Abstract
PURPOSE
To develop a rabbit model for continuous curvilinear capsulorhexis (CCC) instruction.
SETTING
University of California San Francisco, San Francisco, California, USA.
DESIGN
Experimental study.
METHODS
Isolated rabbit lenses were immersed in 2% to 8% paraformaldehyde (PFA) fixative from 15 minutes to 6 hours. Rabbit eyes were treated by substituting aqueous with 2% to 4% PFA for 30 minutes to 6 hours, followed by washes with a balanced salt solution. Treated lenses and eyes were held in purpose-designed holders using vacuum. A panel of 6 cataract surgeons with 5 to 15 years of experience performed CCC on treated lenses and eyes and responded to a questionnaire regarding the utility of these models for resident teaching using a 5-item Likert scale.
RESULTS
The expert panel found that rabbit lenses treated with increasing amounts of fixative simulated CCC on human lens capsules from the third to the seventh decade of life. The panel also found fixative-treated rabbit eyes to simulate some of the experience of CCC within the human anterior chamber but noted a shallower anterior chamber depth, variation in pupil size, and corneal clouding under some treatment conditions.
CONCLUSIONS
Experienced cataract surgeons who performed CCC on these rabbit models strongly agreed that isolated rabbit lenses treated with fixative provide a realistic simulation of CCC in human patients and that both models were useful tools for capsulorhexis instruction. Results indicate that rabbit lenses treated with 8% PFA for 15 minutes is a model with good fidelity for CCC training.
The increasing attention on surgery as a core competency in resident training1,2 underscores the need to ensure resident proficiency in commonly performed procedures such as cataract removal. Several instructional strategies are being used, including virtual simulators,3–5 wet-lab models using animal or cadaver eyes, and curricula that combine didactic instruction, wet labs, and pretesting and posttesting.6,7 Studies have shown that of the steps in cataract surgery, resident trainees consider the creation of the capsulotomy by continuous curvilinear capsulorhexis (CCC) to be one of the technically most challenging parts of the procedure.8–10 Although the capsulorhexis module in virtual simulators can be an effective teaching tool,11–13 the need for additional wet-lab training is widely recognized. Development in this area has progressed from the use of cadaver eyes14,15 to less expensive and more readily available porcine eyes that are treated with a fixative to decrease capsule elasticity.16–18 The porcine eye models typically use a strong fixative with more than 30% formaldehyde and require care to guard against excessive loss of capsule elasticity from prolonged treatment but at the same time to avoid under treatment. Furthermore, the degree to which the treated porcine anterior capsules have biomechanical properties resembling those of the human anterior capsule in patients has not been assessed objectively.
In the present study, we developed an ex vivo isolated rabbit lens model and an intact rabbit eye model for capsulorhexis instruction that can be titrated to simulate human capsules from a range of ages. Furthermore, because the elastic properties of the lens capsule greatly influence the difficulty of CCC, we assembled a panel of experienced cataract surgeons to objectively assess the fidelity of the modified rabbit capsule to simulate the biomechanical properties of the human capsule encountered during surgery.
MATERIALS AND METHODS
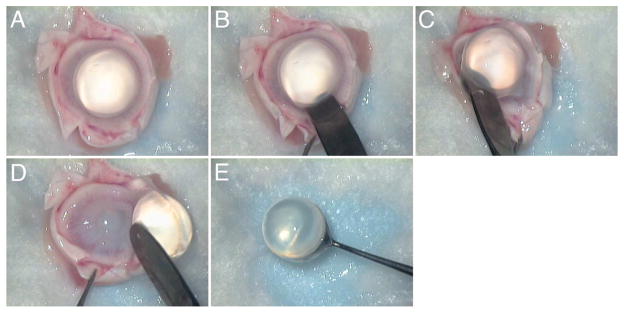
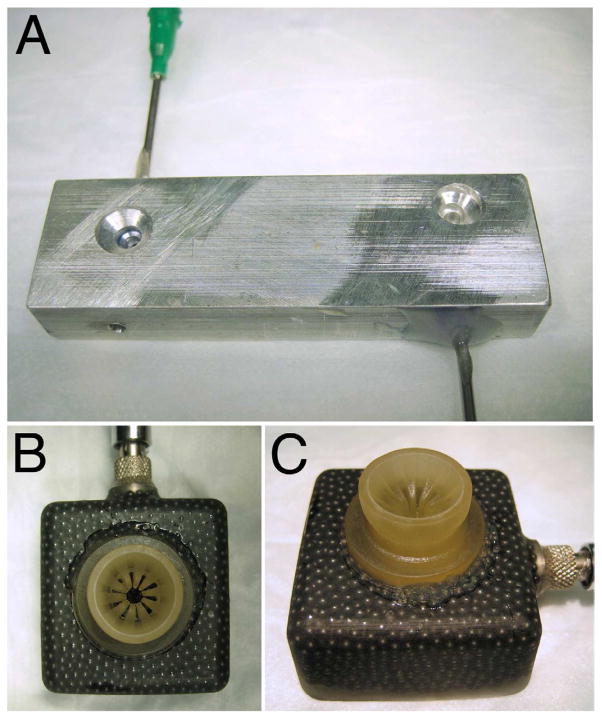
Whole enucleated eyes from New Zealand White rabbits 8 to 12 weeks of age (2.00 to 2.25 kg) were provided by Pel-Freez Biologicals and received by the investigators within 24 hours of harvesting. The rabbit eyes were dissected by incising the globe with a scalpel and cutting it in half just anterior to the equator to obtain isolated lenses for CCC training (Figure 1). A blunt laboratory spatula was then used to firmly separate the lens zonules from the ciliary body in a circumferential manner. This was followed by gentle sweeping of the lens with its capsule intact from the anterior segment. Adherent vitreous was gently cut from the lens capsule. No attempt was made to aggressively remove all attached vitreous because this can cause the capsule to be stripped from the lens. The harvested lenses were collected and subjected to immersion in 1 of 4 fixative treatments at 4°C as follows: (1) 2% paraformaldehyde (PFA) for 6 hours, (2) 4% PFA for 30 minutes, (3) 4% PFA for 1 hour, and (4) 8% PFA for 15 minutes. Up to 20 lenses were processed concurrently in batch format for each condition. The PFA fixative solutions were made in 0.1 M phosphate buffer according to standard recipes. After fixation, the lenses were rinsed with 4 washes of a balanced salt solution and used immediately or stored in balanced salt solution at 4°C for a maximum of 24 hours before testing. Individual lenses were then placed in purpose-designed lens holders (Figure 2, A) and held in place for CCC creation using vacuum (75 to 100 mm Hg) delivered by a small low-cost vacuum pump (Pelco 520, Ted Pella, Inc.).
Figure 1.
Sequence of photographs showing rabbit eye dissection and lens harvesting.
Figure 2.
Custom vacuum holders for isolated rabbit lenses (A) and whole eyes (B and C).
A whole-eye model was also developed for practicing CCC within the confines of the rabbit anterior chamber. Under an operating microscope, the anterior chamber of enucleated rabbit eyes was entered with a 30-gauge needle, the aqueous humor was aspirated, and fixative was injected to reform the chamber. Three fixative conditions were tested: (1) 2% PFA for 6 hours, (2) 4% PFA for 0.5 hour, and (3) 4% PFA for 1 hour; 8% PFA for 15 minutes was not used in whole eyes due to excessive corneal clouding after treatment. After fixative treatment, a paracentesis was made and the anterior chamber washed with a balanced salt solution on a Simcoe irrigation/aspiration cannula. During treatment with fixative, eyes were kept at 4°C and immersed in BSS at all times. Treated eyes were kept at 4°C for up to 24 hours before testing. Testing was performed after securing eyes to custom rabbit eye holders using vacuum (75 to 100 mm Hg) (Figure 2).
A panel of 6 experienced cataract surgeons was assembled to evaluate the isolated-lens model and the whole-eye model for each of the fixative treatment conditions. Members of the expert panel had between 5 years and 15 years of experience in cataract surgery and were requested to perform CCC in the treated eyes and lenses per their usual fashion. Panel members were masked to the fixative treatment condition for each lens or eye and performed their evaluation in the absence of other panel members. After the completion of the CCC, each surgeon was asked to rate the lens capsule biomechanical properties encountered in each specimen in terms of equivalent human age. Surgeons also responded to a questionnaire about the rabbit lens and eye models using a 5-item Likert scale. The mean score of the responses to each question was calculated. The data obtained from the expert panel’s evaluation were statistically analyzed using linear mixed effects regression (with rater as a random effect). The duration and rated ages were categorized because of substantial departures from normality and to prevent observations with long duration from having an excessive influence on the regression.
RESULTS
Table 1 shows the results of the evaluations by the expert panel. The mean equivalent human age with standard error of the mean are indicated for each treatment condition. The data were analyzed by modeling the age category as a function of the category (percentage) of fixative used. Statistical analysis using a linear mixed model showed that an increase in the concentration of fixative used was significantly related to an increase in the rated human-equivalent age of the treated rabbit lens capsules (P = .038).
Table 1.
Results of the evaluations by the expert panel
| Treatment Condition | Samples Tested (n) | Equivalent Human Age (Y) | |
|---|---|---|---|
| Mean | SEM | ||
| Isolated lenses | |||
| 2%PFA,6h | 20 | 23 | 11.0 |
| 4% PFA, 0.5 h | 20 | 58 | 6.8 |
| 4% PFA, 1 h | 20 | 49 | 11.0 |
| 8% PFA, 0.25 h | 20 | 67 | 4.8 |
| Whole eyes | |||
| 2% PFA, 6 h | 17 | 18 | 10.0 |
| 4% PFA, 0.5 h | 17 | 20 | 10.0 |
| 4% PFA, 1 h | 17 | 44 | 9.9 |
PFA = paraformaldehyde
Table 2 shows the opinions of the expert panel on whether creating the CCC in the isolated lenses and in the rabbit whole-eye model resembled creating a CCC in the human eye. Based on their responses, the expert panel found that the lens capsule elasticity and tearing in the isolated rabbit lens model resembled that found in the adult human cataractous lens and that this lens model can be used to simulate the performance of CCC in adult human cataractous lenses. Members of the panel were less uniform in their assessment of the whole-eye model. Some said that the rabbit anterior chamber depth (ACD) was shallower than the human ACD. Because of this perceived difference in ACD, variability in pupil size between rabbit eyes, and some corneal clouding, in particular in samples treated with 4% PFA for 1.0 hour, the panel members said they thought that the whole-eye model only partially replicated CCC creation in an adult human eye. However, when asked about the overall usefulness of both models to surgical training, the expert panel was in general positive.
Table 2.
Expert panel responses on whether creating a CCC in isolated lenses and in the whole rabbit eye model resembled creating a CCC in the human eye
| Question/Metric | Mean Response |
|---|---|
| The elasticity/tearing of the lens replicates that of an adult human cataractous lens. | 4.2 |
| The explanted lens model accurately replicates performance of a CCC in an adult human cataractous lens. | 4.0 |
| The size of the anterior segment in this model is similar to that of a human. | 3.2 |
| The whole eye model accurately replicates performance of a CCC in an adult human. | 3.6 |
| These are useful training models for teaching beginning surgeons the CCC. | 4.6 |
| These models more accurately replicate the experience of performing a CCC in humans than other models you are familiar with. | 4.4 |
| These models represent useful additions to the current methods of training beginning surgeons. | 4.6 |
1 = strongly disagree; 2 = disagree; 3 = neither agree nor disagree; 4 = agree; 5 = strongly agree; CCC continuous curvilinear capsulorhexis
DISCUSSION
The goal of this study was to create reliable and validated rabbit models with good fidelity for resident CCC instruction. To do so, we used several different PFA fixative concentrations and treatment times to modify the biomechanical properties of the rabbit lens capsule. Based on the assessment by a panel of experienced cataract surgeons, the rabbit lens capsule, which is normally highly elastic,19 can be increased in stiffness to resemble that of the human lens capsule by PFA treatment. Furthermore, by varying the fixative concentration and treatment time, a range of capsule stiffness can be obtained that experienced cataract surgeons rated as having biomechanical properties equivalent to those of the human lens capsule from the third to the seventh decades of life.
The rabbit eyes used in our study were obtained from rabbits previously used by the vendor for the production of serum, antibodies, and other tissue products for life science research and in vitro diagnostic manufacturing. Each eye was procured at a relatively low cost (US $3.00). The amount of fixative used (2% to 8% PFA) was a fraction of the amount of formaldehyde used in previous studies of porcine eyes, which ranged from approximately 25% to more than 30%.17,18 Compared with the higher fixative concentrations used in these porcine studies, which mandated more compressed treatment times to avoid overtreatment (1 to 2 minutes,17 unspecified18), the lower fixative concentrations used here allowed expanded treatment times that could be more easily controlled to dial-in a desired human equivalent age for the treated rabbit capsule.
The fixative-treated lenses are easy to prepare, and large numbers can be processed at the same time to facilitate instructional sessions. The objective validation by experienced cataract surgeons that treated lenses simulate the CCC in human capsules across an age range support their utility as a training tool. The treatment protocol of 8% PFA for 15 minutes produced rabbit lens capsule properties that most closely resembled those encountered in adult human patients having cataract surgery. The treated lenses do not have the space constraints imposed by a wound and an anterior chamber and may be best suited for the early stage of teaching surgical concepts and the practice of rescue maneuvers.20 Given the low cost, ease of preparation, and the predictability of this model, it is possible for 1 trainee to perform a significant number of CCCs in the same sitting. Repetition of this crucial step in cataract surgery in a risk-free environment may allow a trainee to quickly become familiar with capsule tissue properties.
The whole-eye model can be further improved. Some panel members commented on the reduced ACD in the rabbit eyes used in this study compared to the human ACD. This likely reflects that the eyes used in the current study were obtained from animals 8 to 12 weeks of age (2.00 to 2.25 kg). Because the rabbit eye continues to grow in life and older rabbits (16 to 18 weeks; 3.5 to 4.0 kg) have a reported central ACD of 2.89 ± 0.09 mm,21 which is more similar to the human central ACD of approximately 3.0 mm,22,23 older rabbits may be substituted to improve chamber dimensions. Some panel members suggested, however, that an instructional model with reduced ACD might be useful for specialized training.
After reviewing comments about the small pupil in the whole-eye model, we reexamined the pupil size in vendor-supplied rabbit eyes and found significant variation in pupil diameter between specimens. In a typical shipment, approximately one third of the specimens have a pupil diameter of 7.0 to 9.0 mm, and these can be selected for use as whole-eye models; the remainder can be used to provide isolated lenses. Intracameral epinephrine injection failed to elicit pupil dilation in the rabbit eyes received from the vendor.
Corneal clouding has been noted in previous studies, especially when high fixative concentrations are used. Methods for mitigation have been described; these include mixing the fixative with an ophthalmic viscosurgical device (OVD) or hydroxyethyl cellulose17 or injecting the fixative under OVD used to protect the corneal epithelium. We expect that inclusion of such methods will also preserve corneal transparency and improve the rabbit whole-eye model.
The 2 rabbit models described here are intended to address a need in CCC instruction and are not suitable in their present form for complete start-to-finish training in cataract surgery, including lens phacoemulsification. Methods to harden animal lenses have been described including the injection of fixative directly into the lens16 and the use of microwave.18 It is possible that the inclusion of such steps will extend the utility of these rabbit models to the subsequent steps of cataract surgery.
WHAT WAS KNOWN
There is a need for CCC teaching models with high fidelity in cataract surgery training.
WHAT THIS PAPER ADDS
Simple aldehyde-based fixatives can be used to convert the elastic rabbit lens capsule into a capsule with increased stiffness.
The biomechanical properties of lens capsules treated with fixative were evaluated by a panel of 6 experienced cataract surgeons who found the treated rabbit lens capsules resembled human capsules from the third to the seventh decade of life.
The panel also indicated that the fixative-treated isolated-lens model and the whole-eye model were useful for surgical training.
Acknowledgments
Supported by unrestricted funds from Research To Prevent Blindness, New York, New York, National Eye Institute grant EY016691, and That Many May See Foundation, San Francisco, California, USA.
Footnotes
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mills RP, Mannis MJ American Board of Ophthalmology Program Directors’ Task Force on Competencies. Report of the American Board of Ophthalmology Task Force on the Competencies [guest editorial] Ophthalmology. 2004;111:1267–1268. doi: 10.1016/j.ophtha.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Lee AG, Volpe N. The impact of the new competencies on resident education in ophthalmology [guest editorial] Ophthalmology. 2004;111:1269–1270. doi: 10.1016/j.ophtha.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair MJ, Peifer JW, Haleblian R, Luxenberg MN, Green K, Hull DS. Computer-simulated eye surgery; a novel teaching method for residents and practitioners. Ophthalmology. 1995;102:517–521. doi: 10.1016/s0161-6420(95)30992-x. [DOI] [PubMed] [Google Scholar]
- 4.Laurell C-G, Söderberg P, Nordh L, Skarman E, Nordqvist P. Computer-simulated phacoemulsification. Ophthalmology. 2004;111:693–698. doi: 10.1016/j.ophtha.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Webster R, Sassani J, Shenk R, Good N. A haptic surgical simulator for the continuous curvilinear capsulorhexis procedure during cataract surgery. Stud Health Technol Inform. 2004;98:404–406. [PubMed] [Google Scholar]
- 6.Lee AG, Greenlee E, Oetting TA, Beaver HA, Johnson AT, Boldt HC, Abramoff M, Olson R, Carter K. The Iowa ophthalmology wet laboratory curriculum for teaching and assessing cataract surgical competency. [Accessed April 1, 2012];Ophthalmology. 2007 114(7):e21–e26. doi: 10.1016/j.ophtha.2006.07.051. Available at: http://www.acgme.org/acwebsite/rrc_240/240_iowa-ophth-wet-lab-prepress.pdf. [DOI] [PubMed] [Google Scholar]
- 7.Rogers GM, Oetting TA, Lee AG, Grignon C, Greenlee E, Johnson AT, Beaver HA, Carter K. Impact of a structured surgical curriculum on ophthalmic resident cataract surgery complication rates. J Cataract Refract Surg. 2009;35:1956–1960. doi: 10.1016/j.jcrs.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Dooley IJ, O’Brien PD. Subjective difficulty of each stage of phacoemulsification cataract surgery performed by basic surgical trainees. J Cataract Refract Surg. 2006;32:604–608. doi: 10.1016/j.jcrs.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 9.Prakash G, Jhanji V, Sharma N, Gupta K, Titiyal JS, Vajpayee RB. Assessment of perceived difficulties by residents in performing routine steps in phacoemulsification surgery and in managing complications. [Accessed April 1, 2012];Can J Ophthalmol. 2009 44:284–287. doi: 10.3129/i09-051. Available at: http://www.eyesite.ca/CJO/4403/i09-051.pdf. [DOI] [PubMed] [Google Scholar]
- 10.Taravella MJ, Davidson R, Erlanger M, Guiton G, Gregory D. Characterizing the learning curve in phacoemulsification. J Cataract Refract Surg. 2011;37:1069–1075. doi: 10.1016/j.jcrs.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 11.Feudner EM, Engel C, Neuhann IM, Petermeier K, Bartz-Schmidt KU, Szurman P. Virtual reality training improves wet-lab performance of capsulorhexis: results of a randomized, controlled study. Graefes Arch Clin Exp Ophthalmol. 2009;247:955–963. doi: 10.1007/s00417-008-1029-7. [DOI] [PubMed] [Google Scholar]
- 12.Le TDB, Adatia FA, Lam W-C. Virtual reality ophthalmic surgical simulation as a feasible training and assessment tool: results of a multicentre study. [Accessed April 1, 2012];Can J Ophthalmol. 2011 46:56–60. doi: 10.3129/i10-051. Available at: http://www.eyesite.ca/CJO/4601/i10-051.pdf. [DOI] [PubMed] [Google Scholar]
- 13.Privett B, Greenlee E, Rogers G, Oetting TA. Construct validity of a surgical simulator as a valid model for capsulorhexis training. J Cataract Refract Surg. 2010;36:1835–1838. doi: 10.1016/j.jcrs.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Auffarth GU, Wesendahl TA, Solomon KD, Brown SJ, Apple DJ. A modified preparation technique for closed-system ocular surgery of human eyes obtained postmortem; an improved research and teaching tool. Ophthalmology. 1996;103:977–982. doi: 10.1016/s0161-6420(96)30576-9. [DOI] [PubMed] [Google Scholar]
- 15.Castellano D, Spraul J, Whitaker TE. A simple, cost-effective method for practicing phacoemulsification in the cadaveric eye. Ophthalmic Surg Lasers. 1998;29:253–256. [PubMed] [Google Scholar]
- 16.Sugiura T, Kurosaka D, Uezuki Y, Eguchi S, Obata H, Takahashi T. Creating cataract in a pig eye. J Cataract Refract Surg. 1999;25:615–621. doi: 10.1016/s0886-3350(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto C, Kurosaka D, Uetsuki Y. Teaching continuous curvilinear capsulorhexis using a postmortem pig eye with simulated cataract. J Cataract Refract Surg. 2001;27:814–816. doi: 10.1016/s0886-3350(00)00728-8. [DOI] [PubMed] [Google Scholar]
- 18.Shentu X, Tang X, Ye P, Yao K. Combined microwave energy and fixative agent for cataract induction in pig eyes. J Cataract Refract Surg. 2009;35:1150–1155. doi: 10.1016/j.jcrs.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Auffarth GU, Wesendahl TA, Newland TJ, Apple DJ. Capsulorhexis in the rabbit eye as a model for pediatric capsulectomy. J Cataract Refract Surg. 1994;20:188–191. doi: 10.1016/s0886-3350(13)80164-2. [DOI] [PubMed] [Google Scholar]
- 20.Little BC, Smith JH, Packer M. Little capsulorhexis tear-out rescue. J Cataract Refract Surg. 2006;32:1420–1422. doi: 10.1016/j.jcrs.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 21.Chen W-L, Shih Y-F, Liao S-L, Hu F-R, Hung P-T. Ultrasound biomicroscopic findings in rabbit eyes undergoing scleral suction during lamellar refractive surgery. [Accessed April 1, 2012];Invest Ophthalmol Vis Sci. 2002 43:3665–3672. Available at: http://www.iovs.org/content/43/12/3665.full.pdf. [PubMed] [Google Scholar]
- 22.Fontana ST, Brubaker RF. Volume and depth of the anterior chamber in the normal aging human eye. Arch Ophthalmol. 1980;98:1803–1808. doi: 10.1001/archopht.1980.01020040655013. [DOI] [PubMed] [Google Scholar]
- 23.Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 1998;105:2091–2098. doi: 10.1016/S0161-6420(98)91132-0. [DOI] [PubMed] [Google Scholar]