Summary
Ischemia-associated oxidative damage leading to necrosis is a major cause of catastrophic tissue loss in human health. Elucidating its signaling mechanism is of paramount importance. p53 is a central stress sensor responding to multiple insults including oxidative stress to orchestrate apoptotic and autophagic types of cell death. Whether p53 can also activate oxidative stress-induced necrosis is unknown. Here we uncover a role of p53 in activating necrosis. In response to oxidative stress, p53 accumulates in the mitochondrial matrix and triggers mitochondrial permeability transition pore (PTP) opening and necrosis by physical interaction with the critical PTP regulator Cyclophilin D (CypD). Intriguingly, a robust p53-CypD complex forms during brain ischemia/reperfusion injury. In contrast, reduction of p53 levels or Cyclosporine A-pretreatment of mice prevents this complex and is associated with effective stroke protection. Our study identifies the mitochondrial p53-CypD axis as an important contributor to oxidative stress-induced necrosis and implicates this axis in stroke pathology.
Introduction
Three morphologically distinct types of cell death occur in physiologic and pathologic processes: apoptosis, autophagy and necrosis (Edinger and Thompson, 2004). p53 is a critical transcriptional activator and exerts additional biochemical functions in the cytoplasm. In its central role as cellular stress sensor that responds to a myriad of signals including DNA damage, oxidative stress and ischemia, p53 controls programs of apoptosis via transcription-dependent and -independent mechanisms to limit the propagation of damaged cells. p53-controlled apoptosis involves transcriptional induction of components of the death receptor and mitochondrial pathways including CD95, Puma, Noxa, Bax and others, which cooperatively promote cell death (Brady et al., 2011; Riley et al., 2008). In addition, p53 protein can directly promote mitochondrial outer membrane permeabilization (MOMP) to trigger apoptosis by modulating the MOMP governing Bcl-2 family (Green and Kroemer, 2009; Vaseva and Moll, 2009). We and others previously identified a p53-protein based mitochondrial apoptosis program. Upon stress, a cytoplasmic pool of p53 rapidly translocates to the mitochondrial surface, where it physically interacts with both anti- and pro-apoptotic Bcl-2 family members to inhibit or activate their respective functions, leading to MOMP and apoptosis. In this role, p53 acts like a BH3-only protein, either as direct activator of the Bax/Bak effectors, or as sensitizer/de-repressor of Bcl-xL/2 and Mcl1 (Vaseva and Moll, 2009). While in principle the pro-apoptotic effects of cytoplasmic p53 are not dependent on transcription by nuclear p53, the latter appears to enhance the function of cytoplasmic p53.
p53 also has a complex role in regulating autophagy which can promote either cell survival under stress or cell death, dependent on context. Activated nuclear p53 can transcriptionally induce AMPK and sestrin1/2 with subsequent mTOR inhibition and autophagy. Autophagy can promote cell death when apoptosis is compromised, or act as reinforcement of apoptotic caspase signaling. The overlap between apoptosis and autophagy in p53-activated cell death is illustrated by the p53 target genes DRAM, Bax and Puma, also shown to be positive autophagy regulators. Conversely, basal levels of p53 in the cytoplasm can directly inhibit autophagosomes (Vousden and Ryan, 2009).
Necrosis is the irreversible tissue destruction due to bioenergetic failure, and central to ischemia/reperfusion injury and oxidative damage as occurs in cerebral stroke and myocardial infarction. The fundamental difference to apoptosis is the rapid loss of cellular membrane potentials due to energy depletion and ion pump/channel failures, leading to swelling, rupture and cytolysis. Mediators of necrosis are excess cytosolic Ca2+ and ROS levels. Rather than being a passive event, necrosis has emerged as a controlled cell death that induces an inflammatory response to stimulate tissue repair by selectively releasing factors like HMGB1 and HDGF from dying cells (Zong and Thompson, 2006). Necrosis in ischemic tissues, which is experimentally modeled by H2O2 treatment of cultured cells, depends on Cyclophilin D (CypD), the key regulator of the mitochondrial permeability transition pore (PTP) at the inner membrane whose opening leads to cell death (Halestrap, 2006). As shown by 4 independent strains, CypD−/− mice are resistant to ischemia-induced necrosis in myocardial infarction and stroke, and CypD-deficient mitochondria and cells are resistant to Ca2+ and H2O2-induced cell death. But notably, they remain sensitive to Bcl-2-family driven apoptosis, emphasizing the two functionally distinct mitochondrial death systems (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005). PTP is a regulated non-selective water- and solute-passing protein channel spanning the inner (IMM) and outer (OMM) mitochondrial membranes at points of contact. The identity of the actual pore-forming proteins of PTP are still debated, since they appear in part dispensable (Baines, 2010) but can encompass VDAC (Voltage Dependent Anion Channel) across the OMM, and ANT (Adenine Nucleotide Translocase) across the IMM. In contrast, the prolyl isomerase CypD in the mitochondrial matrix is the essential regulator of the PTP pore opening, and the only genetically proven indispensable PTP component (Kroemer et al., 2007).
H+ pumping out of the matrix creates the proton gradient ΔΨm across the IMM necessary to maintain mitochondrial respiration and ATP production. In unstressed cells, PTP is closed and the IMM impermeable to ions. Upon oxidative stress, sudden PTP opening causes massive ion influx that dissipates ΔΨm and shuts down oxidative phosphorylation and ATP production. This is called mitochondrial permeability transition (mPT). Concomitantly, water influx causes matrix swelling, rupture of the rigid OMM and release of all sequestered cell death factors. mPT is triggered by mitochondrial matrix sequestration of high levels of cytosolic Ca2+ and ROS generated during oxidative damage. Clinically, CypD-triggered PTP opening and mPT is the driving pathophysiological force behind cerebral stroke, myocardial infarction and other vascular catastrophies that are leading causes of death. How CypD becomes activated to induce mPT remains unclear but depends on its prolyl isomerase activity. Cyclosporine A (CsA) potently and specifically prevents mPT by binding to CypD, inhibiting its isomerase activity and displacing it from the PTP (Kroemer et al., 2007).
It is currently unknown whether p53 can also activate oxidative stress-induced necrosis. Intriguingly, our findings provide genetic, biochemical and pharmacological evidence that fundamentally expands our understanding of p53-mediated cell death networks into necrosis. We identify an unexpected critical role of stress-accumulated mitochondrial p53 protein in directly regulating PTP at the inner membrane. Upon oxidative stress, p53 triggers PTP opening by engaging in a physical interaction with CypD, thereby inducing necrotic cell death in mouse and human cells. Importantly, this pathway is implicated in brain ischemia-reperfusion injury.
Results
In Addition to Bax/Bak Lipid Pores, Purified p53 Also Engages PTP to Induce Mitochondrial Membrane Permeabilization
We previously showed in isolated mitochondria that purified p53 causes robust Cytochrome C (CytoC) release by inducing Bak oligomerization and MOMP with the same efficiency as tBid (Mihara et al., 2003; Wolff et al., 2008). Moreover, we showed that p53 is able to release the entire gamut of soluble and non-soluble apoptogenic factors including non-cleaved AIF and Endo G that are tethered to the IMM by severely disrupting both outer and inner mitochondrial membrane integrity. In contrast, tBid is restricted to releasing only soluble components via MOMP (Wolff et al., 2008). This suggests a stronger permeabilizing effect by p53 than by the prototypical activator of the Bax/Bak pore, tBid. Thus, we hypothesized that p53 is capable of recruiting additional BH3-independent pathways of mitochondrial permeabilization.
To this end, we compared the release response of isolated WT and Bax/Bak double knockout (DKO) mitochondria (Figure S1A) towards highly purified recombinant p53 and tBid. Bax/Bak DKO cells are deprived of the apoptotic gateway to mediate CytoC release for caspase activation (Kim et al., 2006). Indeed, p53 was able to rapidly release CytoC, Smac (soluble) and AIF (membrane tethered) from both WT and Bax/Bak DKO mitochondria, while the tBid-induced release was restricted to WT mitochondria (Figure 1A). The strict Bax/Bak-dependence of tBid-mediated MOMP is completely consistent with the notion of tBid as direct activator of the Bax/Bak lipid pore, tBid’s sole target (Kuwana et al., 2005). In contrast, p53 apparently has additional mitochondrial targets to mediate mitochondrial permeabilization.
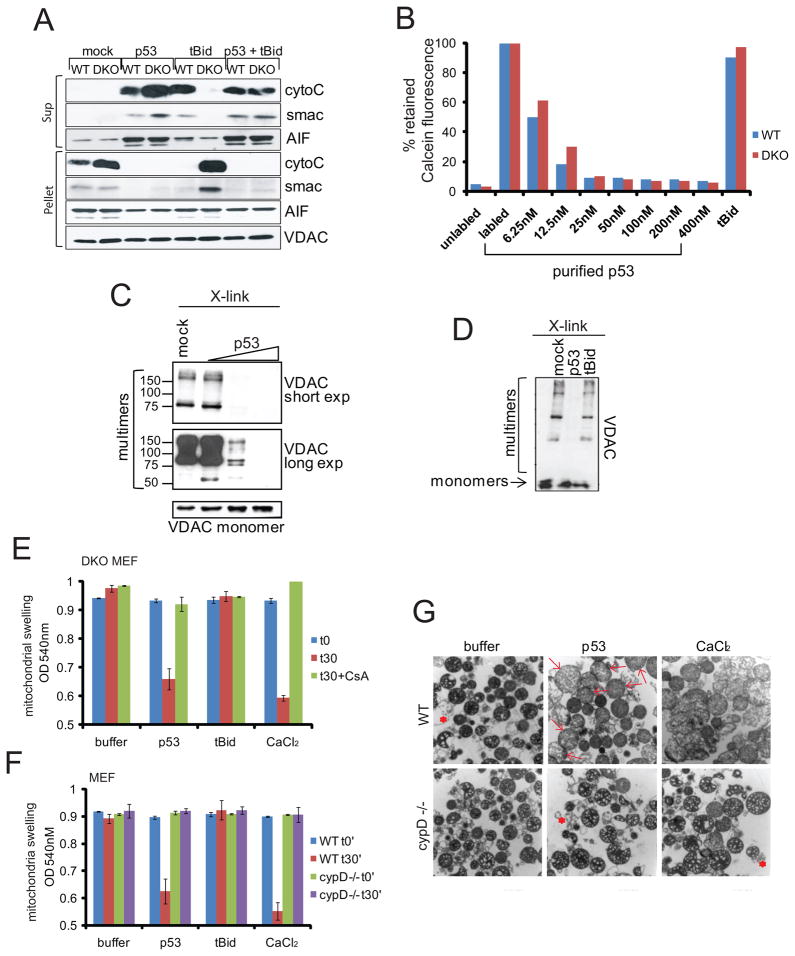
Figure 1. Purified p53 Protein Opens the PTP Pore in Isolated Mitochondria Independent of Bax and Bak but Dependent on CypD.
A. In contrast to tBid, p53’s MOMP activity can also be independent of Bax and Bak. Isolated mitochondria from WT or Bax−/−Bak−/− (DKO) MEFs were incubated with empty elution buffer (mock), purified p53 (100 nM) or tBid (100 nM) for 30 min at 30°C. MOMP was determined by release of CytoC, Smac and AIF into the supernatant (Sup) via immunoblotting. VDAC, loading control.
B. p53, but not tBid opens the PTP pore independently of Bax and Bak. Mitochondria from WT or DKO MEFs were labeled with calcein-AM prior to adding purified p53 (6.25–400 nM) or tBid (400 nM). PTP opening was measured by FACS as loss of retained mitochondrial fluorescence.
C, D. p53 induces physical alterations of VDAC, a proposed structural PTP component. C. p53 drives VDAC-containing complexes ranging from ~ 60 to 300 kDa into high molecular weight complexes that no longer enter the gel. Liver mitochondria were incubated with BSA (mock) or 10, 40 and 100 nM of purified p53 prior to chemical crosslinking and immunoblotting. Co-existing monomeric VDAC as input control. D. p53, but not tBid drives VDAC into high molecular weight complexes. Analysis as in C with p53 and tBid proteins at 100 nM each.
E–G. p53, but not tBid induces mitochondrial swelling in a CypD-dependent and Bax/Bak-independent manner. E. Mitochondria isolated from Bax/Bak DKO MEFs were incubated with buffer, 50 nM of purified p53 or tBid, or 50 μM CaCl2 for 30 min. CypD inhibitor CsA (5 μM) was added prior to proteins or CaCl2 where indicated. Mitochondrial swelling was measured at optical density of 540 nm. F. Mitochondria from WT or cypD−/− MEFs were treated as in E. Three independent experiments; mean ± SD. G. Electron microscopy images of WT or cypD −/− mitochondria treated as in F. p53 induces swelling in WT but not CypD −/− mitochondria which appear large and pale (see arrows). * denotes debris due to isolation procedure.
See also Figure S1.
We reasoned that p53 might also engage the mitochondrial PTP pore. To look for evidence of p53-induced functional alterations of this pore, we used the fluorescent dye calcein, a highly selective indicator of sustained PTP opening in situ (Kroemer et al., 2007). MOMP and the Bax/Bak lipid pore are completely incompetent for calcein release (Petronilli et al., 1998). Adding increasing doses of purified p53 to calcein-prelabeled WT or Bax/Bak DKO mitochondria resulted in a rapid and almost complete loss of signal, indicating PTP opening. In contrast, even the highest dose of tBid lacked any effect (Figure 1B and Figure S1B). Moreover, p53 but not tBid induced structural alterations of PTP components. WT mouse liver mitochondria were incubated with increasing amounts of purified p53 prior to chemical cross-linking. VDAC-containing complexes ranging from ~ 60–300 kDa were readily detected in control mitochondria incubated with buffer or BSA, but increasingly disappeared upon addition of p53 in a dose-dependent fashion (Figure 1C and Figure S1C). Thus, p53 was able to drive VDAC into high molecular weight complexes that no longer entered the gel. This activity was unique to p53, since tBid had no effect on VDAC oligomerization (Figure 1D). Moreover, p53 but not tBid was able to induce swelling (another classical hallmark of PTP opening) of Bax/Bak DKO and WT mitochondria in a CsA-dependent (Figure 1E) and CypD-dependent (Figures 1F, 1G) manner. Thus, in addition to the Bax/Bak lipid pore, p53 can also engage the PTP pore to induce mitochondrial membrane permeabilization.
p53 Protein Interacts with the Essential Regulatory PTP Component CypD
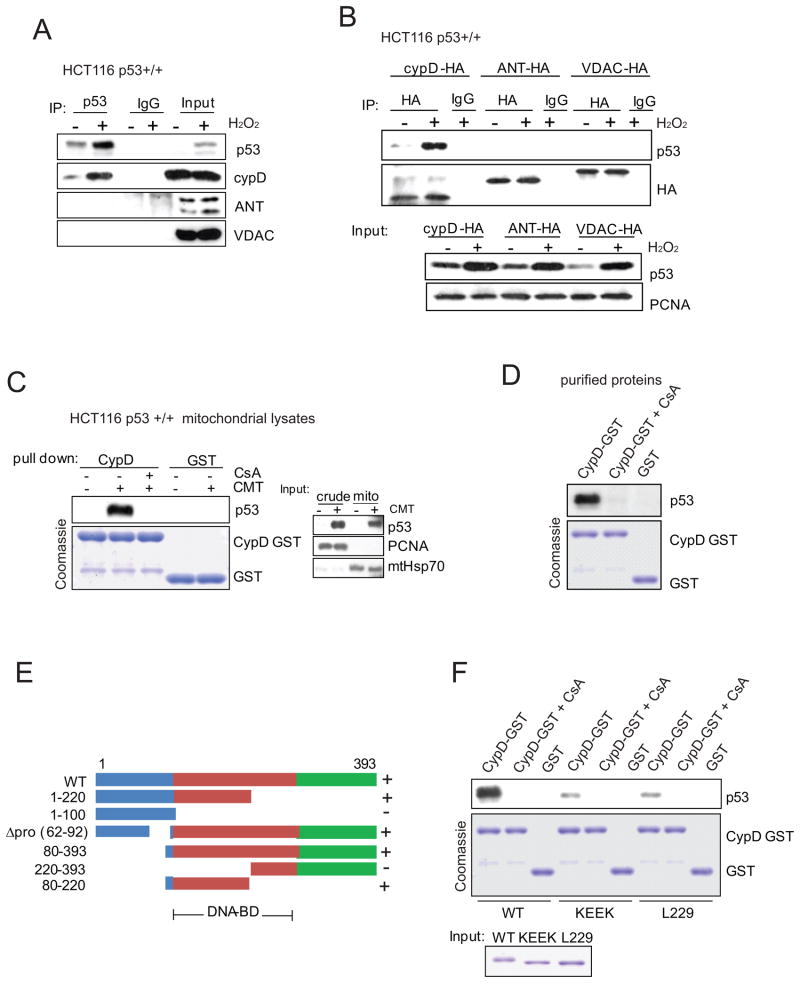
Based on these results, we next asked whether and if so upon which stimulus p53 interacts with components of the PTP complex. Since both p53 and mPT play important roles during oxidative stress (Gudkov and Komarova, 2010; Halestrap, 2006; Vaseva and Moll, 2009), we looked for an interaction between p53 and the PTP components CypD, ANT and VDAC upon H2O2 treatment. Immunoprecipitation detected a prominent oxidative stress-induced endogenous complex between p53 and the essential regulatory PTP component CypD (Figure 2A). No interaction was seen between p53 and the proposed structural components VDAC and ANT (Figure 2A). The H2O2-inducible specific p53-CypD complex was confirmed with pull down assays of HA-tagged CypD, VDAC and ANT expressed in cells with endogenous p53 (Figure 2B). Moreover, endogenous mitochondrial p53 binds to recombinant GST-CypD in a CsA-dependent manner (Figure 2C). Finally, cell-free pull down experiments with recombinant proteins confirmed the direct CsA-sensitive interaction (Figure 2D). Mapping experiments with a series of p53 deletion mutants identified p53 amino acids 80–220 as the region required for CypD interaction (Figures 2E and Figures S2A–S2C). While we failed to identify a single specific subregion, the p53 contact domains that are required for Bcl-xL/Bcl-2 interaction (designated with ‘Δ’) (Mihara et al., 2003) were not required for CypD binding (Figure S2D), underlining structural differences between the two types of interactions. Tetrameric p53 is the preferred partner for CypD interaction (Figure 2F), while it is debated whether mitochondrial p53 that directly triggers Bcl-2 family-mediated apoptosis at the OMM acts as monomer or tetramer (Heyne et al., 2008; Pietsch et al., 2007).
Figure 2. Mitochondrial p53 Interacts with CypD, but not with VDAC or ANT.
A. p53 forms a prominent oxidative stress-induced endogenous complex with CypD, but not with VDAC or ANT. HCT116 p53 +/+ cells were treated with 0.4 mM H2O2 for 6 hrs. Mitochondria were isolated, lysed and immunoprecipitated with p53 antibody or IgG followed by immunoblot.
B. CypD but not VDAC or ANT binds to p53. HCT116 p53 +/+ cells expressing HA-tagged CypD, ANT or VDAC were treated with 0.4 mM H2O2 for 6 hrs, lysed and immunoprecipitated with anti-HA followed by immunoblot.
C. p53 binds to CypD. Binding is blocked by the CypD inhibitor CsA. Mitochondrial lysates of HCT116 p53 +/+ cells (right) treated with 5 μM Camptothecin (CMT) for 3 hrs were incubated with CypD-GST or GST alone (Coomassie) ± CsA (5 μM), washed and immunoblotted for p53.
D. Direct interaction between recombinant p53 and CypD proteins is CsA-dependent. Purified p53 and CypD-GST proteins were used in GST-pull down assays with 5 μM CsA where indicated.
E. Mapping the CypD interaction on p53. The indicated p53 deletion constructs were transfected into HCT116 p53 −/− cells. Binding was assayed with purified CypD-GST protein. Lack (−) or presence (+) of binding is indicated.
F. Tetrameric p53 is the preferred partner for CypD interaction. Purified wild-type, monomeric (KEEK, for F341K, L344E, L348E, A355K) or dimeric (L229K) p53 mutants were used in pull-down assays with purified CypD-GST.
See also Figure S2.
Rapid Stabilization and Mitochondrial Translocation of p53 upon Oxidative Stress
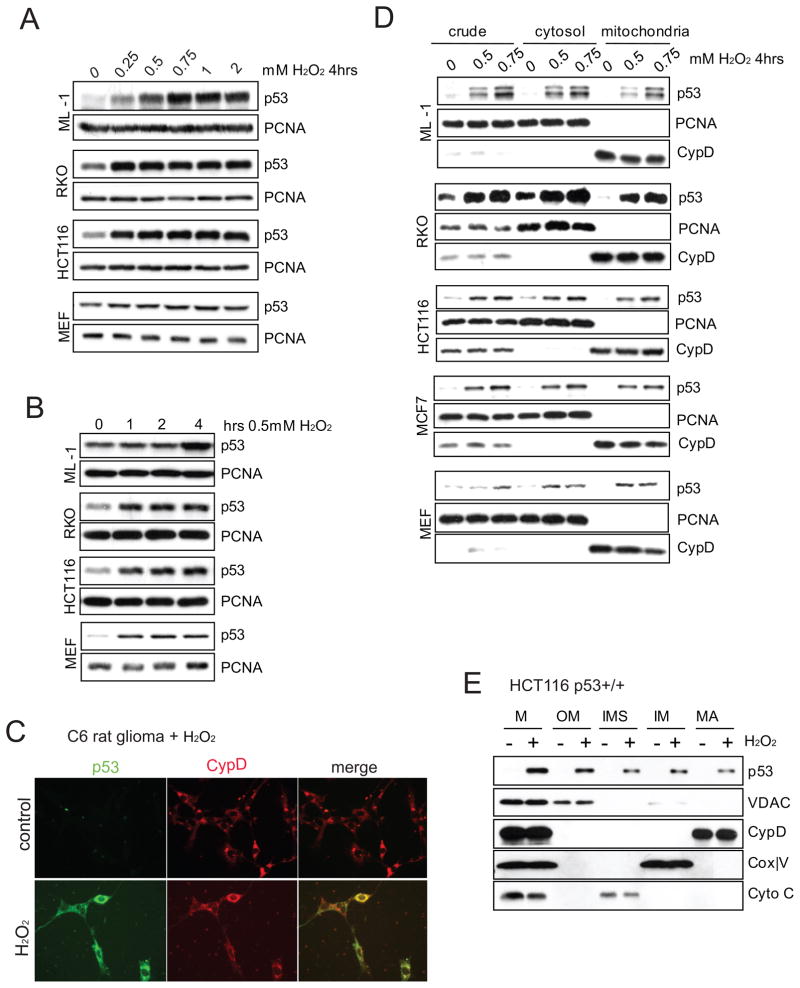
Next we asked whether p53 accumulates in mitochondria upon oxidative stress. Treating various wtp53 harboring cell lines and primary MEFs with 0.25 – 0.75 mM H2O2 uniformly induced robust cellular p53 stabilization (Figure 3A). This oxidative stress-induced p53 response was fast, reaching its peak within 1 hour in most cells (Figure 3B). Immunofluorescence staining of C6 rat glioma cells treated with H2O2 for 4 hrs showed primarily punctate perinuclear p53 accumulation, confirming earlier findings (Bonini et al., 2004), which co-localized with CypD in mitochondria. This suggests an important mitochondrial function of the p53 protein within this window of time during oxidative stress (Figure 3C). Moreover, using sucrose gradient ultracentrifugation, p53 accumulated in mitochondria that were free of detectable contamination within 4 hrs of H2O2 treatment (Figure 3D). Importantly, subsequent submitochondrial fractionation of this mitochondrial starting material (Figure S3A) confirmed that during oxidative stress a fraction of p53 accumulated in the matrix where CypD is located, while some also localized to the surface, as expected (Figure 3E). The observed p53 fractions in the mitochondrial intermembranous space and inner membrane likely represent transport intermediates to the matrix. Moreover, H2O2-induced mitochondrial p53 was also partially protected from trypsin digestion in classical import assays (Figure S3B) and import of p53 required a mitochondrial membrane potential (Figure S3C). This is in agreement with hypoxia-induced endogenous complexes between p53 and the major matrix import proteins mtHSP70 and mtHSP60 (Figure S3D) which we had also reported earlier (Sansome et al., 2001). Of note, the endogenous p53 that accumulates in the mitochondrial matrix is detectable by immunogold electron microscopy (Figure S3E and Table I).
Figure 3. Upon Oxidative Stress p53 is Stabilized and Accumulates in Mitochondria.
A, B. Robust (A) and rapid (B) stabilization of wt p53 in cells treated with H2O2. Doses and times as indicated. Immunoblot, PCNA loading control.
C. Endogenous p53 accumulates in a punctate perinuclear pattern that co-localizes with CypD. C6 rat glioma cells (wt p53) were left untreated or treated with 0.4 mM H2O2 for 6 hrs. Immunofluorescence.
D. Rapid mitochondrial p53 accumulation upon oxidative stress. Cells were treated with H2O2 for 4 hrs. Mitochondrial fractions were prepared by sucrose gradient ultracentrifugation. PCNA serves as indicator of purity of mitochondrial fractions and as loading control for crude and cytosolic fractions. CypD serves as mitochondrial marker and loading control for mitochondrial fractions.
E. Upon oxidative stress, p53 accumulates in the mitochondrial matrix. Isolated mitochondria from HCT116 p53 +/+ cells treated with 0.5 mM H2O2 for 4 hrs shown in (D) were further subjected to submitochondrial fractionation. M, crude mitochondrial; OM, outer membrane; IMS, intermembrane space; IM, inner membrane; MA, matrix.
See also Figure S3.
Oxidative Stress Induces Mitochondrial Permeability Transition in a p53- Dependent Manner
CypD-regulated mPT is a crucial event during H2O2-induced cell death, definitively established by the CypD knockout mice. Our initial results in isolated mitochondria had shown that purified p53 protein was able to engage PTP in a CypD-dependent manner (Figure 1). However, an mPT-regulatory role of p53 in vivo remained to be established. We therefore asked whether H2O2 - induced PTP opening in cells is p53-dependent.
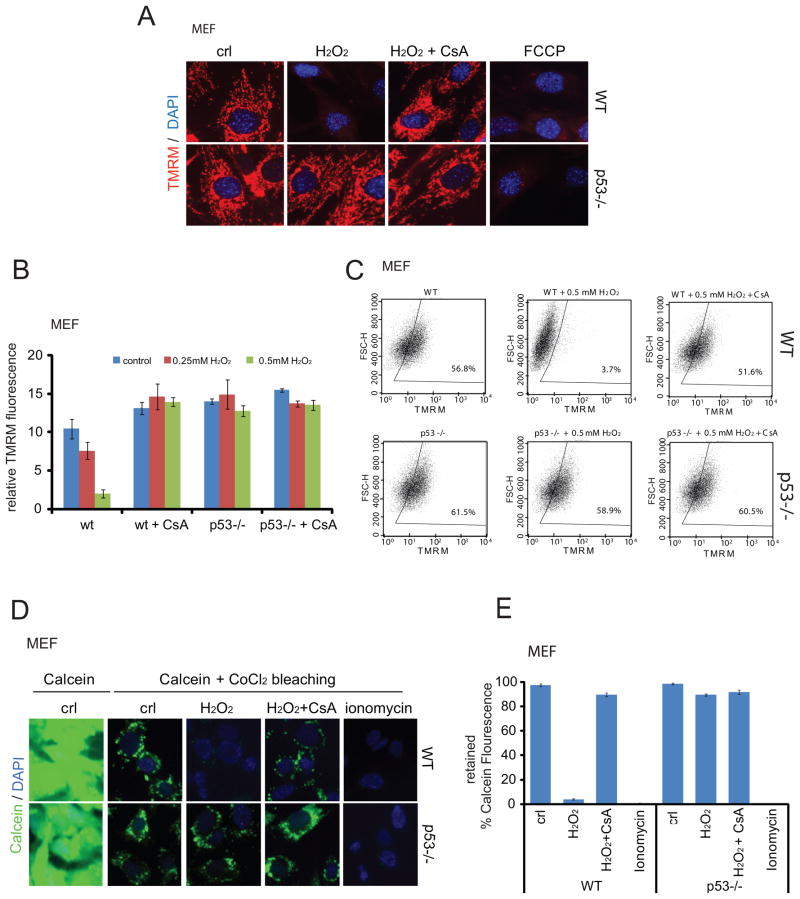
Opening of the PTP pore and subsequent mPT results in dissipation of the mitochondrial membrane potential ΔΨm across the IMM. The cationic fluorescent dye tetramethylrhodamine methyl ester (TMRM) is widely used to assess changes in ΔΨm and mPT. TMRM is readily sequestered by healthy mitochondria, but its fluorescence is rapidly lost when ΔΨm is dissipated. Indeed, in response to H2O2 treatment WT MEFs completely lost TMRM fluorescence. This occurred in a CypD-dependent manner, since CypD inhibitor CsA completely blocked this event. In contrast, p53−/− MEFs remained completely unaffected (Figures 4A–4C). Conversely, acute restoration of p53 re-sensitizes p53-null cells to H2O2 (Figures S4A and S4B). This strong p53-dependent response was also observed in the isogenic HCT116 p53+/+ and p53−/− pair (Figure S4C). These results were further confirmed by direct in situ assessment of PTP pore opening via Calcein release, which again showed p53 and CypD dependence (see CsA, Figures 4D, 4E and Figure S4D). Of note, while CsA can block other cytosolic cyclophilins besides mitochondrial matrix-specific CypD, none of them play any role in mitochondrial mPT. In sum, the mPT-regulatory activity of p53 in cells in response to oxidative stress is also CypD-dependent. In contrast, p53 does not respond to calcium overload, and calcium overload-induced PTP opening and necrosis does not require p53 (Figures S4E–S4H).
Figure 4. Oxidative stress induces mPT and PTP Opening in a p53-Dependent Manner.
A–C. p53 deficiency renders cells resistant to H2O2-induced and CsA-dependent loss of mitochondrial membrane potential (ΔΨm). WT and p53 −/− MEFs were treated with 0.4 mM H2O2 for 8 hrs ± 2 μM CsA and stained with the ΔΨm-sensitive dye TMRM (Tetramethyl Rhodamine Methyl Ester). A. TMRM assessed by fluorescence microscopy. Protonophore FCCP as control. B. Mean TMRM fluorescence of 3 independent experiments ± SD measured by AxioVision Automatic Quantitation. C. TMRM fluorescence of MEFs treated as in A, measured by FACS.
D, E. p53 deficiency renders primary MEFs resistant to PTP opening. WT and p53 −/− MEFs were treated with 0.4 mM H2O2 for 8 hrs ± 2 μM CsA. Calcein release assay. D. Representative images by fluorescence microscopy. E. Mean Calcein fluorescence from 3 independent experiments ± SD, measured by FACS. Ionophore Ionomycin as control.
See also Figure S4.
Oxidative Stress Induces Necrosis that Concomitantly Depends on p53 and CypD
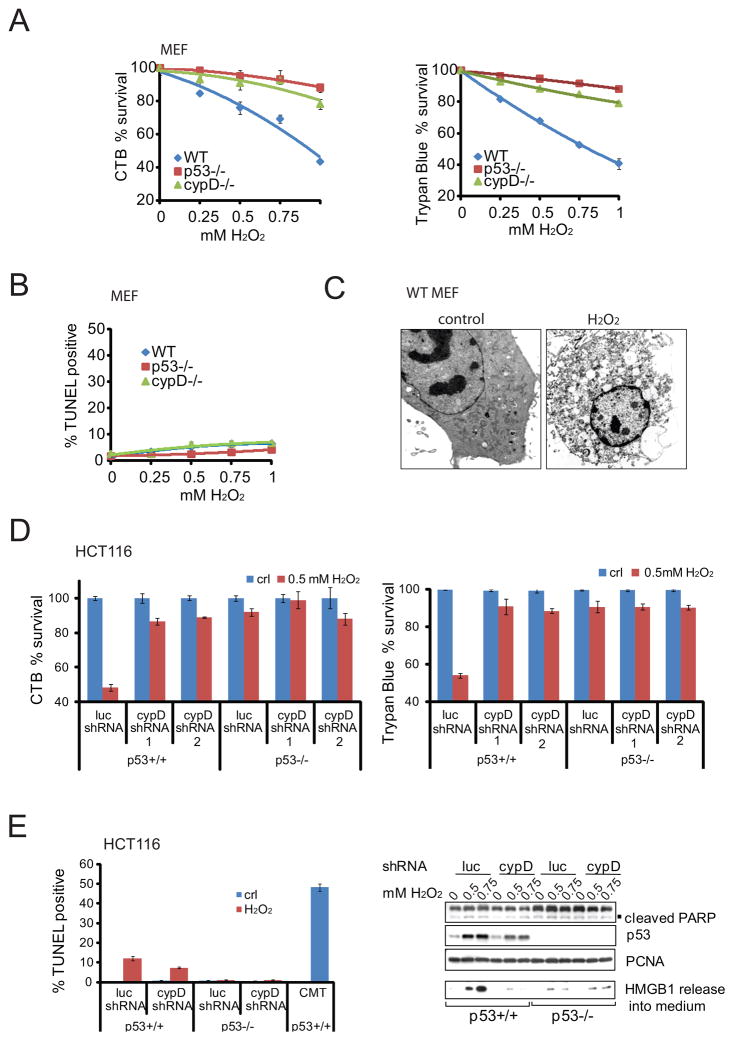
Both necrosis and apoptosis are oxidative stress-induced cell death modes (Javadov and Karmazyn, 2007). Upon oxidative stress, CypD primarily mediates necrotic death via PTP and mPT (Baines et al., 2005; Nakagawa et al., 2005), and evidence exists that CypD can actively suppress apoptosis (Eliseev et al., 2009; Li et al., 2004; Machida et al., 2006). On the other hand, while p53 protein is a well-established apoptosis regulator, almost nothing is known if and when p53 participates in regulating necrotic cell death. Importantly, primary p53-deficient MEFs were equally well protected from H2O2-induced cell death as CypD-deficient MEFs, while 60% of WT MEFs died, as indicated by cell viability assays measuring metabolic activity and membrane integrity (Figure 5A). To determine the nature of this cell death we first measured apoptosis. Notably, only negligible TUNEL positivity was detected in all genotypes and H2O2 conditions used (Figure 5B). Thus, upon oxidative stress the protection of primary MEFs bestowed by loss of p53 and CypD, and conversely the death of WT MEFs, is largely unrelated to apoptosis. Instead, electron microscopy of H2O2-treated WT MEFs revealed all signatures of necrosis, such as loss of plasma membrane integrity, organelle swelling, massive intracellular vacuoles and lack of nuclear fragmentation (Figure 5C and Figure S5A). The same holds true for cancer cells, as shown by the HCT116 p53+/+ and p53 −/− colorectal carcinoma pair (Figure 5D). Lack of p53 or silencing of CypD by shRNA (see Figure S5B) caused significant resistance to H2O2 -induced cell death, while 50% of WT cells were killed. On the other hand, a similar rescue effect was obtained by eliminating/silencing p53 alone or together with CypD, i.e. p53 was epistatic to CypD, further supporting that they act on the same biochemical pathway. Again, apoptosis levels in HCT116 p53+/+ cells were very low (10%), confirmed by the complete absence of cleaved PARP induction (Figure 5E), and did not account for the observed 50% of cell death (Figure 5D). Instead, H2O2-treated cells released large quantities of High Mobility Group Box 1 (HMGB1) protein into the culture medium in a dose-dependent, p53-dependent and CypD-dependent manner (Figure 5E right). Released HMGB1 is the classical biochemical hallmark specific for necrosis. In contrast, In apoptotic cells HMGB1 binds irreversibly to the condensed chromatin in the nucleus (Bianchi and Manfredi, 2004). While stabilization of p53 alone was sufficient to induce p53 accumulation at mitochondria, it was not sufficient to induce PTP opening in the absence of oxidative stress (Figure S5C). In sum, this data indicated that the predominant mode of cell death in response to H2O2 was p53/CypD-mediated necrotic cell death. Moreover, in addition to Camptothecin-induced apoptosis (Figure 5E left), Camptothecin also triggered some necrosis, as indicated by HMGB1 release that was p53- and CypD-dependent (Figure S5D). This is consistent with the observed Camptothecin-induced p53-CypD complex (Figure 2C).
Figure 5. Oxidative Stress Induces Necrosis that Concomitantly Depends on p53 and CypD.
A. p53 −/− and CypD −/− cells are resistant to H2O2-induced cell death. Primary MEFs of the indicated genotype were treated with H2O2 for 24 hrs. Cell death was measured by CTB viability (left) or trypan blue exclusion assays (right).
B. H2O2 does not induce significant apoptosis. Primary MEFs were treated with H2O2 for 24 hrs as in (A). Apoptosis was assessed by TUNEL assays.
C. WT MEFs were treated with 0.5 mM H2O2 for 24 hrs and the morphological hallmarks of necrotic cell death confirmed by electron microscopy.
D. p53 deficiency and silencing of CypD protects cells from H2O2-induced necrosis. Cell death of HCT116 p53 +/+ versus HCT116 p53 −/− cells treated with H2O2 for 24 hrs in the presence of control shRNA (luc) or two independent cypD shRNAs. CTB viability (left) and trypan blue exclusion assays (right).
E. Absence of significant apoptosis in cells from (D), as measured by TUNEL (left) and absence of PARP cleavage (right). In contrast, H2O2-treated cells release large quantities of necrosis indicator HMGB1 into the medium in a dose-, p53- and CypD-dependent manner. Immunoblot. B–E, Data are mean ± SD.
See also Figure S5.
Direct Targeting of p53 to the Mitochondrial Matrix Induces mPT and Necrosis in a CypD-Dependent Manner. Oxidative Stress-Induced PTP Opening and Necrosis is Largely Transcription-Independent
Although the above data strongly suggest a direct necrosis-regulating action of p53 at the mitochondria, oxidative stress also induces p53 target genes, some of which might contribute to regulate the observed H2O2 response in a CypD-dependent manner. For example, proteins participating in pro-or anti-oxidation that modulate cellular ROS levels are transcriptional p53 targets (Hussain et al., 2004; Macip et al., 2003; Sablina et al., 2005). Thus, direct proof was necessary to substantiate the local action of mitochondrial p53, as opposed to its nuclear action.
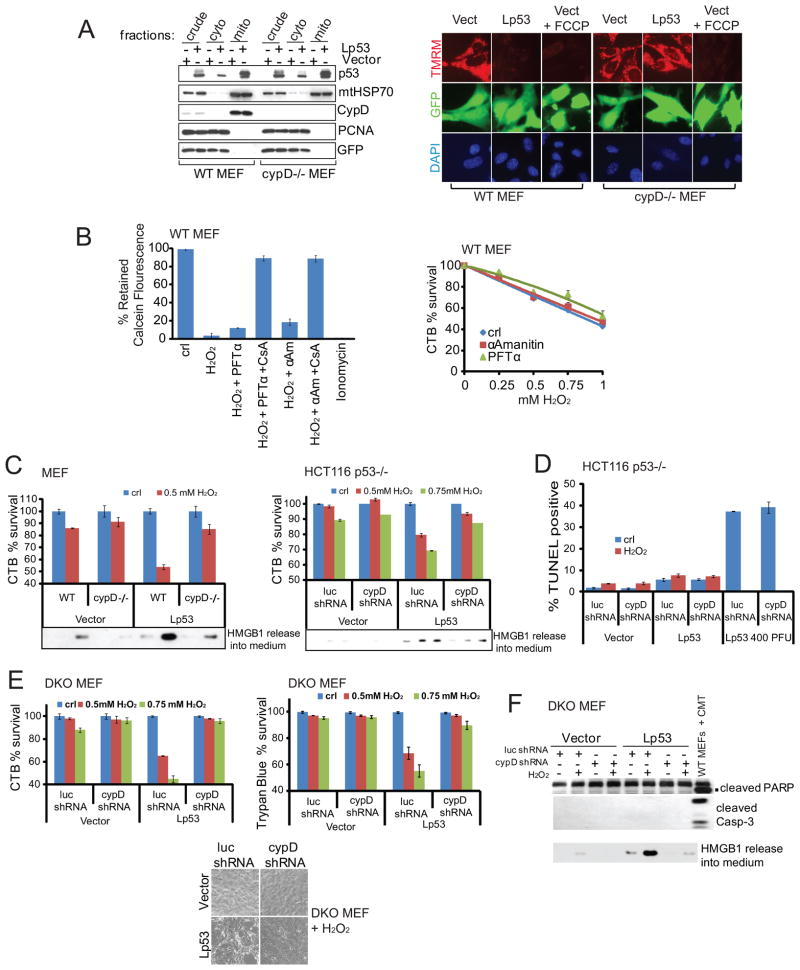
To this end we first used direct targeting of p53 to the mitochondrial matrix. We had previously generated various mitochondrially targeted versions of wild-type p53 (Mihara et al., 2003). One of them, called Lp53 (wt p53 fused to the mitochondrial import leader sequence of ornithine transcarbamylase) delivers p53 specifically and exclusively to the mitochondrial matrix, where the leader sequence gets cleaved off (Mihara et al., 2003) (Figure 6A left and Figure S6A). Importantly, Lp53 completely bypasses the nucleus and is devoid of any transcriptional activities (Mihara et al., 2003). Indeed, adenoviral delivery of Lp53 into the mitochondrial matrix caused a major loss of ΔΨm in WT MEFs, indicated by loss of TMRM fluorescence. In contrast, Lwtp53 had no effect in CypD−/− MEFs (Figure 6A right). This indicates that p53 in the mitochondrial matrix can regulate PTP opening in cells independent of p53 transcription. This direct p53 action requires CypD. Moreover, PFTα, an inhibitor of p53-mediated transcription, or α-Amanitin, a powerful general transcriptional inhibitor of RNA Polymerase II, did not significantly prevent oxidative stress-induced PTP opening or cell death in WT MEFs (Figure 6B and Figure S6B–S6C), confirming the largely transcription-independent effect of p53 in this context. Moreover, at early time points (e.g. 12 hrs) when H2O2 had not yet killed many cells, Lp53 sensitized WT MEFs and HCT116 p53−/− cells in a CypD-dependent manner (Figure 6C and Figures S6D–S6F). Again, robust p53- and CypD-dependent HMGB1 release into the medium (Figure 6C) and lack of significant apoptosis (Figure 6D) confirmed the primarily necrotic nature of the cell death.
Figure 6. Direct Targeting of p53 to Mitochondrial Matrix Induces mPT and Necrosis in a CypD-Dependent Manner. Oxidative Stress-Induced PTP Opening and Necrosis is Largely Transcription-Independent.
A. Mitochondrial matrix-targeted p53 (Lp53) causes collapse of ΔΨm in WT but not in CypD −/− MEFs. WT and CypD −/− MEFs were infected with Ad5-GFP (vect) or Ad5-Lp53-GFP that targets p53 exclusively to the mitochondrial matrix. Left, mitochondrial fractions from these cells show localization of Lp53 at mitochondria independent of CypD. Right, 24 hrs after infection TMRM was added to the medium to visualize loss of ΔΨm.
B. Transcriptional blockade does not prevent H2O2-induced PTP opening and necrosis. Left, Loss of mitochondrial calcein indicates PTP opening after treatment of WT MEFs with 0.5 mM H2O2 for 8 hrs in the presence of α-Amanitin or PFTα ± CsA. Right, survival of WT MEFs treated with H2O2 for 24 hrs. CTB viability assay.
C, D. Mitochondrial matrix-targeted p53 (Lp53) sensitizes cells to H2O2-induced CypD-dependent necrosis. C. Survival and HMGB1 release of WT or CypD −/− MEFs (left) or HCT116 p53 −/− cells stably expressing luc shRNA or cypD shRNA (right) infected with vector or Lp53 (100 PFU each) after H2O2 treatment for 12 hrs. CTB assay. At this early time point, H2O2 had not yet killed many cells. D. Only minimal apoptosis in HCT116 p53 −/− cells from C. Positive control, infection with 400 PFU Lp53 for 48 hrs. TUNEL assay.
E, F. As shown by early time points, Lp53 sensitizes Bax/Bak DKO MEFs to H2O2 in a CypD-dependent manner. E. Bax/Bak DKO MEFs stably expressing luc shRNA or cypD shRNA were infected with adenoviral Lp53 or vector and treated with H2O2 for 12 hrs. Cell viability measured as above. Bottom, bright field image of cells from E. B–E, Data are mean ± SD.
F. Strong release of cellular HMGB1 into the medium depends on the presence of both Lp53 and H2O2. This effect is reversed by downregulation of CypD. PARP and caspase-3 cleavage is absent. Bax/Bak DKO MEFs treated for 12 hrs as in E. WT MEFs treated with Camptothecin serve as positive control for PARP and Caspase-3 cleavage. Immunoblot.
See also Figure S6.
To further support the role of mitochondrial p53 in oxidative stress-induced necrotic death, we used Bax/Bak DKO MEFs. Adenovirally delivered Lp53 in Bax/Bak DKO MEFs localized to mitochondria (Figure S6G) and importantly, bound to endogenous CypD (Figure S6H). As already seen for WT MEFs (Figure 6C), Lp53 greatly sensitized DKO cells to oxidative stress at early time points, and this sensitivity was abolished upon CypD knockdown (Figure 6E and Figure S6I). Cell death was again due to necrosis and not apoptosis, as indicated by HMGB1 release that depended on the presence of CypD, as well as the absence of Casp3 and PARP cleavage (Figures 6F and Figure S6J). In sum, the physical interaction between mitochondrial p53 and CypD is critical to induce necrotic cell death upon oxidative stress. This p53 action is independent of Bax and Bak.
Pathologic p53-CypD Complex Formation During Ischemic Stroke-Induced Brain Tissue Necrosis
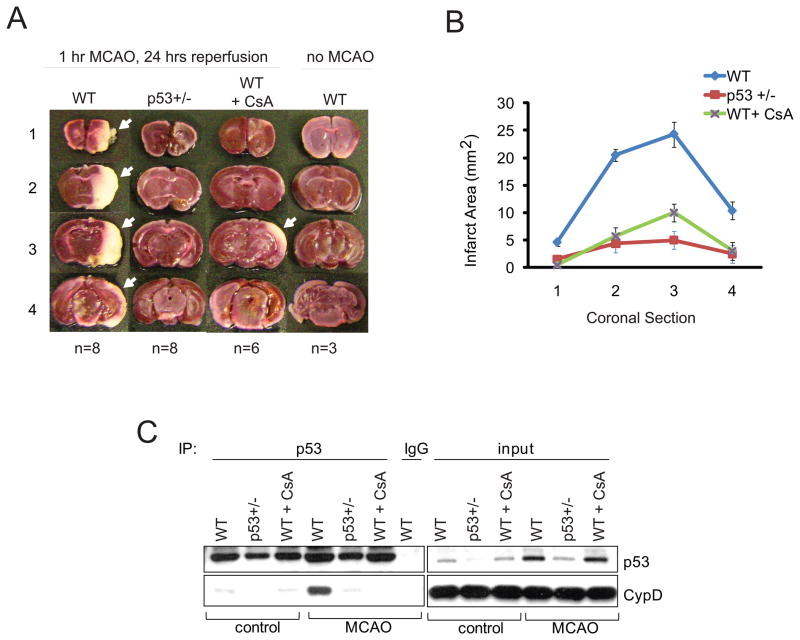
In vivo, the parameters required for induction of mPT are fulfilled during stroke (transient ischemia - reperfusion injury of the brain). During brain ischemia, the absence of glucose and oxygen causes mitochondrial dysfunction, intracellular calcium uptake and a sharp decrease in ATP levels. Subsequent brain reperfusion generates the conditions required for mPT as mitochondria repolarize, sequester excess cytosolic calcium accumulated during the ischemic period, and generate ROS and oxidative stress. Cells (mainly neurons but also glia) die largely by necrosis, although in the peripheral penumbra apoptosis also takes place. Importantly, CypD deficiency confers significant protection against ischemic brain injury in permanent and transient ischemic stroke models in mice (Schinzel et al., 2005).
Since our cell culture data identified a physical and functional interaction between mitochondrial p53 and CypD that is critical for oxidative stress-induced necrosis, we investigated the pathophysiologic role of the PTP-regulating function of p53 in transient ischemia/reperfusion stroke. To this end, WT, p53+/− and WT control mice pre-treated with CsA were subjected to carefully controlled unilateral acute middle cerebral artery occlusion (MCAO) for 1 hr, followed by 24 hrs of reperfusion. Infarct size was measured by staining coronal brain slices with triphenyl-tetrazolium chloride (TTC), a dye that is oxidized by intact mitochondrial dehydrogenase to yield the red product formazan. In the infarcted area (white), mitochondria are uncoupled and dysfunctional and no longer stain with TTC. WT brains (n=8) were grossly infarcted. Intriguingly, p53 +/− brains (n=8) were strongly protected from stroke, similar to WT control mice protected by intraperitoneal injection of CsA 15 min prior to MCAO (n=6) (Figures 7A, 7B). Most importantly, a pathologic p53-CypD complex was robustly induced in infarcted WT brains after MCAO, as shown by co-immunoprecipitation with a specific p53 antibody but not with IgG (Figure 7C, ‘MCAO’). Intriguingly, the induced p53-CypD complex was undetectable in CsA-pretreated, stroke-protected WT control mice. Moreover, the complex was only barely detectable in p53+/− mice, whose stroke stress-mediated p53 induction did not exceed the baseline p53 levels of WT control mice, and which were also strongly protected from infarction (Figure 7C). Thus, a strong association exists between the formation of the pathologic p53-cypD complex and ischemia-reperfusion brain infarction. These data support the idea that this novel p53-CypD axis is an important pathophysiologic contributor to ischemic stroke.
Figure 7. The p53-CypD Complex has a Pathophysiologic Role in Ischemic Stroke.
A–C. Ischemia/reperfusion-mediated brain injury. Littermates (6–8 weeks old males) of WT, heterozygous p53+/− and WT control mice pre-treated with CsA (15 mg/kg i.p, 15 min prior to MCAO) were subjected to middle cerebral artery occlusion (MCAO, right side) for 1 hr followed by reperfusion for 24 hrs under cerebral blood flow and temperature-controlled conditions. Control mice (‘no MCAO’) underwent all steps of the surgery without blockade of the MCA. A. Representative TTC-stained coronal brain sections; white indicates infarcted tissue. The number of mice is indicated. B. Mean infarct size ± SD. In contrast to infarcted WT brains (n=8), p53 +/− brains (n=8) are strongly stroke-protected and phenocopy the brain protection seen in cyclosporine A-pretreated WT mice (n=6).
C. In both cases, stroke protection directly correlates with the absence of an ischemia-induced p53-CypD complex in brain tissue. Co-immunoprecipitation with p53 antibody or non-specific IgG from the 25 hrs necrotic (‘MCAO’) or corresponding contralateral control hemispheres from representative brains treated as in A. immunoblotting for CypD. Input shown.
See also Figure S7.
Discussion
Here we identify a new role of p53 in activating necrotic cell death. In response to oxidative stress and ischemia, p53 protein accumulates in the mitochondrial matrix and triggers PTP opening, mPT and necrosis by physical interaction with the critical PTP regulator CypD. This p53 action is transcription-independent and inhibited by the specific CypD inhibitor CsA, and by genetic CypD deletion or knockdown. In support, in contrast to the prototypical MOMP-inducer tBid, purified p53 protein has efficacy that goes beyond activating the Bax/Bak lipid pore at the outer membrane. p53 is able to also rapidly open the PTP pore at the inner membrane in healthy Bax−/−Bak−/− mitochondria where tBid fails. Similarly, p53 causes structural alterations of the PTP pore, while tBid does not. Using gene knockout and knockdown in mouse and human cells, we show that oxidative stress induces PTP opening, mPT and necrosis that concomitantly depend on the presence of p53 and CypD. Importantly, WT, Bax/Bak DKO and p53−/− cells are all (further) sensitized to H2O2-induced PTP opening and necrotic death upon direct delivery of p53 into the mitochondrial matrix. This sensitivity is reversed by knockdown of CypD, confirming the physiological p53-CypD cross-talk. Most intriguingly, a robust p53-CypD complex is formed during necrosis in brain tissue upon ischemia/reperfusion stroke injury. In contrast, genetic reduction of p53 levels (p53+/− mice) or CsA-pretreatment of WT mice prevents this complex from forming, and is associated with effective stroke protection. This strongly supports a pathophysiologic role of the mitochondrial p53-CypD axis in ischemic stroke.
Aside from p53, very few extramitochondrial proteins are known to bind CypD and trigger pathologic PTP opening. A compelling analogy to p53, albeit acting chronically, is the highly toxic, soluble intracellular form of β amyloid peptide (Aβ), a key protein in Alzheimer’s disease (AD) that progressively accumulates in brain mitochondria of AD patients and AD transgenic mice. In AD, Aβ forms a critical complex with CypD that promotes PTP opening, thereby boosting Ca2+-induced mitochondrial damage and neuronal injury. Inhibiting PTP or ablating CypD in AD mice renders their cortical brain mitochondria resistant to PTP opening and improves their cognitive functions (Du et al., 2008).
In response to many insults, a continuum of apoptosis and necrosis exists. Such insults induce apoptosis at lower doses and necrosis at higher doses, a scenario that is clearly the case in ischemic brain infarction with an apoptotic penumbra surrounding a necrotic center (Zong and Thompson, 2006). The long-standing paradigm had been that p53 controls apoptosis but plays no role in necrosis. Only recently the very first link between p53 and necrosis was reported. Upon etoposide-mediated DNA damage Bax/Bak DKO MEFs die via slow necrosis, which is largely controlled by p53-mediated transcription of cathepsin Q in cooperation with DNA damage-induced ROS (Tu et al., 2009). Interestingly, however, this transcriptional p53-cathepsin Q axis only plays a minor role in the corresponding WT MEF necrosis. In contrast, H2O2-induced necrosis in DKO MEFs was found to be completely transcription-independent (Tu et al., 2009), supporting our finding of direct CypD-mediated necrotic signaling by p53. Thus, in response to oxidative stress our data establish a necrotic mitochondrial p53 program, in addition to the well-characterized mitochondrion-based apoptotic p53 program. The decision whether to engage one and/or the other appears to depend on the type and dose of stress. Clearly though, necrotic signaling also uses p53-independent pathways, depending on the insult. Examples are necrosis induced by DNA alkylating agents which signals through PARP and necrosis signaling via the TNFR/RIP1 pathway (Zong and Thompson, 2006).
In oxidative damage, our data shows that p53 can control a CypD-mediated necrotic program in various cell types in vitro and in brain tissue in vivo. The surprisingly strong brain protection of p53+/− mice from ischemia/reperfusion injury (Figures 7A, B) is likely due to two components: The absence of a destructive p53-CypD complex, thereby protecting the PTP pore (Figure 7C), and the presence of an only weak p53 apoptosis program such as e.g. Puma and Noxa (Figure S7A). Importantly though, the absence of the p53-CypD complex appears to be the dominant factor in stroke protection. This is indicated by CsA-protected WT brains that do not form the p53-CypD complex, yet concomitantly mount a full-blown p53 apoptosis program including Puma and Noxa (Figure S7A). Conversely, p53−/− MEFs but not PUMA−/− MEFs are protected from oxidative stress-induced cell death, confirming that Puma does not play a significant role in this process (Figure S7B). Parenthetically, the p53 homolog p73, which is robustly expressed in adult mouse and human brain, was not induced in the ischemic brain tissues (Figure S7C). Finally, although low or uninduced levels of p53 can clearly mount a transcriptional anti-oxidant defense by regulating expression of e.g. sestrins, glutathione peroxidase and TIGAR, this is highly tissue-specific. Notably, in brain tissue p53 is constitutively pro-oxidant in both physiologic and pathologic conditions due to transcriptional repression of antioxidant genes (Chatoo et al., 2009). Thus, a protective p53 anti-oxidant program is likely not a factor in the observed p53+/− stroke protection. Interestingly, for reasons that are currently not clear, p53−/− brains were not as protected as p53+/− brains (not shown), consistent with a previous report (Crumrine et al., 1994).
CypD−/− mice show strong protection against focal ischemia-reperfusion brain injury (Schinzel et al., 2005). Similarly, mice and rats treated with CsA or non-immunosuppressive derivatives that do not bind to calcineurin are also protected from stroke (Khaspekov et al., 1999; Korde et al., 2007; Matsumoto et al., 1999). Clinically, CsA is chronically given to organ transplant patients as a time-tested immunosuppressant. Our data suggest that acute temporary blockade of the destructive p53-CypD complex by CsA-type inhibitors may be a therapeutic strategy to limit infarct extent in the rising number of ischemic stroke patients where reperfusion of the occluded artery can be reestablished by interventional thrombolysis.
Experimental Procedures
Cell culture
Primary WT and p53−/− mouse embryo fibroblasts MEF) were established from E13.5 embryos. Primary CypD−/− MEFs were purchased (ArtisOptimus). Bax/Bak DKO and corresponding WT MEFs were a gift of Dr. Weixing Zong, Stony Brook Univ. MEFs (all SV40 immortalized) and cancer cell lines were maintained in DMEM/10% FBS. Cyclosporine A, αAmanitin, Pifithrin-α, ionomycin and FCCP were purchased from Sigma.
Plasmids and recombinant proteins
pGEX-CypD were a gift from Dr. Dario Altieri, Univ. of Massachusetts (Kang et al., 2007). Human cypD, ANT-2 and VDAC-1 cDNAs in pHA vectors were gifts from Dr. Stefan Grimm, Max-Planck-Institute, Germany (Bauer et al., 1999). Immunoaffinity purified recombinant human p53 expressed in human cells was purchased (Origene). Recombinant adenovirus (Ad5-GFP) was generated as described previously (Palacios et al., 2008) under the control of a CMV promoter. Luc shRNA and CypD shRNA plasmids were purchased (Dharmacon).
Mitochondrial isolation
Mitochondria from cultured cells and mouse liver were isolated by sucrose gradients as we described (Mihara et al., 2003). Mitochondria (35 μg) were subjected to chemical crosslinking with BMH (1,6-bis-maleimidohexane, Pierce, 10 mM in DMSO for 10 min) or mock-treated (DMSO only). Submitochondrial fractionation was performed by phosphate swelling-shrinking as described (Kang et al., 2007) and detailed in supplemental material. For mitochondrial assays see also supplemental material.
Biochemical assays
For immunoprecipitation, isolated mitochondria or cells were lysed in PBS containing 1% Triton X-100 and protease inhibitors (Roche). After centrifugation at 13,000x g for 10 min at 4°C, the supernatant was precleared with Protein G-agarose beads (Roche) for 2h at 4°C, and 500 μg of precleared protein extracts were incubated with antibody to p53 or cypD for 16 h at 4°C. Precipitated complexes were washed in lysis buffer and bound proteins analyzed by immunoblots. For GST-pull down experiments, bead -bound GST-CypD was blocked with H-buffer (20 mM Hepes pH 7.7, 75 mM KCl, 0.1 mM EDTA, 2.5 mM MgCl2, 0.05% NP40, 1 mM DTT, 1 mg/ml BSA). Blocked beads were incubated with cell/mitochondria lysates or recombinant proteins for 16 h at 4°C in the presence of 5 μM CsA or vehicle. Pelleted beads were washed in H-buffer and bound proteins analyzed by immunoblots. For HA-pull down assays, HA-antibody (Abcam) was used to precipitate protein complexes from cells expressing HA-tagged CypD, VDAC or ANT.
Cell death assays
Cell viability was assessed by Cell Titer Blue (CTB) and Trypan Blue Exclusion assays (Sigma). Apoptosis was measured by TUNEL (Kit TMR Red, Roche). Nuclei were counterstained with Hoechst 33342. Apoptosis was quantified as the proportion of red/blue areas from 3 representative images (Zeiss Axioskope with AxioVision v4.3).
HMGB1 release assay
Medium from treated cells was harvested, spun at 800x g for 5 min and supernatant filtered (0.45 μm). Proteins were precipitated with trichloroacetic acid and analyzed by HMGB1 immunoblots.
Transient Focal Cerebral Ischemia (MCAO)
Male 6–8 week old littermate WT and p53+/−(Sv129) were used for all experiments. Focal cerebral ischemia was induced in anesthetized mice by inserting an intraluminal filament in the middle cerebral artery for 60 min, followed by 24 hrs of reperfusion. During the course of the experiment blood flow and body temperature of the animals were monitored by an experienced investigator (KJ) who was naive to the genetic identity of individual mice. Mock injured animals were kept under the same conditions. Where indicated, Cyclosporine A (15 mg/kg) was administered 15 mins prior to MCAO by intraperitoneal injection. All animal work was done in accordance with Stony Brook University IACUC.
Supplementary Material
Highlights.
Necrosis in oxidative stress and ischemia depends on mitochondrial PTP pore opening
PTP opening is triggered by p53 interaction with CypD in the mitochondrial matrix
A p53-CypD complex is formed in necrotic brain tissue in a stroke model in mice
Blocking formation of the p53-CypD complex is associated with stroke protection
Acknowledgments
We thank Susan Vanhorn and Ioana Russ for technical assistance, and Franz X. Schmid and Philipp Schmidpeter for discussion. This work was funded by a grant from the National Cancer Institute (CA060664) to U.M.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bauer MK, Schubert A, Rocks O, Grimm S. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J Cell Biol. 1999;147:1493–1502. doi: 10.1083/jcb.147.7.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME, Manfredi A. Chromatin and cell death. Biochim Biophys Acta. 2004;1677:181–186. doi: 10.1016/j.bbaexp.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Bonini P, Cicconi S, Cardinale A, Vitale C, Serafino AL, Ciotti MT, Marlier LN. Oxidative stress induces p53-mediated apoptosis in glia: p53 transcription-independent way to die. J Neurosci Res. 2004;75:83–95. doi: 10.1002/jnr.10822. [DOI] [PubMed] [Google Scholar]
- Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Kenzelmann Broz D, Basak S, Park EJ, McLaughlin ME, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–583. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatoo W, Abdouh M, David J, Champagne MP, Ferreira J, Rodier F, Bernier G. The polycomb group gene Bmi1 regulates antioxidant defenses in neurons by repressing p53 pro-oxidant activity. J Neurosci. 2009;29:529–542. doi: 10.1523/JNEUROSCI.5303-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab. 1994;14:887–891. doi: 10.1038/jcbfm.1994.119. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem. 2009;284:9692–9699. doi: 10.1074/jbc.M808750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol. 2010;2:a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Heyne K, Schmitt K, Mueller D, Armbruester V, Mestres P, Roemer K. Resistance of mitochondrial p53 to dominant inhibition. Mol Cancer. 2008;7:54. doi: 10.1186/1476-4598-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, Ichimiya M, Sengupta S, Mechanic L, Okamura S, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20:1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Khaspekov L, Friberg H, Halestrap A, Viktorov I, Wieloch T. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur J Neurosci. 1999;11:3194–3198. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Korde AS, Pettigrew LC, Craddock SD, Pocernich CB, Waldmeier PC, Maragos WF. Protective effects of NIM811 in transient focal cerebral ischemia suggest involvement of the mitochondrial permeability transition. J Neurotrauma. 2007;24:895–908. doi: 10.1089/neu.2006.0122. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Johnson N, Capano M, Edwards M, Crompton M. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochem J. 2004;383:101–109. doi: 10.1042/BJ20040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K, Ohta Y, Osada H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J Biol Chem. 2006;281:14314–14320. doi: 10.1074/jbc.M513297200. [DOI] [PubMed] [Google Scholar]
- Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1999;19:736–741. doi: 10.1097/00004647-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Palacios G, Crawford HC, Vaseva A, Moll UM. Mitochondrially targeted wild-type p53 induces apoptosis in a solid human tumor xenograft model. Cell Cycle. 2008;7 doi: 10.4161/cc.7.16.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V, Miotto G, Canton M, Colonna R, Bernardi P, Di Lisa F. Imaging the mitochondrial permeability transition pore in intact cells. Biofactors. 1998;8:263–272. doi: 10.1002/biof.5520080314. [DOI] [PubMed] [Google Scholar]
- Pietsch EC, Leu JI, Frank A, Dumont P, George DL, Murphy ME. The tetramerization domain of p53 is required for efficient BAK oligomerization. Cancer Biol Ther. 2007;6:1576–1583. doi: 10.4161/cbt.6.10.4719. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansome C, Zaika A, Marchenko ND, Moll UM. Hypoxia death stimulus induces translocation of p53 protein to mitochondria. Detection by immunofluorescence on whole cells. FEBS Lett. 2001;488:110–115. doi: 10.1016/s0014-5793(00)02368-1. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HC, Ren D, Wang GX, Chen DY, Westergard TD, Kim H, Sasagawa S, Hsieh JJ, Cheng EH. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc Natl Acad Sci U S A. 2009;106:1093–1098. doi: 10.1073/pnas.0808173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Wolff S, Erster S, Palacios G, Moll UM. p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008;18:733–744. doi: 10.1038/cr.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.