Abstract
Cutaneous melanoma is a tumor with rising incidence and a very poor prognosis at the disseminated stage. Melanomas are characterized by frequent mutations in BRAF and also by overexpression of fibroblast growth factor 2 (FGF2), offering opportunities for therapeutic intervention. We investigated inhibition of FGF signaling and its combination with dacarbazine or BRAF inhibitors as an antitumor strategy in melanoma. The majority of melanoma cell lines displayed overexpression of FGF2 but also FGF5 and FGF18 together with different isoforms of FGF receptors (FGFRs) 1–4. Blockade of FGF signals with dominant-negative receptor constructs (dnFGFR1, 3, or 4) or small-molecule inhibitors (SU5402 and PD166866) reduced melanoma cell proliferation, colony formation, as well as anchorage-independent growth, and increased apoptosis. DnFGFR constructs also significantly inhibited tumor growth in vivo. Combination of FGF inhibitors with dacarbazine showed additive or antagonistic effects, whereas synergistic drug interaction was observed when combining FGFR inhibition with the multikinase/BRAF inhibitor sorafenib or the V600E mutant-specific BRAF inhibitor RG7204. In conclusion, FGFR inhibition has antitumor effects against melanoma cells in vitro and in vivo. Combination with BRAF inhibition offers a potential for synergistic antimelanoma effects and represents a promising therapeutic strategy against advanced melanoma.
INTRODUCTION
Melanoma incidence has increased significantly during the last decades, and despite intensive research, treatment options at the disseminated stage are still very limited (Gogas et al., 2007). Mutations in proteins involved in signal transduction pathways, such as BRAF, NRAS, or CKIT, have been identified in the majority of melanomas (Ibrahim and Haluska, 2009), but their exploitation as therapy targets has only recently started to translate into prolonged patient survival in clinical settings. Development of additional treatment options is therefore an urgent requirement to improve the prognosis of patients with advanced melanoma.
Overexpression of growth and survival-promoting factors is an important hallmark of neoplastic cells and a major driving force for tumor progression and dissemination. Expression of fibroblast growth factor 2 (FGF2) has been identified as an important characteristic of melanoma cells in contrast to normal melanocytes (NMs; Halaban et al., 1988) and has been linked to tumor progression in melanoma and multiple other malignancies (Jeffers et al., 2002). The role of other FGFs is widely unexplored in melanoma so far. FGFs constitute a structurally conserved family of polypeptide growth factors, with 22 members in humans (Beenken and Mohammadi, 2009; Powers et al., 2000). FGFs transduce signals through binding to transmembrane receptor tyrosine kinases, named FGF receptors (FGFR1-4), and also bind with lower affinity to heparin-like glycosaminoglycans of the extracellular matrix (McKeehan et al., 1998). After ligand binding, FGFRs activate major cellular growth and survival pathways including, for example, mitogen-activated protein kinase and phosphoinositide 3-kinase signal cascades (Acevedo et al., 2009).
Several members of the FGF family have crucial roles in embryonic and postnatal development and are implicated in wound healing and tissue maintenance (Powers et al., 2000). Similar to EGF-receptor, there is also convincing evidence that hyperactivation of FGFR signaling is associated with—and functionally important for—the growth and progression of several types of human cancer (Jeffers et al., 2002). In addition to an autocrine effect on tumor cell survival and proliferation, FGFs also have important roles in neoangiogenesis, thereby supporting tumor vascularization and metastasis (Presta et al., 2005).
In the present report, we show that the concomitant overexpression of several FGF and FGFR family members is a common feature of human melanoma cells derived from primary tumors or metastatic lesions. Blockade of FGFR signals by genetic constructs or specific tyrosine kinase inhibitors (TKIs) strongly reduces malignant growth of melanoma cells and synergistically enhances the antimelanoma effect of BRAF inhibition in vitro and in vivo.
RESULTS
Multiple FGF and FGFR molecules are co-expressed in melanoma cells
To get a systematic overview of the presence of FGF ligands and receptors in melanoma cells, we screened the expression of all 4 FGFR and 22 FGF genes by conventional and real-time reverse transcription PCR in 12 cell lines from primary and metastatic melanoma established from surgery specimens. Except for VM47, all cell lines harbored the V600E mutation of BRAF. The majority of the cell lines expressed all the four FGFR genes, including both mesenchymal IIIc and epithelial IIIb isoforms of FGFR1-3 (Figure 1a). Expression of FGFR1 and 4 was especially prominent. Although FGFR1 was high in NMs as well, FGFR2 and 4 were upregulated in the majority of melanoma cell lines (Figure 1b). No obvious difference was seen between cell lines derived from primary tumors and those from metastatic lesions. In addition to the widespread expression of different receptor variants, melanoma cell lines also expressed multiple ligands of the FGF family, suggesting the presence of autocrine signaling loops (Figure 1c and d, Supplementary Table S1 online). FGF2 was universally upregulated, reaching more than 100-fold transcript levels in 50% of our melanoma cell lines when compared with NM. FGF5 was almost undetectable in NM but highly expressed in 6 of 12 melanoma cell lines. No increase of expression was seen for FGF8 compared with NM (not shown), and about equal numbers of cell lines displayed increased and decreased expression of FGF18, another FGF with oncogenic potential (Sonvilla et al., 2008). Expression of FGF5, FGF18, and FGFR1 protein was confirmed by immunofluorescence analysis showing cytoplasmic staining compatible with transport along the secretory pathway and comparable to previous results (Figure 1d; Allerstorfer et al., 2008).
Figure 1. Multiple receptor variants and ligands of the fibroblast growth factor/FGF receptor (FGF/FGFR) axis are expressed in melanoma cells.
(a) Presence of all four FGFR transcripts and the different immunoglobulin III-domain isoforms of FGFR1–3 was assessed by reverse transcription PCR in a panel of 12 melanoma cell lines with specific primer pairs. Percentages of cell lines positive for the analyzed transcripts are shown. (b) Expression level of FGFR1–4 and (c) FGF2, 5, and 18 was analyzed with Taqman assays in melanoma cell lines derived from primary tumor (PT) lymph node (LN) or brain (BR) metastases or malignant effusion (ME) and in normal melanocytes (NM). (d) Immunofluorescence staining of VM21 and VM24 cells for FGF5, FGF18, and FGFR1. For controls (Co), primary antibodies were replaced by non-immune serum. Bar = 20 μm. dCT, delta threshold cycle.
FGFs stimulate growth and migration of melanoma cells
To test whether FGF signals stimulate the neoplastic behavior of melanoma cells, we investigated the effect of FGF treatment on cell growth and migration. When VM1, 21, 24, and 48 cells were treated with FGF2, cell growth increased by 25–50% in all cell lines (Figure 2a). The effect was strongest in VM48 cells with the lowest endogenous FGF2 level and weaker in VM24 and VM1, which have 10- to 20-fold higher endogenous FGF2 expression levels than VM48. Moreover, migration of melanoma cells in transwell assays was increased upon treatment with a combination of FGF2 and FGF5 in VM48 cells, whereas in VM24 cells the same treatment did not reach a significant stimulation of migration (Figure 2b). These data suggest that FGF signals enhance growth capacity and migratory potential of melaoma cells.
Figure 2. Fibroblast growth factor (FGF) enhances viability and migration of melanoma cell lines.
(a) Melanoma cells were seeded in 96-well plates and treated with FGF2 (20 ng ml −1) in a medium with 5% fetal calf serum (FCS) for 5 days. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. (b) Melanoma cells were seeded in transwell chambers in a medium with 5% FCS and treated for 72 hours with FGF2 or FGF5 (20 ng ml −1) or a combination of FGF2 and FGF5 (100 ng ml −1 each, FGF high). Cells that had migrated to the bottom of the lower chamber were stained with crystal violet and staining quantified with Lucia software. a, P<0.05 versus untreated control.
Inhibition of FGF signals inhibits melanoma cell growth in vitro and in vivo
To determine the dependency of melanoma cells on FGF signals, we blocked FGFR-mediated signals with adenoviruses expressing dominant-negative (dn)FGFR1, 3, and 4. Expression of dn receptors resulted in up to 50% inhibition in short-term growth assays (Figure 3a and b; Supplementary Figure S1a and b online) and was even more pronounced in clonogenic assays (Supplementary Figure S1c and d online). The strongest effect was generally seen with dnFGFR1, whereas dnFGFR3 did not lead to significant inhibition of growth in any of the cell lines. Transduction with dnFGFR1 also had a very pronounced effect on anchorage-independent growth, almost completely inhibiting colony formation in soft agar in three of four cell lines (Figure 3c). Microscopic examination of VM1 cells after transduction revealed a high number of rounded-up and detached cells in dnFGFR1—but not green fluorescent protein (GFP)—transduced cultures (Supplementary Figure S1e online). Western blotting revealed processing of caspase-3 and poly(ADP-ribose) polymerase-1 cleavage in the dnFGFR1-transduced cultures, thus demonstrating induction of apoptosis by dnFGFR1 expression (Figure 3d). In a xenotransplantation experiment of VM1 cells, tumor growth was markedly delayed by dnFGFR1 or dnFGFR4 compared with the GFP control group (Figure 3e). This resulted in a significantly prolonged survival of mice carrying the dnFGFR1 xenografts (1.3-fold, Figure 3f).
Figure 3. Dominant-negative (dn) fibroblast growth factor receptor (FGFR) expression inhibits melanoma cell growth in vitro and in vivo, and induces apoptosis.
(a, b) Melanoma cells were transduced with dnFGFR1, dnFGFR3, dnFGFR4, or green fluorescent protein (GFP) adenovirus. Cell number was determined after 2 and 5 days. (c) Melanoma cells transduced with dnFGFR1 or GFP adenovirus were reseeded in soft agar. Number of clones was determined after 14 days. (d) Cell lysates from transduced or untransduced (UT) VM21 cells were immunoblotted for procaspase-3, cleaved poly(ADP-ribose) polymerase (PARP)-1, and β-actin. (e) Tumor growth in severe combined immunodeficient mice was recorded from VM1 cells transduced with dnFGR1, dnFGFR4, or GFP. (f) Survival analysis of mice bearing tumors from dnFGFR1- or GFP-transduced VM1 cells. a, P<0.05; b, P<0.01 versus GFP.
FGFR inhibitors enhance the effect of antimelanoma drugs
For clinical application, blockade of FGFR signals with TKIs is more feasible than the use of dn receptor constructs. Therefore, we tested the impact of two TKIs inhibiting FGFR, SU5402, and PD166866 on the melanoma lines (Figure 4a and b). Both inhibitors reduced cell viability, with the strongest effect in VM21 (PD166866) and VM24 (SU5402). Only modest effects were seen in the brain metastasis-derived cell line VM48. The pan-FGFR inhibitor SU5402 had a higher efficacy than the FGFR1-specific inhibitor PD166866. As FGF signals have been implicated in cell survival and chemoresistance, we tested whether FGFR-specific TKIs could enhance the effect of antimelanoma drugs. Addition of SU5402 to dacarbazine, the standard chemotherapeutic drug used in melanoma, revealed a statistically significant but modest increase in efficacy only in VM48 cells (Figure 4c). In contrast, synergistic melanoma cell growth inhibition was observed for combinations of FGFR inhibitors with the multikinase/BRAF inhibitor sorafenib (Figure 4d). In VM48 cells, the FGFR–TKIs alone were largely ineffective. Nevertheless, the combination of sorafenib and PD166866 produced a pronounced effect. A similar efficacy of PD166866 as in VM21 cells and a moderate synergism with sorafenib (combination index (CI): 0.581 at 10 μm) was observed also in VM47 cells harboring wild-type BRAF (not shown). Clonogenic assays with PD166866 and sorafenib in VM21 cells further confirmed the synergistic nature of drug interaction (Figure 4e and f).
Figure 4. Fibroblast growth factor receptor (FGFR) kinase inhibitors reduce melanoma cell growth and show synergism with sorafenib.
Melanoma cells were treated with 2.5–25 μM SU5402 (a) or PD166866 (PD; b) for 5 days and analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. (c, d) For combination treatment, cells were simultaneously exposed to 10 μm dacarbazine (DTIC) or sorafenib plus 10 μm PD166866 or SU5402 and analyzed as above. (e) Representative examples and (f) quantification of clonogenic assays of VM21 cells treated with PD166866 plus sorafenib (1 μm each). Combination index (CI) values <0.9 indicating synergism are shown. For VM48 cells, inefficacy of FGFR-tyrosine kinase inhibitors alone precluded calculation of CI values and significant differences compared with antimelanoma drugs alone are shown instead. a, P<0.05; b, P<0.01. Co, control; Sor, sorafenib.
Combination of PD166866 and sorafenib blocks survival signals and enhances apoptosis
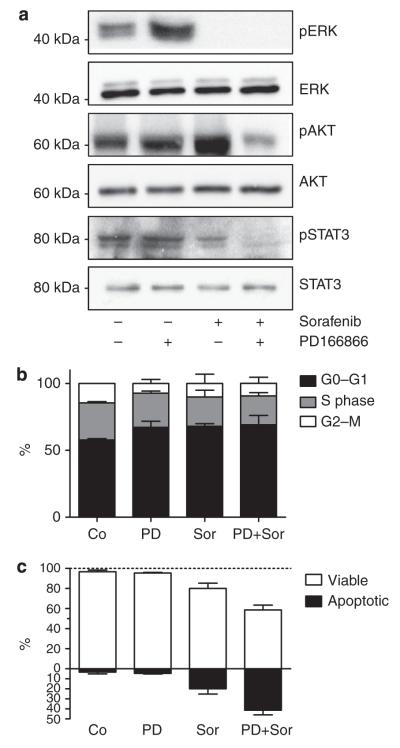
To shed light on the mechanism underlying the synergism between FGFR inhibitors and sorafenib, we analyzed the effects on downstream signal transduction in VM21 cells (Figure 5a). As expected, sorafenib alone was sufficient to abrogate extracellular signal-regulated kinase phosphorylation, likely via BRAF inhibition. However, only the combination treatment led to inhibition of phosphorylation of Akt (acutely transforming retrovirus AKT8 in rodent T-cell lymphoma) and STAT3, two important mediators of cell survival. With respect to cell cycle distribution, both drugs increased the fraction of cells in the G0/G1 phase and decreased the S-phase population, but the combination did not further enhance these effects (Figure 5b). Regarding apoptosis induction, PD166866 alone was not effective at 10 μm but more than doubled the apoptosis-inducing potential of sorafenib (Figure 5c). These results imply FGFs as survival factors in melanoma cells and suggest that increased cell death as a consequence of pronounced survival pathway inhibition underlies the synergism between sorafenib and FGFR-targeting agents.
Figure 5. Combination of fibroblast growth factor receptor (FGFR) inhibition with sorafenib treatment blocks survival signals and enhances apoptosis.
(a) VM21 cells were treated with 10 μm PD166866 (PD), 10 μm sorafenib (Sor), or a combination of both agents in medium with 5% fetal calf serum for 6 hours. Cell lysates were immunoblotted with the indicated antibodies, and representative blots of at least three different experiments are shown (b) VM21 cells were treated as above for 18 hours and analyzed by flow cytometry. Means and SD of duplicates from two experiments are shown. (c) Cells were treated as above for 48 hours, and percentages of viable and apoptotic cells were determined after staining with Hoechst 33258 and propidium iodide. Values represent means and SD of duplicates from two experiments. Co, control; ERK, extracellular signal-regulated kinase; pAKT, phosphorylated acutely transforming retrovirus AKT8 in rodent T cell lymphoma; pERK, phosphorylated extracellular signal-regulated kinase; pSTAT3, phosphorlylated signal transducer and activator of transcription 3.
FGFR inhibition enhances efficacy of sorafenib in vivo and potentiates the activity of the BRAF V600E-specific inhibitor RG7204
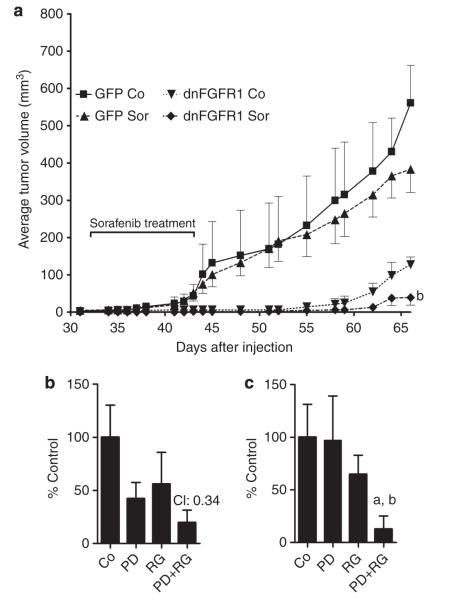
As a next step, we investigated the combination of FGFR inhibition and sorafenib in vivo in the VM1 human melanoma xenograft model (Figure 6a). DnFGFR1 alone again significantly reduced tumor growth (compare with Figure 3e). In contrast, sorafenib induced only a modest reduction of tumor growth in the GFP control group. However, when combined with dnFGFR1, sorafenib further significantly reduced growth of VM1 xenograft tumors in severe combined immunodeficient mice.
Figure 6. Fibroblast growth factor receptor (FGFR) inhibition enhances efficacy of sorafenib (Sor) in vivo and shows synergism with RG7204.
(a) VM1 cells transduced with dominant-negative (dn)FGFR1 or green fluorescent protein (GFP) adenovirus were injected into severe combined immunodeficient mice (eight per group) and treated with Sor or solvent during the indicated period. b, P<0.01 versus GFP Sor, dnFGFR1 control (Co). Clonogenic assays of VM21 (b) and VM1 (c) cells treated with 1 μm PD166866 (PD) and 0.1 μm RG7204 (RG) for 14 days. For VM21 cells, the combination index (CI) value indicating synergism is shown. For VM1 cells, inefficacy of 1 μm PD166866 alone precluded calculation of a CI value and P-values for reduction of clonal growth are shown instead. a, P<0.05 versus RG; b, P<0.01 versus PD.
Finally we tested, whether FGFR inhibition may also enhance the efficacy of drugs targeting mutated BRAF. Combination of PD166866 with the V600E BRAF-specific inhibitor RG7204 (PLX4032) potently reduced clonogenic growth of VM21 and VM1 cells (Figure 6b and c), suggesting this combination for further (pre)clinical evaluation as antimelanoma therapy.
DISCUSSION
During the last decade, molecularly targeted therapies have revolutionized the treatment of many cancer types and improved patient survival even in cancers largely resistant to classical chemotherapy such as hepatocellular carcinoma (Llovet et al., 2008). In melanoma, FGF2 was identified as an autocrine growth factor for melanoma cells (Halaban et al., 1988), and the forced expression of FGF2 was shown to contribute to a transformed phenotype in melanocytes (Nesbit et al., 1999). Moreover, inhibition of FGF2 signal transduction by antisense oligonucleotides or receptor blockage has confirmed the importance of FGF2/FGFR1 for melanoma growth in vitro and in vivo (Becker et al., 1992; Becker et al., 1989; Ozen et al., 2004; Wang and Becker, 1997; Yayon et al., 1997). Despite this important role of FGF-mediated signals for melanoma development and progression, no targeting strategies against the FGFR axis have so far found their way into clinical settings. More recently, activating mutations in BRAF have attracted much interest in melanoma and raised hopes for the successful application of molecularly oriented targeting approaches (Davies et al., 2002). So far however, clinical trials with the multikinase/ BRAF inhibitor sorafenib have been largely disappointing (Eisen et al., 2006), and consequently improved targeting strategies are still eagerly anticipated. The data shown here demonstrate that inhibition of FGF receptors could be an important therapeutic approach for melanoma treatment especially in combination with additional kinase inhibitors.
The expression data from this study are in line with previous reports showing widespread overexpression of FGF2 in melanoma (Birck et al., 1999; Scott et al., 1991), but also highlight that a considerable fraction of melanoma cells express additional FGFs with known oncogenic activity, such as FGF5 and FGF18 (Allerstorfer et al., 2008; Sonvilla et al., 2008). Interestingly, these FGFs both contain classical signal peptides (Beenken and Mohammadi, 2009) and are readily secreted, whereas FGF2 lacks a signal peptide and was shown to be partly retained in the cytoplasm, for instance, in non-small cell lung cancer cells (Berger et al., 1999). Similar to FGF2, FGF5 also was recently recognized as a potent inducer of proliferation and tube formation of endothelial cells (Allerstorfer et al., 2008), and could therefore be an important player in melanoma cell-induced angiogenesis. FGF18 expression is controlled by Wnt pathway activity in colorectal carcinoma (Shimokawa et al., 2003; Sonvilla et al., 2008) and may be driven by similar mechanisms in melanoma, where deregulated Wnt signaling has been described in up to one-third of patients (Larue and Delmas, 2006). On the receptor side, all melanoma cell lines investigated express multiple FGFR types and isoforms, enabling them to make use of a wide spectrum of different ligands with receptor isoform-specific binding affinities (Zhang et al., 2006). For instance, FGF18 shows only limited stimulation of FGFR1 IIIc and all IIIb variants but binds with high affinity to both FGFR3 IIIc and FGFR4 (Zhang et al., 2006). The latter is strongly expressed in our melanoma cell lines and was previously found to be associated with reduced patient survival in melanoma (Streit et al., 2006).
In multiple tumor types, FGF signals have been implicated in cell survival (Turner and Grose, 2010) and this is confirmed here by the apoptosis-inducing effect shown for dnFGFR1. It has been proposed that FGFs may function as potent rescue signals especially when other signaling molecules are blocked. For instance, in non-small cell lung cancer, FGF signals were implicated in intrinsic resistance against EGFR inhibitors (Kono et al., 2009), and FGFR inhibitors showed a synergistic activity with the avian erythroblastosis oncogene B-targeting drugs erlotinib and lapatinib (Fischer et al., 2008). Similar to these results, we observed synergistic growth inhibition in our melanoma cell lines also when combining FGFR inhibitors with erlotinib (not shown). Stronger synergism, however, was observed in our study upon combining FGFR inhibitors with sorafenib. This inhibitor targets BRAF, vascular endothelial growth factor receptor, and platelet-derived growth factor receptor (Wilhelm et al., 2004) and has shown therapeutic efficacy in renal cell and hepatocellular carcinoma (Escudier et al., 2009; Llovet et al., 2008), leading to its clinical approval for these malignancies. As BRAF is mutated in over 60% of melanomas (Davies et al., 2002), sorafenib was considered a promising agent for melanoma treatment. However, sorafenib showed no clinical benefit in melanoma when given as single agent (Eisen et al., 2006); further, the combination with chemotherapy showed only modest effects (Hauschild et al., 2009; McDermott et al., 2008). Remarkably, our study also did not reveal any synergism between chemotherapy with dacarbazine and FGFR inhibition comparable to recent data on combination of chemotherapy and FGFR inhibitors in non-small cell lung cancer (Fischer et al., 2008). These data suggest that a combination of several kinase inhibitors with different targets may be more promising in melanoma than a combination of kinase inhibitors with cytotoxic drugs. Second-generation inhibitors of BRAF have a higher inhibitory potency especially against the mutated form of BRAF and have shown increased efficacy in early clinical evaluation (Tsai et al., 2008; Wellbrock and Hurlstone, 2010). However, the inhibition of additional BRAF-independent pathways like the phosphoinositide 3-kinase/AKT pathway may still be required for efficient cell killing in melanoma cells, as suggested by studies combining inhibitors of these two pathways (Smalley et al., 2006; Tran et al., 2008). Our data showing strong inhibition of Akt and STAT3 phosphorylation preceding significantly increased apoptosis induction upon combination of the FGF inhibitor PD166866 and sorafenib clearly support this hypothesis. The kinase inhibitor RG7204 specifically targeting mutated BRAF has recently shown an unprecedented response rate of 80% in advanced melanoma harboring the BRAF mutation (Bollag et al., 2010). Nevertheless, tumor regrowth occurred in many of the patients. Thus, the authors suggested combination of RG7204 with additional agents as strategy to increase durability of the response. The synergistic potential of combining RG7204 with PD166866 observed in this study indicates that inhibition of the universally hyperactivated FGFR system, which feeds into several major survival pathways, in combination with the targeted blockade of a tumor-specifically activated downstream pathway may represent a promising approach for melanoma therapy.
In summary, our data highlight the importance of the FGF/FGFR axis as significant therapeutic target in melanoma and suggest that especially the combination of FGFR blockade with BRAF inhibition may offer a considerable potential for synergistic antitumor effects.
MATERIALS AND METHODS
Chemicals
The FGFR1-specific inhibitor PD166866 (Panek et al., 1998) was supplied by Pfizer (Groton, CT). The pan-FGFR inhibitor SU5402 was obtained from Calbiochem (La Jolla, CA), sorafenib from LC Laboratories (Woburn, MA), and RG7204 from Selleck Chemicals (Houston, TX). Recombinant human FGF2 was from Chemicon International (Temecula, CA) and FGF5 from Strathmann Biotec AG (Hamburg, Germany). All other reagents were from Sigma (St Louis, MO).
Cell culture
Twelve cell lines have been established from surgical specimens of primary melanoma (VM7, VM10, VM21, VM23, and VM30) or metastatic melanoma from lymph node (VM1, VM8, and VM24) or brain (VM28, VM47, and VM48; Pirker et al., 2003). One cell line (VM31) was derived from a malignant effusion. Histopathologically, cell lines were derived from superficial spreading melanoma (SSM; VM1 and VM10), nodular melanoma (VM7 + 8, VM21 + 23, VM24, VM30, VM31, VM47, and VM48), mixed SSM/NM histology (VM30), or unknown subtype of the primary tumor (VM28). Cells were grown in RPMI medium with 10% fetal calf serum at 37 °C in a humidified atmosphere containing 5% CO2. Cells were authenticated using microsatellite marker analysis and regularly checked for mycoplasma contamination.
Real-time reverse transcription PCR
Total RNA was extracted from 106 cells with TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse transcription, PCR and Taqman analyses were performed as published (Grusch et al., 2006). RNA from normal human epidermal neonatal melanocytes (Lonza, Basel, Switzerland) as non-malignant cell counterparts was used for comparison. Primer sequences and Taqman assay IDs are given in Supplementary Table S2 and S3 online. For normalization, threshold cycle (Ct) values of 18S were subtracted from the Ct values of the respective FGFR/FGF genes and the resulting delta Ct (dCt) values transformed to relative expression levels by the formula 2(−dCt) × 105.
Immunofluorescence
Cells were grown on collagen-coated microscope slides in quadriperm chambers (Sigma) and fixed with methanol/acetone (3:1) for 10 minutes at −20 °C. Cells were incubated with primary antisera (anti-FGFR1, anti-FGF18 (Santa Cruz Biotechnology (Santa Cruz, CA), sc-121, and sc-16830), anti-FGF5 (R&D Systems (Minneapolis, MN), AF-237 NA) diluted 1:25 in phosphate-buffered saline with 1% BSA) for 1 hour, followed by FITC- or Cy3-coupled secondary antibodies (1:500, Jackson Laboratories, West Grove, PA). Cells were counterstained with TOPRO3, mounted in Mowiol (Sigma), and photographed on a Leica TCS-SP confocal microscope (Leica Microsystems, Wetzlar, Germany).
In vitro assays
For cytotoxicity assays, exponentially growing cells were seeded into 96-well plates at a density of 2 × 103 cells per well in 100 μl medium containing 10% fetal calf serum. At 24 hours later, another 100 μl serum-free medium containing FGF2, FGF5, or the indicated drugs were added. Controls were vehicle-treated only. Cell viability was assessed by MTT assay (EZ4U, Biomedica, Vienna, Austria). Five wells were analyzed per treatment condition, and experiments were repeated at least three times. Effects of drug combinations were analyzed by exposing tumor cells in parallel to the two investigated drugs as single agents and in combination. CI values indicating additive (0.9<CI<1.1), antagonistic (CI>1.1), or synergistic (CI<0.9) drug interaction were calculated with Calcusyn software (Biosoft, Cambridge, UK; Chou and Talalay, 1984). For clonogenic assays, 1.25 × 102 cells cm −2 were exposed to viral constructs or drugs for 14 days. Clones were stained with crystal violet and CI values calculated as above. For details on additional in vitro assays, see Supplementary Materials and Methods online.
DnFGFR adenoviruses
Adenoviral expression vectors for dnFGFR1 and dnFGFR3 have been described previously (Fischer et al., 2008; Sonvilla et al., 2010). A plasmid encoding human FGFR4 cDNA was kindly provided by S Ezzat. To generate a kinase-truncated dn variant, the FGFR4 open reading frame was subcloned into pENTR1A (Invitrogen, Carlsbad, CA) with KpnI/XhoI and the kinase domain removed by digestion with BglII/NotI and replaced with the likewise digested cyan fluorescent protein sequence. The resulting FGFR4-cyan fluorescent protein chimera was transferred into pAd/CCMV/V5-Dest by Gateway recombination (Invitrogen). Virus amplification was done as described and an adenovirus-expressing GFP was used as control (Losert et al., 2006). Virus titers were determined with the Adeno-X Rapid Titer Kit (Clontech, Mountain View, CA).
Western blot analysis
Western blotting and immunodetection were done as described (Sonvilla et al., 2008). For details, see Supplementary Materials and Methods online.
Tumor formation in severe combined immunodeficient mice
Cells were transduced with dnFGFR1, dnFGFR4, or GFP adenovirus on cell culture plates. Subsequently, 106 cells in 50 μl phosphate-buffered saline were subcutaneously injected into the rear flanks of severe combined immunodeficient BALB/c recipient mice (female, age 4 weeks; Harlan Winkelmann, Borchen, Germany). In one experiment, animals were treated orally with sorafenib (2.5 mg kg −1 in cremophor EL) or solvent five times a week for 2 weeks starting from day 34 after injection. Tumor size was determined using a vernier caliper and used for calculation of tumor volume (smaller diameter2 × larger diameter × 0.5). Animals were killed when tumors exceeded a volume of 5,000 mm3. Experiments were carried out according to the Austrian and FELASA guidelines for animal care and protection.
Statistical analysis
Unless stated otherwise, data are presented as means and SD of at least three experiments. Statistical significance between treatments was analyzed with GraphPad Prism software using Student’s t-test or one- or two-way analysis of variance for comparison of two or multiple groups, respectively. Survival analysis was performed using Kaplan-Meier curves and log-rank test. In all cases, P≤0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Initiative for Cancer Research of the Medical University of Vienna, the Fund of the City of Vienna for Interdisciplinary Cancer Research, the Fellinger Foundation for Cancer Research, and Austrian Science Fund (FWF) Projects P17630-B12 and P19920-B12.
Abbreviations
- CI
combination index
- dn
dominant negative
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- GFP
green fluorescent protein
- NM
normal melanocyte
- TKI
tyrosine kinase inhibitor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–8. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allerstorfer S, Sonvilla G, Fischer H, et al. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene. 2008;27:4180–90. doi: 10.1038/onc.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Lee PL, Rodeck U, et al. Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene. 1992;7:2303–13. [PubMed] [Google Scholar]
- Becker D, Meier CB, Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. Embo J. 1989;8:3685–91. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Setinek U, Mohr T, et al. Evidence for a role of FGF-2 and FGF receptors in the proliferation of non-small cell lung cancer cells. Int J Cancer. 1999;83:415–23. doi: 10.1002/(sici)1097-0215(19991029)83:3<415::aid-ijc19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Birck A, Kirkin AF, Zeuthen J, et al. Expression of basic fibroblast growth factor and vascular endothelial growth factor in primary and metastatic melanoma from the same patients. Melanoma Res. 1999;9:375–81. doi: 10.1097/00008390-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–8. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- Fischer H, Taylor N, Allerstorfer S, et al. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7:3408–19. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–64. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- Grusch M, Drucker C, Peter-Vorosmarty B, et al. Deregulation of the activin/follistatin system in hepatocarcinogenesis. J Hepatol. 2006;45:673–80. doi: 10.1016/j.jhep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Halaban R, Kwon BS, Ghosh S, et al. bFGF as an autocrine growth factor for human melanomas. Oncogene Res. 1988;3:177–86. [PubMed] [Google Scholar]
- Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Haluska FG. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol. 2009;4:551–79. doi: 10.1146/annurev.pathol.3.121806.151541. [DOI] [PubMed] [Google Scholar]
- Jeffers M, LaRochelle WJ, Lichenstein HS. Fibroblast growth factors in cancer: therapeutic possibilities. Expert Opin Ther Targets. 2002;6:469–82. doi: 10.1517/14728222.6.4.469. [DOI] [PubMed] [Google Scholar]
- Kono SA, Marshall ME, Ware KE, et al. The fibroblast growth factor receptor signaling pathway as a mediator of intrinsic resistance to EGFR-specific tyrosine kinase inhibitors in non-small cell lung cancer. Drug Resist Updat. 2009;12:95–102. doi: 10.1016/j.drup.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–42. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Losert A, Mauritz I, Erlach N, et al. Monitoring viral decontamination procedures with green fluorescent protein-expressing adenovirus. Anal Biochem. 2006;355:310–2. doi: 10.1016/j.ab.2006.04.034. [DOI] [PubMed] [Google Scholar]
- McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–85. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- McKeehan WL, Wang F, Kan M. The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol. 1998;59:135–76. doi: 10.1016/s0079-6603(08)61031-4. [DOI] [PubMed] [Google Scholar]
- Nesbit M, Nesbit HK, Bennett J, et al. Basic fibroblast growth factor induces a transformed phenotype in normal human melanocytes. Oncogene. 1999;18:6469–76. doi: 10.1038/sj.onc.1203066. [DOI] [PubMed] [Google Scholar]
- Ozen M, Medrano EE, Ittmann M. Inhibition of proliferation and survival of melanoma cells by adenoviral-mediated expression of dominant negative fibroblast growth factor receptor. Melanoma Res. 2004;14:13–21. doi: 10.1097/00008390-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Panek RL, Lu GH, Dahring TK, et al. In vitro biological characterization and antiangiogenic effects of PD 166866, a selective inhibitor of the FGF-1 receptor tyrosine kinase. J Pharmacol Exp Ther. 1998;286:569–77. [PubMed] [Google Scholar]
- Pirker C, Holzmann K, Spiegl-Kreinecker S, et al. Chromosomal imbalances in primary and metastatic melanomas: over-representation of essential telomerase genes. Melanoma Res. 2003;13:483–92. doi: 10.1097/00008390-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Scott G, Stoler M, Sarkar S, et al. Localization of basic fibroblast growth factor mRNA in melanocytic lesions by in situ hybridization. J Invest Dermatol. 1991;96:318–22. doi: 10.1111/1523-1747.ep12465203. [DOI] [PubMed] [Google Scholar]
- Shimokawa T, Furukawa Y, Sakai M, et al. Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res. 2003;63:6116–20. [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, et al. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Sonvilla G, Allerstorfer S, Heinzle C, et al. Fibroblast growth factor receptor 3-IIIc mediates colorectal cancer growth and migration. Br J Cancer. 2010;102:1145–56. doi: 10.1038/sj.bjc.6605596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonvilla G, Allerstorfer S, Stattner S, et al. FGF18 in colorectal tumour cells: autocrine and paracrine effects. Carcinogenesis. 2008;29:15–24. doi: 10.1093/carcin/bgm202. [DOI] [PubMed] [Google Scholar]
- Streit S, Mestel DS, Schmidt M, et al. FGFR4 Arg388 allele correlates with tumour thickness and FGFR4 protein expression with survival of melanoma patients. Br J Cancer. 2006;94:1879–86. doi: 10.1038/sj.bjc.6603181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MA, Gowda R, Sharma A, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–49. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Wang Y, Becker D. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med. 1997;3:887–93. doi: 10.1038/nm0897-887. [DOI] [PubMed] [Google Scholar]
- Wellbrock C, Hurlstone A. BRAF as therapeutic target in melanoma. Biochem Pharmacol. 2010;80:561–7. doi: 10.1016/j.bcp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- Yayon A, Ma YS, Safran M, et al. Suppression of autocrine cell proliferation and tumorigenesis of human melanoma cells and fibroblast growth factor transformed fibroblasts by a kinase-deficient FGF receptor 1: evidence for the involvement of Src-family kinases. Oncogene. 1997;14:2999–3009. doi: 10.1038/sj.onc.1201159. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.