Abstract
Background
To investigate the effect of helminth infections and their treatment during pregnancy on HIV load, we conducted a 2×2 factorial randomised controlled trial of albendazole versus placebo and praziquantel versus placebo in pregnant women in Entebbe, Uganda
Methods
Two hundred and sixty-four HIV-infected women from the Entebbe Mother and Baby Study (ISRCTN32849447) were included in this analysis. Women were tested for helminth infections at enrolment and mean HIV load was compared between infected and uninfected groups. The effect of anthelminthic treatment on HIV load was evaluated at six weeks post-treatment and at delivery using linear regression and adjusting for enrolment viral load.
Results
Hookworm and Trichuris infections were associated with higher mean viral load at enrolment (adjusted mean difference 0.24log10 copies/ml, 95% confidence interval (CI): 0.01 to 0.47, p=0.03 and 0.37log10 copies/ml, 95%CI: 0.00 to 0.74, p=0.05, respectively). There were no associations between viral load and other helminth species. There was some evidence that albendazole reduced viral load at six weeks post-treatment (adjusted mean difference −0.17, 95% CI: −0.36 to 0.01, p=0.07), however this effect did not differ according to mother’s hookworm infection status and had diminished at delivery (adjusted mean difference −0.11, 95% CI: −0.28 to 0.07, p=0.23). There was no effect of praziquantel treatment on HIV load at any time point.
Conclusions
Infection with some soil-transmitted helminth species is associated with increased HIV load in pregnancy. Treatment with albendazole causes a small decrease in HIV load, however this may not represent a direct effect of worm removal.
Keywords: HIV, viral load, helminths, anthelminthic treatment, clinical trial
Introduction
In 2010, 34 million people worldwide were estimated to be living with HIV, with 7000 new infections daily 1. Antiretroviral therapy (ART) slows disease progression in people living with HIV (PLHIV) by suppressing viral replication, and may prevent both horizontal and vertical transmission of the disease 2,3. Current WHO guidelines are that ART be initiated in all PLHIV with a CD4 count ≤350 cells/mm3 4. In addition, the provision of ART for the prevention of mother-to-child transmission is recommended in all pregnant women with HIV, regardless of their symptoms 5. Although there have been dramatic improvements in treatment access over the last decade, 9 million individuals who are eligible to receive ART are still estimated not to do so 1. In parallel with continued efforts to improve ART coverage, other interventions that may impact on HIV disease progression and transmission should be considered.
Sub-Saharan Africa bears a disproportionate burden of the HIV epidemic, with approximately 68% of infected individuals estimated to live in the region 6. Co-infection with other pathogens including helminths is common 7,8, and it has been suggested that persistent co-infections may have a detrimental impact on PLHIV. Helminths have profound effects on the host immune system, and these effects may spill over to impact on immune responses to other pathogens 9. Specifically, helminth infection induces a prominent type 2 response profile which inhibits the type 1 response profile needed to combat and control viral antigens such as HIV. It is hypothesised that removal of helminth infections, which can be achieved with cheap and widely available anthelminthic treatments, would reverse these associations.
Observational studies of the relationship between helminths and HIV have found conflicting results 10-13 but a recent systematic review 14, pooled data from three randomised controlled trials 15-17 and concluded there was evidence of a beneficial effect of anthelminthic treatment on markers of HIV disease progression. Level of HIV load during pregnancy is a key determinant of vertical transmission risk and there is some observational evidence for a positive association between helminth infection and increased risk of mother-to-child transmission of HIV 18, however we have previously reported finding no benefit of anthelminthic treatment during pregnancy for vertical HIV transmission in a randomised controlled trial 19. To further elucidate the relationship between helminth co-infection and HIV, we report results from the same trial, investigating the impact of helminths and their treatment during pregnancy on viral load in HIV infected women six weeks post-treatment and after delivery.
Methods
Study area and participants
Participants were HIV infected pregnant women enrolled in the Entebbe Mother and Baby Study (EMaBS; ISRCTN32849447) 20, a randomised controlled trial of anthelminthic treatment during pregnancy conducted in Entebbe Municipality and Katabi subcounty, a peninsula in Lake Victoria, Uganda, with a high burden of parasitic infections 21. Pregnant women presenting at the government-funded antenatal clinic at Entebbe General Hospital, who were resident in the study area, planning to deliver in the hospital, willing to know their HIV status, and in the second or third trimester of pregnancy, were eligible for inclusion in EMaBS. Exclusion criteria were evidence of possible helminth-induced pathology (haemoglobin <8g/dl, clinically apparent severe liver disease, diarrhoea with blood in stool), history of adverse reaction to anthelminthics, prior enrolment in an earlier pregnancy or abnormal pregnancy as assessed by the midwife.
The primary outcomes for EMaBS were response to immunisation and incidence of infectious diseases in the offspring, and vertical transmission of HIV; results for these have been reported elsewhere 19. In this analysis, we examine the effect of anthelminthic treatment on HIV load in the HIV-1 positive mothers in the cohort. The primary outcome for this analysis is viral load during pregnancy, measured at six weeks post treatment, with secondary outcome viral load at delivery. Women were eligible for inclusion in this analysis if they were HIV-1 positive and had viral load measured at enrolment and were excluded if known to be taking ART.
Study design and procedures
After obtaining written, informed consent, demographic and clinical details were recorded, blood samples obtained and women requested to return with a stool sample. Women were then randomised in a 1:1:1:1 ratio to single-dose albendazole 400 mg or matching placebo and praziquantel 40 mg/kg or matching placebo in a 2×2 factorial design. The randomisation code was generated by the trial statistician in blocks of 100. Sealed envelopes containing the study intervention were prepared by colleagues at the Medical Research Council Unit in Entebbe with no other involvement in the trial. Treatments were allocated in numerical order by trained interviewer-counsellors and taken under observation. All participants and staff were blinded to treatment allocation. After enrolment, women continued to receive standard antenatal care, including haematinics, tetanus immunization, and intermittent presumptive treatment for malaria (with sulfadoxine-pyrimethamine) after the first trimester.
Prior to the start of the project, a programme for prevention of mother-to-child HIV transmission was established at Entebbe Hospital. In accordance with guidelines current at the time, HIV positive women were counselled and given intrapartum and neonatal single-dose nevirapine for prevention of mother-to-child HIV transmission 22. From March 2005 onwards, when ART provision became available, HIV infected women were offered a repeat full blood count and CD4 T cell count and referred to the Entebbe Hospital ART services if indicated. Those for whom ART was not yet indicated at enrolment had repeat CD4 counts performed annually (or earlier if clinically indicated), to allow referral when necessary.
Blood samples were collected six weeks after enrolment from mothers who had not yet delivered . Blood samples were also collected from the mother after delivery, and from cord blood. Blood samples were taken from offspring of HIV infected women at six weeks and 18 months of age to assess their HIV-1 status.
Stool samples were collected approximately six weeks after delivery to assess effectiveness of anthelminthic treatment; thereafter all mothers were treated with both praziquantel and albendazole.
Parasitology and virology
Stool samples were examined for helminth ova using the Kato-Katz method 23 and by charcoal culture for Strongyloides 24; two Kato-Katz slides were prepared from each sample and examined within 30 minutes for hookworm ova, the following day for other species. Urine examination for S. haematobium was not conducted due to the low prevalence of this species in the study area. Hookworm and Schistosoma mansoni infections were classified into low, medium and high intensities according to WHO guidelines 25,26. Blood samples were examined by a modified Knott’s method for Mansonella perstans 27 and by thick film for malaria parasites. Quality control for Kato-Katz analyses was provided by the Vector Control Programme of the Ministry of Health, Uganda, and for malaria parasitology through the United Kingdom National External Quality Assessment Schemes.
HIV serology was performed for mothers by rapid test algorithm 28. For viral load assessment, plasma and whole blood cell pellet were separated by centrifugation and stored at −80°C until assays were performed. Plasma HIV load was measured using either Bayer Versant branched DNA assay version 3.0 (Bayer HealthCare, Germany) or Roche Amplicor HIV-1 RNA Monitor test Version 1.5 (Roche Molecular Systems Inc., USA). Methods used to detect HIV-1 proviral DNA in infants at six weeks are described elsewhere 19. Eighteen month samples were tested for HIV using rapid test algorithm.
Ethical approval
Ethical approval was given by the Uganda Virus Research Institute, Uganda National Council for Science and Technology, and the London School of Hygiene & Tropical Medicine.
Statistical analysis
The sample size for EMaBS was determined for the primary outcomes of the full trial 20. Data were double entered into Microsoft Access (Redmond, WA, USA) and analysed using Stata version 11 (College Station, TX, USA). Viral load data were log10 transformed for analysis. Baseline characteristics of the mothers included in this study were summarised overall and by randomisation arm.
An observational analysis of the association between infection with each helminth and HIV load was conducted by comparing log10 viral load enrolment samples from mothers with and without infection, using linear regression with adjustment for potential confounders (CD4 count, age, asymptomatic malaria infection at enrolment) to calculate mean differences with 95% confidence intervals. Likelihood ratio tests were used to calculate p-values.
Trial analysis was done by intention-to-treat. The effects of albendazole treatment and of praziquantel treatment on HIV load in mothers at six weeks post-treatment and at delivery were examined separately using linear regression, including treatment group as a covariate and adjusting for baseline (enrolment) viral load, and any factors that showed imbalance between treatment arms and that were thought, a priori, likely to impact on viral load. Since the study was designed as a factorial trial, we checked for interaction between albendazole and praziquantel treatments on the two viral load outcomes by fitting interaction terms in regression models.
Since not all women were infected with all helminths at enrolment, a planned subgroup analysis was conducted, comparing the effect of albendazole treatment versus placebo in women who had hookworm (the most prevalent helminth treated by albendazole) at enrolment, and the effect of praziquantel treatment versus placebo in women who had S. mansoni at enrolment, using linear regression models adjusted for baseline HIV-1 load and any factors that showed imbalance between treatment arms. Differences between subgroups were examined by fitting interaction terms in regression models. All p-values are two-sided with no adjustment made for multiple comparisons.
Results
Between April 2003 and November 2005, 2507 women were enrolled in EMaBS. Of these, 299 (12%) tested positive for HIV. Five women on HAART and 30 women for whom no viral load measurement was available at enrolment were excluded from the analysis, leaving 264 women suitable for inclusion (Figure 1). Women who were HIV infected were on average older, less educated, more likely to be widowed or divorced, to already have children, and to be infected with asymptomatic malaria, and less likely to be infected with hookworm, compared to HIV negative women. At enrolment, 67% of the 264 women were infected with at least one helminth species, with individual prevalences: hookworm 39%, Mansonella perstans 23%, Schistosoma mansoni 18%, Strongyloides stercoralis 11%, Trichuris trichiura 9%, Ascaris lumbricoides 2%. The prevalences of all helminth species other than hookworm were comparable between HIV infected and uninfected women 21. Helminth infections were generally mild amongst HIV infected women: of hookworm infections, 92% were classified as light (<1000 eggs per gram of stool (epg); of S. mansoni infections, 65% were light (<100 epg). Characteristics of the 264 women at enrolment were broadly similar between the four randomisation arms (Table 1), with the exception that women allocated to albendazole had lower mean HIV load, and were less likely to have malaria parasitaemia. In addition, women allocated to albendazole were more likely to have viral load quantified by the Bayer assay at six weeks post-treatment. These chance imbalances were taken into account in the analysis. Numbers of serious adverse events are reported elsewhere 29 and were distributed evenly between treatment groups.
Figure 1.
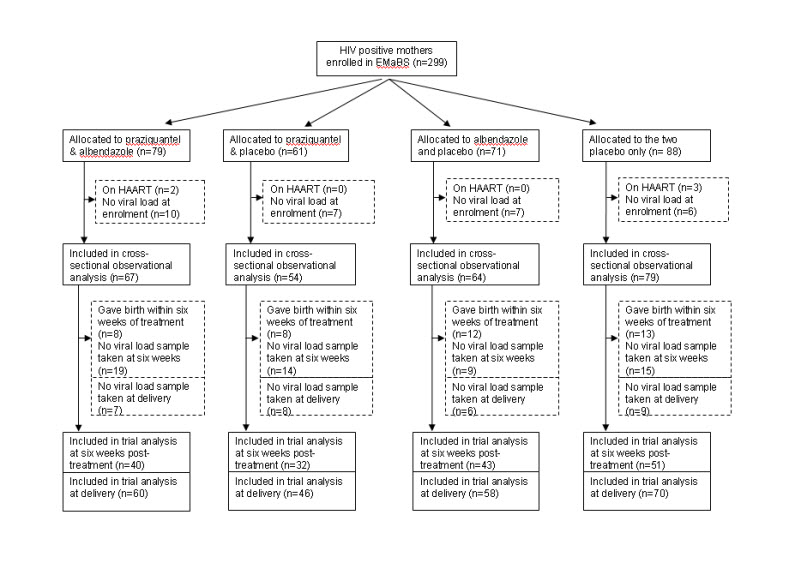
Flowchart of participants through the study
Table 1.
Characteristics of 264 HIV-1 infected women by treatment arm
| Albendazole + praziquantel |
Praziquantel only |
Albendazole only |
Placebo | |
|---|---|---|---|---|
| Number HIV infected women enrolled | 67 | 54 | 64 | 79 |
| Age in years | ||||
| <20 | 9 (13%) | 8 (15%) | 9 (14%) | 10 (13%) |
| 20-24 | 23 (34%) | 21 (39%) | 18 (28%) | 29 (37%) |
| 25-29 | 18 (27%) | 15 (28%) | 23 (36%) | 22 (28%) |
| ≥30 | 17 (25%) | 10 (19%) | 15 (22%) | 18 (23%) |
| Education | ||||
| None | 9 (13%) | 1 (2%) | 3 (5%) | 5 (6%) |
| Primary | 39 (58%) | 33 (61%) | 36 (56%) | 45 (57%) |
| Secondary | 12 (18%) | 16 (30%) | 22 (34%) | 26 (33%) |
| Tertiary | 7 (10%) | 4 (7%) | 3 (5%) | 3 (4%) |
| Marital status | ||||
| Married | 49 (73%) | 46 (85%) | 52 (81%) | 63 (80%) |
| Single | 12 (18%) | 5 (9%) | 8 (13%) | 9 (11%) |
| Widowed/divorced/separated | 6 (9%) | 3 (6%) | 5 (6%) | 7 (9%) |
| Gravidity | ||||
| 1 | 9 (13%) | 10 (19%) | 14 (22%) | 11 (14%) |
| 2-4 | 41 (61%) | 30 (56%) | 33 (52%) | 50 (63%) |
| ≥5 | 17 (25%) | 14 (26%) | 17 (27%) | 18 (23%) |
| Helminth infections | ||||
| Hookworm | 20 (30%) | 24 (44%) | 25 (39%) | 33 (42%) |
| S. mansoni | 13 (19%) | 8 (15%) | 14 (22%) | 13 (16%) |
| M. perstans | 13 (19%) | 14 (26%) | 15 (23%) | 20 (25%) |
| S. stercoralis (1mv) | 10 (15%) | 8 (15%) | 4 (6%) | 6 (8%) |
| T. trichiura | 6 (9%) | 4 (8%) | 7 (11%) | 7 (9%) |
| A. lumbricoides | 1 (1%) | 0 (0%) | 3 (5%) | 2 (3%) |
| Any helminth | 42 (63%) | 35 (65%) | 44 (69%) | 54 (69%) |
| Malaria parasitaemia (3 mv) a | ||||
| Positive | 7 (11%) | 8 (15%) | 8 (13%) | 22 (28%) |
| CD4 count | ||||
| <300 | 13 (19%) | 10 (19%) | 9 (14%) | 14 (18%) |
| 300-499 | 25 (37%) | 11 (20%) | 16 (25%) | 19 (24%) |
| >=500 | 18 (27%) | 19 (35%) | 26 (41%) | 30 (38%) |
| Not done | 11 (16%) | 14 (26%) | 13 (20%) | 16 (20%) |
| HIV-1 log10 load at enrolment | ||||
| Mean (SD) | 4.00 (0.91) | 4.11 (0.96) | 3.96 (0.85) | 4.27 (0.96) |
| Assay used to measure HIV load at baseline | ||||
| Bayer | 60 (90%) | 49 (91%) | 60 (94%) | 68 (86%) |
| Roche | 7 (10%) | 5 (9%) | 4 (6%) | 11 (14%) |
| Assay used to measure HIV load at six weeks post-treatment (166 samples tested) | ||||
| Bayer | 16 (40%) | 11 (34%) | 19 (44%) | 14 (27%) |
| Roche | 24 (60%) | 21 (66%) | 24 (56%) | 37 (73%) |
| Assay used to measure HIV load at delivery (234 samples tested) | ||||
| Bayer | 59 (98%) | 46 (100%) | 56 (97%) | 66 (94%) |
| Roche | 1 (2%) | 0 (0%) | 2 (3%) | 4 (6%) |
mv=missing values
Associations between helminth infections and HIV-1 load at enrolment
The mean (standard deviation, SD) viral load at baseline was 4.09 (0.93) log10 copies/mL. The mean viral load in women infected with hookworm was 0.22 log10 higher than in uninfected women (Table 2). After adjustment for age, CD4 count, and asymptomatic malaria infection, the difference in viral load was 0.24 log10 (95% confidence interval [CI]: 0.01, 0.47; p=0.03). There was some evidence that women infected with T. trichiura had higher mean viral loads than those who were uninfected (adjusted mean difference (95% CI): 0.37 log10 (0.00, 0.74); p=0.05). There was no evidence of a difference in log10 viral load for any other helminth infection (Table 2). For hookworm and S. mansoni, we found no evidence of an association between infection intensity and viral load.
Table 2.
Association between helminth infections and HIV-1 load at enrolment
| Exposure | Mean (SD) log HIV load |
Crude mean difference (95% CI) |
p | Adjusted mean difference (95% CI)a |
p |
|---|---|---|---|---|---|
| Hookworm | |||||
| Uninfected (n=162) | 4.01 (0.93) | ||||
| Infected (n=102) | 4.23 (0.91) | 0.22 (−0.01, 0.45) | 0.06 | 0.24 (0.01, 0.47) | 0.03 |
| S. mansoni | |||||
| Uninfected (n=216) | 4.11 (0.94) | ||||
| Infected (n=48) | 4.01 (0.87) | −0.11 (−0.40, 0.18) | 0.53 | −0.05 (−0.33, 0.24) | 0.75 |
| M. perstans | |||||
| Uninfected (n=202) | 4.10 (0.92) | ||||
| Infected (n=62) | 4.06 (0.96) | −0.04 (−0.31, 0.22) | 0.74 | −0.01 (−0.27, 0.26) | 0.95 |
| S. stercoralis | |||||
| Uninfected (n=235) | 4.11 (0.91) | ||||
| Infected (n=28) | 3.95 (1.05) | −0.16 (−0.53, 0.20) | 0.38 | −0.12 (−0.48, 0.24) | 0.51 |
| T. trichiura | |||||
| Uninfected (n=240) | 4.07 (0.94) | ||||
| Infected (n=24) | 4.34 (0.76) | 0.27 (−0.12, 0.66) | 0.17 | 0.37 (0.00, 0.74) | 0.05 |
| A. lumbricoides | |||||
| Uninfected (n=258) | 4.08 (0.93) | ||||
| Infected (n=6) | 4.55 (0.84) | 0.47 (−0.29, 1.22) | 0.22 | 0.39 (−0.34, 1.11) | 0.29 |
Adjusted for CD4 count, age, concurrent malaria parasitaemia;
SD=standard deviation; CI=confidence interval
Effect of anthelminthic treatment on HIV-1 load during pregnancy and post-delivery
At six weeks post-enrolment, 41 women had given birth and one had miscarried. Of the remaining 222 women, HIV-1 load measurements were obtained for 166 (75%) at a median (IQR) of 42 (41-45) days after treatment; mean (SD) viral load was 4.06 (0.91) log10. Based on the single stool samples after delivery, single-dose albendazole and praziquantel treatments were estimated to be 81% and 63% effective in removing hookworm and S. mansoni, respectively.
There was no evidence of interaction between albendazole and praziquantel treatments, therefore the effects of each treatment were examined independently. At six weeks post-treatment women who had received albendazole had lower mean viral load than those who received placebo (mean difference −0.40 log10, 95% CI: −0.68, −0.13), however after adjusting for baseline viral load, asymptomatic malaria and assay used for viral load quantification at six weeks post-treatment, this difference was reduced to −0.17 log10 (95% CI: −0.36, 0.01), p=0.07 (Table 3). The effect of albendazole treatment was similar in women with hookworm infection at enrolment compared to those uninfected (interaction p=0.44).
Table 3A.
Effect of anthelminthic treatment on HIV-1 load
| A. Six weeks post-treatment | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Group | Mean (SE) log10 HIV load at baselinea |
Mean (SE) log10 HIV load at six weeks post-treatment |
Crude mean difference (95% CI) |
Adjusted mean difference (95% CI)b |
p | ||
| Placebo | Albendazole | Placebo | Albendazole | ||||
|
| |||||||
| All women | 4.21 (0.11) | 3.92 (0.10) | 4.26 (0.10) | 3.85 (0.09) | −0.40 (−0.68, −0.13) | −0.17 (−0.36, 0.01) | 0.07 |
| Hookworm infected | 4.32 (0.18) | 3.99 (0.18) | 4.18 (0.16) | 3.85 (0.16) | −0.33 (−0.77, 0.11) | −0.12 (−0.46, 0.22) | 0.44c |
| Hookworm uninfected | 4.13 (0.13) | 3.88 (0.13) | 4.31 (0.13) | 3.85 (0.12) | −0.46 (−0.81, −0.10) | −0.22 (−0.44, −0.01) | |
|
| |||||||
| Placebo | Praziquantel | Placebo | Praziquantel | ||||
|
| |||||||
| All women | 4.08 (0.10) | 4.05 (0.11) | 4.06 (0.09) | 4.05 (0.11) | −0.01 (−0.29, 0.27) | 0.01 (−0.18, 0.20) | 0.94 |
| S. mansoni infected | 3.89 (0.22) | 4.17 (0.27) | 3.80 (0.22) | 4.12 (0.27) | 0.32 (−0.38, 1.02) | 0.07 (−0.27, 0.41) | 0.56c |
| S. mansoni uninfected | 4.11 (0.11) | 4.02 (0.12) | 4.11 (0.10) | 4.03 (0.12) | −0.08 (−0.39, 0.23) | −0.02 (−0.24, 0.20) | |
For those with viral load measured at six weeks post-treatment
For effect of albendazole treatment, results were adjusted for baseline viral load, baseline malaria parasitaemia and viral load assay used at six weeks; for effect of praziquantel treatment, results were adjusted for baseline viral load
Interaction p-value
SE=standard error;CI=confidence interval
Table 3B.
| B. At delivery | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Group | Mean (SE) log10 HIV load at baselinea |
Mean (SE) log10 HIV load at delivery |
Crude mean difference (95% CI) |
Adjusted mean difference (95% CI)b |
p | ||
| Placebo | Albendazole | Placebo | Albendazole | ||||
| All women | 4.16 (0.09) | 4.05 (0.08) | 4.10 (0.09) | 3.83 (0.08) | −0.27 (−0.51, −0.03) | −0.11 (−0.28, 0.07) | 0.23 |
| Hookworm infected | 4.27 (0.13) | 4.12 (0.14) | 4.10 (0.14) | 3.87 (0.14) | −0.24 (−0.63, 0.16) | −0.00 (−0.25, 0.25) | 0.43c |
| Hookworm uninfected | 4.09 (0.12) | 4.03 (0.10) | 4.10 (0.12) | 3.81 (0.10) | −0.29 (−0.60, 0.03) | −0.17 (−0.42, 0.07) | |
|
| |||||||
| Placebo | Praziquantel | Placebo | Praziquantel | ||||
|
| |||||||
| All women | 4.04 (0.08) | 4.05 (0.09) | 3.94 (0.08) | 3.99 (0.09) | 0.05 (−0.20, 0.29) | 0.04 (−0.13, 0.21) | 0.64 |
| S. mansoni infected | 3.86 (0.17) | 4.12 (0.21) | 3.69 (0.20) | 4.04 (0.24) | 0.35 (−0.27, 0.97) | 0.10 (−0.22, 0.42) | 0.50c |
| S. mansoni uninfected | 4.09 (0.09) | 4.03 (0.10) | 4.00 (0.09) | 3.98 (0.10) | 0.02 (−0.25, 0.29) | 0.01 (−0.19, 0.21) | |
For those with viral load measured at delivery
For effect of albendazole treatment, results were adjusted for baseline viral load and baseline malaria parasitaemia; for effect of praziquantel treatment, results were adjusted for baseline viral load
Interaction p-value
SE=standard error; CI=confidence interval
HIV load was measured in 234 (89%) women post-delivery, at a median (IQR) time of 105 (67-133) days post-treatment; this figure was comparable between treatment groups. Adjusting for viral load and malaria parasitaemia at enrolment, the effect of albendazole treatment on viral load had diminished compared to that observed at six weeks post-treatment during pregnancy (adjusted mean difference −0.11 log10 (95% CI: −0.28, 0.07; p=0.23). There weres no effects of praziquantel treatment on viral load at either time point, nor any evidence of a differential effect of either anthelminthic treatment by susceptible helminth infection (Table 3).
Effect of anthelminthic treatment on HIV vertical transmission and viral load in cord blood
Amongst the 294 HAART-naïve women included in this analysis, 16 were lost to follow-up before delivery, five had miscarriages and nine had stillbirths, leaving information available on 264 live deliveries. We have previously reported that six-week blood samples were available from 211 infants of whom 39 (18%) were diagnosed with HIV infection, and that there were no effects of treatment with albendazole or praziquantel on vertical HIV transmission 19. Two further HIV transmissions were detected in blood samples taken from children at 18 months, inclusion of these transmissions in the analysis had no impact on results.
Discussion
There are limited data on the relationship between HIV load and helminth infection during pregnancy. We have previously shown, and confirmed in this analysis, that anthelminthic treatment during pregnancy does not significantly reduce the risk of vertical transmission, however this finding is in the context of three quarters of women taking nevirapine at delivery, and power to detect small to moderate sized reductions in risk of vertical transmission was limited. Therefore by analysing the effect of anthelminthic treatment on viral load during pregnancy and at delivery, we sought to further understand the relationship between helminths during pregnancy and factors that could lead to vertical transmission of HIV.
We have shown that infection with the soil-transmitted intestinal helminths, hookworm and T. trichiura, during pregnancy is associated with higher HIV load. However, although there was some evidence that albendazole treatment led to a small reduction in viral load six weeks after treatment, this was not sustained to delivery. There was no evidence for a role of other helminth species in HIV load modulation, although power to detect associations for species which were rare in our study population, such as A. lumbricoides, was limited, therefore we cannot discount a role for this species in viral load modulation.
The size of the effect of albendazole treatment was modest, conferring a reduction of 0.17 log10 in viral load. However, modelling studies have shown that even small reductions in HIV load at the population level could translate to reductions in disease incidence; for example a decrease in viral load of 0.3log10 has been estimated to decrease HIV transmission by 20% and HIV progression by 25% 30.
We found no differential effect of albendazole treatment on HIV load amongst mothers who were diagnosed with hookworm infection at enrolment compared to mothers with no detectable hookworm, raising questions about the mechanism of the effect of albendazole. If the effect was mediated by hookworm removal, we would expect the benefit to be greatest in the hookworm-infected women and to persist to delivery (since stool samples at delivery showed a reduction in hookworm prevalence from 45 to 5% amongst women treated with albendazole 29). One possible explanation is the low sensitivity of a single Kato Katz stool sample31,32: women in the hookworm “uninfected” group may have had low intensity hookworm infection, or infections with other albendazole-susceptible helminth species. However data available from a subgroup of women in the study who provided three stool samples indicate that sensitivity was high for hookworm. Albendazole treatment was not effective in the treatment of T. trichiura, therefore the effect of albendazole treatment cannot be mediated through removal of this worm. Another possible explanation relates to the fact that albendazole has a broad spectrum of action and may clear other infections such as malaria 33,34 which may themselves impact on HIV load 35, thus an effect of anthelminthic treatment is seen in both the helminth “infected” and “uninfected” mothers; in this case weakening of the effect by the time of delivery could be accounted for by the provision of intermittent presumptive treatment for malaria to all women in the latter part of pregnancy.
We found no evidence for a role of S. mansoni or M. perstans in HIV load modification, in contrast to previous randomised trials that have shown a reduction in viral load after treatment of schistosomiasis 15 and filariasis 16, and our own observational study which showed a transient increase in viral load after treatment of schistosomiasis 13. For M. perstans, this was not unexpected since the single-dose anthelminthic treatments administered in this trial were not effective in its treatment, however praziquantel treatment was 63% effective in treating S. mansoni thus we would have expected to see any important effects of the removal of this worm.
An association between S. haematobium infection and HIV prevalence has been documented 36,37, suggesting a role for this helminth species in HIV transmission dynamics 38. We could not examine this possibility since S. haematobium was not prevalent in the study area; similar studies conducted in regions where S. haematobium infection is endemic are required to elucidate this relationship.
In conclusion, soil-transmitted helminth co-infections may play a role in HIV load modulation during pregnancy, but we found no evidence for involvement of other helminth species. Treatment with albendazole caused a small decrease in HIV load, however the observation that this decrease occurred regardless of whether the mother had an albendazole-susceptible worm taken together with the diminution of the effect of albendazole treatment by delivery indicate that any impact of albendazole treatment on HIV load may not be directly mediated through worm removal.
Acknowledgements
A. Elliott conceived, designed and led the study. J. Kysoiimire-Lugemwa conducted the virological investigations, with contributions from P. Nkurunziza. E. Webb conducted the statistical analysis and drafted the report, with contributions from A. Elliott. D Kizito participated in the collection and curation of samples. S. Lule contributed to recruitment and follow up of participants and to clinical care. L. Muhangi contributed to data management and analysis. M. Muwanga contributed to the design and conduct of the study. P. Kaleebu was in charge of the virology, and contributed to the manuscript. All authors reviewed the final paper.
We thank all staff and participants of the Entebbe Mother and Baby Study, the midwives of the Entebbe Hospital Maternity Department, the community field team in Entebbe and Katabi, and the staff of the Clinical Diagnostic Services Laboratory at the MRC/UVRI Uganda Research Unit on AIDS.
The study was funded by Wellcome Trust grant numbers 064693 and 079110; Medical Research Council staff were supported by MRC programme grant E743; E. Webb was supported by the UK MRC; albendazole and matching placebo were provided by GlaxoSmithKline.
Footnotes
Sources of Funding: The study was funded by Wellcome Trust grant numbers 064693 and 079110; Medical Research Council staff were supported by MRC programme grant E743; E. Webb was supported by the UK MRC; albendazole and matching placebo were provided by GlaxoSmithKline.
Conflicts of Interest The authors declare no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS . AIDS at 30: Nations at the crossroads. WHO; Geneva: 2011. [Google Scholar]
- 2.Treating HIV-infected People with Antiretrovirals Protects Partners from Infection. 2011 http://www.niaid.nih.gov/news/newsreleases/2011/Pages/HPTN052.aspx.
- 3.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000 Mar 1;283(9):1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 4.WHO Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010. [PubMed]
- 5.WHO Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. 2010. [PubMed]
- 6.UNAIDS . AIDS Epidemic Update. WHO; Geneva: 2009. [Google Scholar]
- 7.Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers--a review. Int J Parasitol. 2010 Aug 15;40(10):1137–1144. doi: 10.1016/j.ijpara.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fincham JE, Markus MB, Adams VJ. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop. 2003 May;86(2-3):315–333. doi: 10.1016/s0001-706x(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 9.Borkow G, Leng Q, Weisman Z, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000 Oct;106(8):1053–1060. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown M, Mawa PA, Joseph S, et al. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J Infect Dis. 2005 May 15;191(10):1648–1657. doi: 10.1086/429668. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinipour MC, Napravnik S, Joaki G, et al. HIV and parasitic infection and the effect of treatment among adult outpatients in Malawi. J Infect Dis. 2007 May 1;195(9):1278–1282. doi: 10.1086/513274. [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Karanja DM, Mwinzia P, et al. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS. 2000 Nov 10;14(16):2437–2443. doi: 10.1097/00002030-200011100-00004. [DOI] [PubMed] [Google Scholar]
- 13.Brown M, Kizza M, Watera C, et al. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J Infect Dis. 2004 Nov 15;190(10):1869–1879. doi: 10.1086/425042. [DOI] [PubMed] [Google Scholar]
- 14.Walson JL, Herrin BR, John-Stewart G. Deworming helminth co-infected individuals for delaying HIV disease progression. Cochrane Database Syst Rev. 2009;3:CD006419. doi: 10.1002/14651858.CD006419.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallestrup P, Zinyama R, Gomo E, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis. 2005 Dec 1;192(11):1956–1961. doi: 10.1086/497696. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen NO, Simonsen PE, Dalgaard P, et al. Effect of diethylcarbamazine on HIV load, CD4%, and CD4/CD8 ratio in HIV-infected adult Tanzanians with or without lymphatic filariasis: randomized double-blind and placebo-controlled cross-over trial. Am J Trop Med Hyg. 2007 Sep;77(3):507–513. [PubMed] [Google Scholar]
- 17.Walson JL, Otieno PA, Mbuchi M, et al. Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial. AIDS. 2008 Aug 20;22(13):1601–1609. doi: 10.1097/QAD.0b013e32830a502e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher M, Malhotra I, Mungai PL, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005 Nov 4;19(16):1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 19.Webb EL, Mawa PA, Ndibazza J, et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant’s response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Jan 1;377(9759):52–62. doi: 10.1016/S0140-6736(10)61457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott AM, Kizza M, Quigley MA, et al. The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood [ISRCTN32849447] Clin Trials. 2007;4(1):42–57. doi: 10.1177/1740774506075248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodburn PW, Muhangi L, Hillier S, et al. Risk Factors for Helminth, Malaria, and HIV Infection in Pregnancy in Entebbe, Uganda. PLoS Negl Trop Dis. 2009;3(6):e473. doi: 10.1371/journal.pntd.0000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999 Sep 4;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 23.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972 Nov-Dec;14(6):397–400. [PubMed] [Google Scholar]
- 24.Friend J. Helminths. In: Collee J, Frase A, Marmion B, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. Churchill Livingstone; Edinburgh: 1996. [Google Scholar]
- 25.WHO . Report of the WHO informal consultation on hookworm infection and anaemia in girls and women. Geneva: 1994. [Google Scholar]
- 26.WHO . Monitoring Helminth Control Programmes. Guidelines for monitoring the impact of control programmes aimed at reducing morbidity caused by soil-transmitted helminths and schistosomes, with particular reference to school-age children. World Health Organisation; Geneva, Switzerland: 1999. [Google Scholar]
- 27.Melrose WD, Turner PF, Pisters P, Turner B. An improved Knott’s concentration test for the detection of microfilariae. Trans R Soc Trop Med Hyg. 2000 Mar-Apr;94(2):176. doi: 10.1016/s0035-9203(00)90266-9. [DOI] [PubMed] [Google Scholar]
- 28.Muhangi L, Woodburn P, Omara M, et al. Associations between mild-to-moderate anaemia in pregnancy and helminth, malaria and HIV infection in Entebbe, Uganda. Trans R Soc Trop Med Hyg. 2007 Sep;101(9):899–907. doi: 10.1016/j.trstmh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndibazza J, Muhangi L, Akishule D, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clin Infect Dis. 2010 Feb 15;50(4):531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008 Oct 18;22(16):2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopp S, Rinaldi L, Khamis IS, et al. A single FLOTAC is more sensitive than triplicate Kato-Katz for the diagnosis of low-intensity soil-transmitted helminth infections. Trans R Soc Trop Med Hyg. 2009 Apr;103(4):347–354. doi: 10.1016/j.trstmh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Utzinger J, Booth M, N’Goran EK, Muller I, Tanner M, Lengeler C. Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology. 2001 May;122(Pt 5):537–544. doi: 10.1017/s0031182001007752. [DOI] [PubMed] [Google Scholar]
- 33.Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990 Apr;6(4):112–115. doi: 10.1016/0169-4758(90)90227-u. [DOI] [PubMed] [Google Scholar]
- 34.Skinner-Adams TS, Davis TM, Manning LS, Johnston WA. The efficacy of benzimidazole drugs against Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1997 Sep-Oct;91(5):580–584. doi: 10.1016/s0035-9203(97)90035-3. [DOI] [PubMed] [Google Scholar]
- 35.Barnabas RV, Webb EL, Weiss HA, Wasserheit JN. The role of co-infections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS. 2011 May 30; doi: 10.1097/QAD.0b013e3283491e3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downs JA, Mguta C, Kaatano GM, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg. 2011 Mar;84(3):364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjetland EF, Ndhlovu PD, Gomo E, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006 Feb 28;20(4):593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 38.Gibson LR, Li B, Remold SK. Treating cofactors can reverse the expansion of a primary disease epidemic. BMC Infect Dis. 2010;10:248. doi: 10.1186/1471-2334-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]


