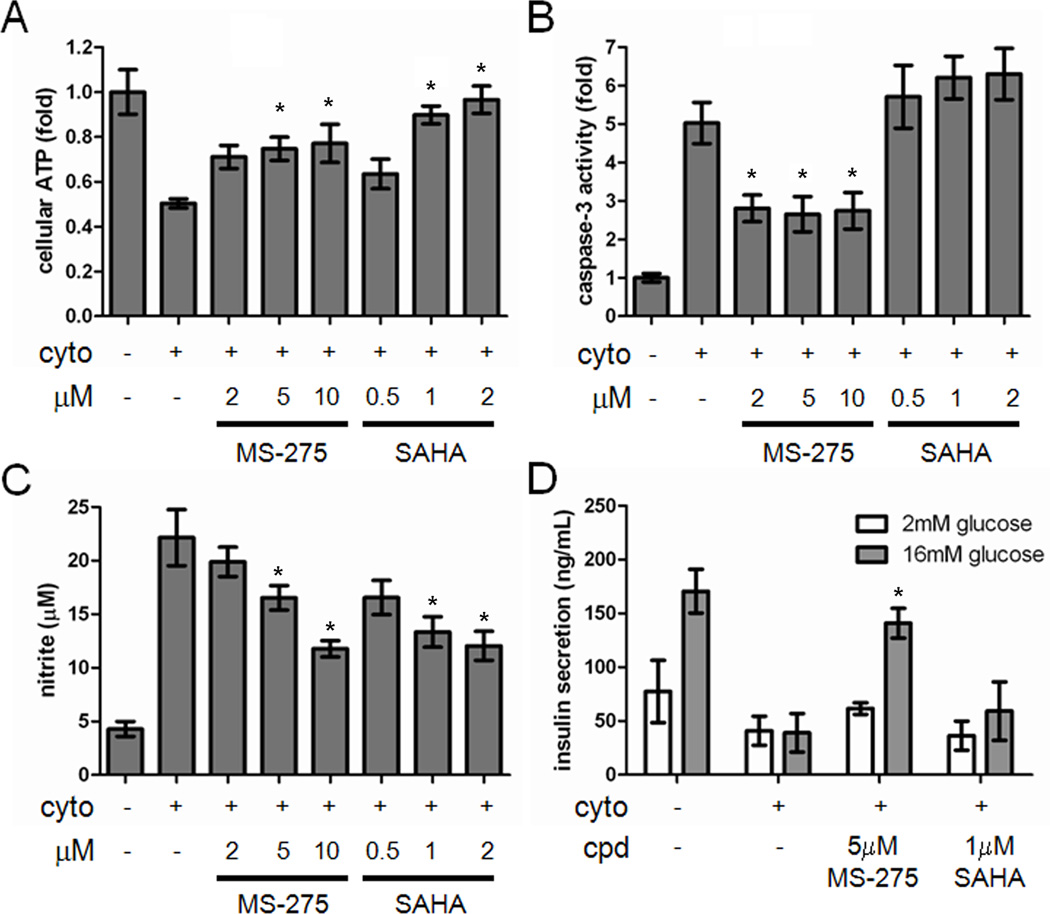
These measurements were made at a single compound concentration, the highest at which the cells remained viable (Figure S2). In order to determine concentration dependence, we tested MS-275 and SAHA as exemplars of class I (HDAC1, 2, 3) and broad-spectrum inhibition, respectively, over a 4- to 5-fold range of concentrations. Both MS-275 and SAHA restored ATP levels in INS-1E cells treated with cytokines, with SAHA resulting in a greater suppression (Figure 2A). However, SAHA slightly increased caspase-3 activity, while MS-275 reduced caspase-3 activity in the presence of cytokines by approximately 50% (Figure 2B). MS-275 was also more effective in restoring mitochondrial membrane potential, another readout of apoptosis, than SAHA (Figure S3). MS-275 and SAHA resulted in comparable reduction of nitrite production in the presence of cytokines (Figure 2C). Consistent with previous reports that SAHA could not restore the impairment in GSIS caused by treatment with cytokines (Larsen et al., 2007), we confirmed that 1 µM SAHA had no effect on GSIS, while 5 µM MS-275 restored GSIS in the presence of cytokines (Figure 2D). These results suggest that MS-275 may be a superior suppressor of cytokine-induced beta-cell apoptosis than less selective HDAC inhibitors.
Figure 2. Cytokine-induced apoptosis is suppressed by MS-275 but not SAHA.
(A) Effects of MS-275 and SAHA on cellular ATP levels in INS-1E cells after 48 hours in the presence of the cytokine cocktail. (B) Effects of MS-275 and SAHA on caspase-3 activity after 48-h treatment. (C) Effects of MS-275 and SAHA on the cellular production of nitrite after 48-h treatment. (D) Effects of MS-275 and SAHA on glucose-stimulated insulin secretion after 48-h treatment. Data represent the mean ± standard deviation of 8 independent wells for insulin secretion and 24 independent wells for others. * indicates p < 0.01 as compared to the cytokine treatment alone. See also Figure S3.
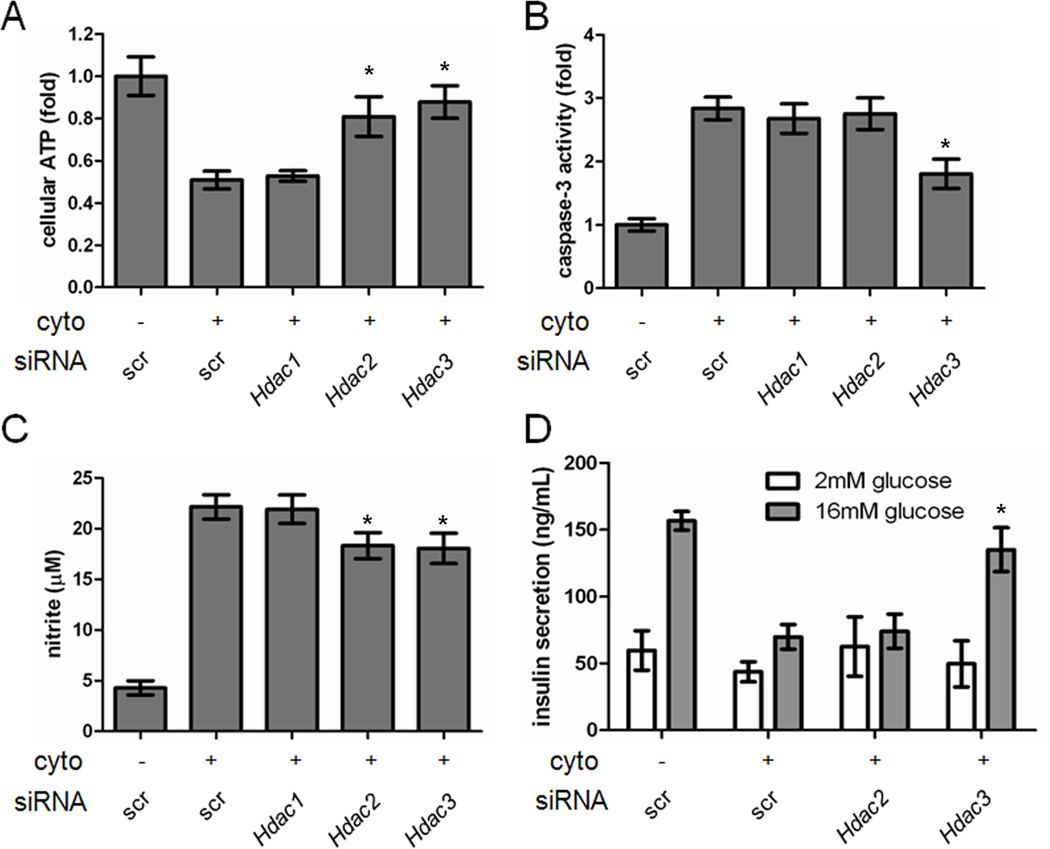
We then sought to determine the HDAC isoform(s) responsible for suppression of cytokine-induced beta-cell apoptosis. MS-275 is a selective inhibitor of HDAC1, 2, and 3, while SAHA additionally inhibits HDAC6 and 8 (Bradner et al., 2010). We reasoned that inhibition of HDAC1, 2, or 3 led to the protective effects of MS-275. To test this hypothesis, we performed gene-silencing experiments targeting these HDACs using small-interfering RNA (siRNA). siRNA duplexes specific for Hdac1, Hdac2, or Hdac3 were transfected into INS-1E cells, leading to a selective decrease in protein expression of the appropriate HDAC (Figure S4). Each knock-down alone had no effect on viability (Figure S5). Knock-down of Hdac2 or Hdac3 in the presence of cytokines each resulted in a 35% restoration of ATP levels, while knock-down of Hdac1 had no effect (Figures 3A, S6). We thus proceeded to examine the effects of knocking down Hdac2 or Hdac3. Only knock-down of Hdac3 was sufficient to reduce caspase-3 activity (Figure 3B). Knock-down of either Hdac2 or Hdac3 resulted in slight but statistically significant reduction in nitrite production (Figure 3C) and increase in mitochondrial membrane potential (Figure S4), while again knock-down of Hdac1 had no effect. We observed similar effects using a cell-death ELISA to measure DNA-histone complexes released into the cytoplasm (Figure S7). Importantly, knock-down of Hdac3 alone led to restoration of GSIS (Figure 3D), showing that the beta cells are fully functional. Knock-down of Hdac1 resulted in a moderate but statistically insignificant restoration of GSIS in INS-1E cells (Figure S8), and the restoration was to a lesser extent than Hdac3 knock-down. These results indicate that inhibition of HDAC3 is primarily responsible for the protective effects of MS-275 on beta cells in the presence of inflammatory cytokines. Further, insulin secretion does not appear to be correlated with ATP levels alone, but rather is more related to caspase activity.
Figure 3. Genetic knock-down of Hdac3 suppresses beta-cell apoptosis.
The effects of knocking down Hdac1, 2, or 3 on (A) cellular ATP levels, (B) caspase-3 activity, (C) nitrite production, or (D) glucose-stimulated insulin secretion. Data represent the mean ± standard deviation of 8 independent wells for insulin secretion and 24 independent wells for others. * indicates p < 0.01 as compared to the cytokine treatment alone. See also Figures S4-S8.
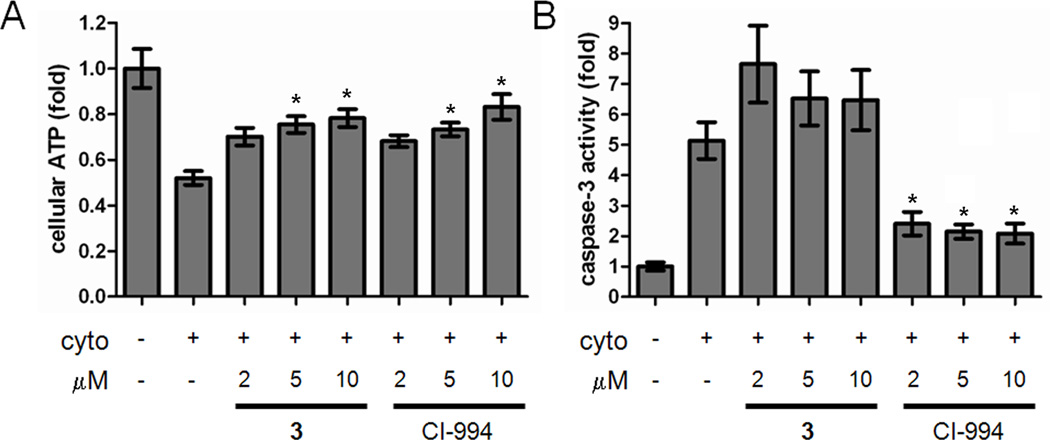
To further demonstrate the importance of HDAC3 in protecting insulin-secreting cells from cytokine-induced apoptosis, we used small-molecule inhibitors to chemically isolate HDAC3 activity. CI-994, an inhibitor selective for HDAC1, 2, and 3, restored ATP levels and reduced caspase-3 activity in INS-1E cells (Figure 4A, B). A 2-thiophenyl analog of CI-994, 3 (Figure 1B), exhibits inhibitory activity toward HDAC1 and 2 only (IC50 7 and 49 nM, respectively, vs. 10 µM for HDAC3) (Methot et al., 2008). Thus, we could indirectly assess the effects of HDAC3 inhibition by comparing the activities of 3 and CI-994. Although 3 restored ATP levels (Figure 4A), it did not reduce caspase-3 activity (Figure 4B). Mitochondrial membrane potential was completely restored by CI-994, but not by 3 (Figure S9). These results further demonstrate the crucial role of HDAC3 in cytokine-induced beta-cell apoptosis. Importantly, these data are limited to beta-cell lines; study in primary islets will enable target validation for future therapeutic development.
Figure 4. Analysis of chemical HDAC3 inhibition on beta-cell apoptosis.
Effects of 3 and 4 (CI-994) on (A) cellular ATP levels and (B) caspase-3 activity in the presence of cytokines. Data represent the mean ± standard deviation of 24 independent wells. * indicates p < 0.01 as compared to the cytokine treatment alone. See also Figure S9.
In conclusion, we evaluated a panel of eleven clinically advanced HDAC inhibitors and found that MS-275 and CI-994 suppress cytokine-induced beta-cell apoptosis, while the less selective inhibitors SAHA and TsA do not. By comparing these results to biochemical activities, we narrowed down the possible HDAC targets to a combination of HDAC1, 2, or 3. Using siRNA reagents and isoform-selective inhibitors, we observed that inhibition of HDAC3 appears to be critical for the protective effects of HDAC inhibitors, while the inhibition of additional isoforms may be deleterious to beta-cell function. It is possible there may be further protective effects on beta-cell physiology after HDAC1 inhibition as well. The lack of correlation between ATP levels and caspase activity after treatment with some of these compounds suggests that these assays are reading out different aspects of cell toxicity. This notion is consistent with a previous analysis of the lack of overlap between viability assay readouts in hepatoma cells (Miret et al., 2006). This study suggests the development of more selective HDAC3 inhibitors, and exploration of their potential uses in protecting pancreatic beta cells from inflammatory attack during the development of type-1 diabetes.
Significance
Illuminating the mechanism of inflammatory cytokine-induced beta-cell apoptosis will be important for understanding the etiology of type-1 diabetes. Small molecules capable of suppressing this apoptotic process may be suitable candidates for developing therapies to protect beta cells from autoimmune attack. Initial reports demonstrated that inhibition of histone deacetylase (HDAC) activity may be protective to beta cells (Larsen et al., 2007). However, broad-spectrum HDAC inhibitors cannot restore critical beta-cell functions such as glucose-stimulated insulin secretion, and may be toxic on their own. Thus, we sought to determine the isoform(s) responsible for beta-cell survival in this process. Here, we have demonstrated that both chemical and genetic inhibition of HDAC3 are sufficient to suppress apoptosis in a rat beta-cell line. Small molecules selective for HDAC1, 2, or 3 were more restorative to beta-cell function than broad-spectrum inhibitors like SAHA, suggesting that inhibition of additional HDAC isoforms causes additional toxicity to these cells. Most importantly, glucose-stimulated insulin secretion was restored by selective compounds such as MS-275, and by genetic knock-down of Hdac3. Future efforts to determine the cellular events regulated by HDAC3 in response to cytokine treatment, why inhibition of other isoforms may be toxic, and assessment of this effect in primary beta cells, will help clarify the pathways involved in the development of type-1 diabetes.
Experimental Procedures
Cell culture and reagents
INS-1E cells (generously provided by Claes Wollheim and Pierre Maechler, University of Geneva, Switzerland) were maintained in culture medium (RPMI 1640) containing 11 mM glucose, 10% fetal bovine serum, 10 mM HEPES, 50 µM 2-mercaptoethanol, 1 mM sodium pyruvate) and cultivated at 37C with 5% CO2 in a humidified atmosphere. In all compound-treatment experiments, final DMSO concentrations were kept to less than 0.5%. Recombinant rat IL-1β and recombinant mouse TNF-α were purchased from R&D Systems. Recombinant mouse IFN-γ and Griess reagent were purchased from Sigma. CellTiter-Glo and Caspase-Glo 3/7 reagents were purchased from Promega. Rabbit antibodies for HDAC1, 2 and 3 were from CellSignaling. Mouse monoclonal antibody to tubulin was from Sigma. Secondary horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit antibodies were from Thermo Fisher Scientific. HDAC inhibitors were purchased from Sigma or synthesized in-house.
Measurement of cellular ATP levels
INS-1E cells were seeded at 10,000 cells/well using a Multidrop Combi (Thermo Labsystems) in white optical 384-well plates (Corning Life Sciences). After overnight incubation, medium was removed and 50 µL RPMI containing the treated compound, 1% FBS and a combination of cytokines (10 ng/mL IL-1β, 50 ng/mL IFN-γ, 25 ng/mL TNF-α) was added to every well. After incubation for 48 hr, medium was removed and 20 µL CellTiter-Glo reagent was added. Luminescence was measured after 10-min incubation using an EnVision plate reader (PerkinElmer).
Measurement of cellular nitrite production
INS-1E cells were seeded at 10,000 cells/well using a Multidrop Combi (Thermo Labsystems) in white optical 384-well plates (Corning Life Sciences). After overnight incubation, medium was removed and 50 µL RPMI containing the treated compound, 1% FBS and a combination of cytokines (10 ng/mL IL-1β, 50 ng/mL IFN-γ, 25 ng/mL TNF-α) was added to every well. After treatment with cytokine and compounds for 48 hr, 10 µL modified Griess reagent (1:1 mixture of 1% sulfanilamide in 30% acetic acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 60% acetic acid) was added to each well. After 5-min incubation at room temperature, the absorbance at 540 nm was measured using an Envision plate reader (PerkinElmer).
Measurement of mitochondrial membrane potential
Upon depolarization, the JC-1 dye is converted from a diffuse green form to red fluorescent J-aggregates. The ratio of red to green fluorescence serves as a readout of the mitochondrial membrane potential (Reers et al., 1995). INS-1E cells were seeded at 10,000 cells/well using a Multidrop Combi (Thermo Labsystems) in white optical 384-well plates (Corning Life Sciences). After overnight incubation, medium was removed and 50 µL RPMI containing the treated compound, 1% FBS and a combination of cytokines (10 ng/mL IL-1β, 50 ng/mL IFN-γ, 25 ng/mL TNF-α) was added to every well. After treatment with cytokine and compounds for 48 hr, 20 µL of 3.25µM JC-1 was added to each well. After 3hr incubation at 37°C, the cells were gently washed three times with 50 µL per well of 1X calcium- and magnesium-free PBS. Fluorescence was measured with an EnVision plate reader (PerkinElmer) at the rhodamine spectra (excitation/emission 530 nm/580 nm) followed by fluorescein (excitation/emission 485 nm/530 nm). The ratio of rhodamine to fluorescein intensity was determined and represents the degree of mitochondrial membrane potential.
Caspase-3 activity assay
INS-1E cells were seeded at 5,000 cells/well using a Multidrop Combi (Thermo Labsystems) in white optical 384-well plates (Corning Life Sciences). After overnight incubation, medium was removed and 50 µL RPMI containing the treated compound, 1% FBS and a combination of cytokines (10 ng/mL IL-1β, 50 ng/mL IFN-γ, 25 ng/mL TNF-α) was added to every well. After treatment with cytokines and compounds for 48 hr, medium was removed and 20 µL Caspase-Glo 3/7 reagent was added. Luminescence was measured after 2-hr incubation using an Envision plate reader (PerkinElmer).
RNA interference and Western blotting
Small-interfering RNAs against Hdac1, 2 and 3 were obtained from Dharmacon. siRNAs (100 nM) were transfected into INS-1E cells (5,000 cells/well in a 384-well plate) using Dharma FECT reagent. Transfected cells were cultured for 72hr, then collected for Western blot analysis and cell-based assays. For Western blotting, cells were lysed in RIPA buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin). Total protein was separated by 4–12% SDS-PAGE and transferred to a PVDF membrane. Blots were developed using the chemiluminescence detection system SuperSignal (Thermo Fisher Scientific) and light emission was captured using an Imaging Station 4000MM (Carestream).
Glucose-stimulated insulin secretion
INS-1E cells were seeded in 96-well plates at 20,000 cells/well in 100 µL RPMI and incubated for 48 hr in 100 µL fresh RPMI containing 1% FBS and the cytokine cocktail in the presence or absence of 5 µM MS-275. Cells were washed and incubated for 2 hr in KRBH buffer (135 mM NaCl, 3.6 mM KCl, 5 mM NaHCO3, 0.5 mM NaH2PO4, 0.5 mM MgCl2, 1.5 mM CaCl2, 10 mM HEPES, pH 7.4, 0.1% BSA) lacking glucose. Cells were subsequently incubated with KRBH buffer containing 2 mM or 16 mM glucose for 1 hr. The supernatant was collected for measurement of secreted insulin. Insulin was measured with a rat insulin ELISA kit (Alpco).
Supplementary Material
Highlights.
Broad-spectrum inhibitors of histone deacetylases only partially suppress cytokine-induced beta-cell apoptosis
Inhibitors selective for HDAC1, 2, and 3 are most effective in suppressing apoptosis
Genetic knock-down of HDAC3 alone suppresses beta-cell apoptosis and restores physiological function
Acknowledgements
This work was supported by a Type 1 Diabetes Pathfinder Award (NIH-NIDDK, to B.K.W.) and the Juvenile Diabetes Research Foundation (S.L.S., B.K.W.). D.H.-C.C. is a Vertex scholar and a recipient of HTK fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, Mazitschek R. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DH, Bodycombe NE, Carrinski HA, Lewis TA, Clemons PA, Schreiber SL, Wagner BK. Small-Molecule Suppressors of Cytokine-Induced beta-Cell Apoptosis. ACS Chem Biol. 2010;5:729–734. doi: 10.1021/cb100129d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DP, Dahllof M, Lundh M, Rasmussen DN, Nielsen MD, Billestrup N, Grunnet LG, Mandrup-Poulsen T. HDAC inhibition as a novel treatment for diabetes mellitus. Mol Med. 2011 doi: 10.2119/molmed.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia. 1998;41:1101–1108. doi: 10.1007/s001250051036. [DOI] [PubMed] [Google Scholar]
- Fornoni A, Pileggi A, Molano RD, Sanabria NY, Tejada T, Gonzalez-Quintana J, Ichii H, Inverardi L, Ricordi C, Pastori RL. Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia. 2008;51:298–308. doi: 10.1007/s00125-007-0889-4. [DOI] [PubMed] [Google Scholar]
- Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes. 2009;58:1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L, Tonnesen M, Ronn SG, Storling J, Jorgensen S, Mascagni P, Dinarello CA, Billestrup N, Mandrup-Poulsen T. Inhibition of histone deacetylases prevents cytokine-induced toxicity in beta cells. Diabetologia. 2007;50:779–789. doi: 10.1007/s00125-006-0562-3. [DOI] [PubMed] [Google Scholar]
- Lewis EC, Blaabjerg L, Storling J, Ronn SG, Mascagni P, Dinarello CA, Mandrup-Poulsen T. The oral histone deacetylase inhibitor ITF2357 reduces cytokines and protects islet beta-cells in vivo and in vitro. Mol Med. 2010 doi: 10.2119/molmed.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–678. doi: 10.1210/en.2003-1099. [DOI] [PubMed] [Google Scholar]
- Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, et al. Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2) Bioorg Med Chem Lett. 2008;18:973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Miret S, De Groene EM, Klaffke W. Comparison of in vitro assays of cellular toxicity in the human hepatic cell line HepG2. J Biomol Screen. 2006;11:184–193. doi: 10.1177/1087057105283787. [DOI] [PubMed] [Google Scholar]
- Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- Soldevila G, Buscema M, Doshi M, James RF, Bottazzo GF, Pujol-Borrell R. Cytotoxic effect of IFN-gamma plus TNF-alpha on human islet cells. J Autoimmun. 1991;4:291–306. doi: 10.1016/0896-8411(91)90025-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.