Abstract
Neurons in layer 4 of the primary auditory cortex receive convergent glutamatergic inputs from thalamic and cortical projections that activate different groups of postsynaptic glutamate receptors. Of particular interest in layer 4 neurons are the Group II metabotropic glutamate receptors (mGluRs), which hyperpolarize neurons postsynaptically via the downstream opening of GIRK channels. This pronounced effect on membrane conductance could influence the neuronal processing of synaptic inputs, such as those from the thalamus, essentially modulating information flow through the thalamocortical pathway. To examine how Group II mGluRs affect thalamocortical transmission, we used an in vitro slice preparation of the auditory thalamocortical pathways in the mouse to examine synaptic transmission under conditions where Group II mGluRs were activated. We found that both pre- and post-synaptic Group II mGluRs are involved in the attenuation of thalamocortical EPSP/Cs. Thus, thalamocortical synaptic transmission is suppressed via the presynaptic reduction of thalamocortical neurotransmitter release and the postsynaptic inhibition of the layer 4 thalamorecipient neurons. This could enable the thalamocortical pathway to autoregulate transmission, via either a gating or gain control mechanism, or both.
1. Introduction
Auditory information ascending from the cochlea is transmitted through several brainstem and midbrain stations before eventually reaching neurons in layer 4 of the primary auditory cortex via projections from the ventral division of the medial geniculate body (Lee and Winer, 2008; Webster, 1992). These thalamocortical synapses reliably convey auditory information to the cortex by activating postsynaptic ionotropic glutamate receptors (iGluRs) (Rose and Metherate, 2005), which result in large excitatory postsynaptic potentials (EPSPs) that depress in response to repetitive stimulation (Lee and Sherman, 2008). These synaptic properties are analogous to those found at other information-bearing synapses in the auditory system (Bartlett and Smith, 2002; Covic and Sherman, 2011; Lee and Sherman, 2010), as well as in other sensory modalities (Reichova and Sherman, 2004; Sherman and Guillery, 1998; Sherman and Guillery, 2006)
In addition to activation by iGluRs, layer 4 neurons also express different groups of metabotropic glutamate receptors (mGluRs) that are activated postsynaptically by intracortical and corticocortical projections (Covic and Sherman, 2011; Lee and Sherman, 2009a; Lee and Sherman, 2009b). In particular, we have recently demonstrated that neurons in layer 4 of the primary auditory cortex, as well as other sensory cortices, can be inhibited postsynaptically through the activation of Group II mGluRs, which results in the prolonged hyperpolarization of the membrane potential mediated by G-protein coupled inwardly rectifying potassium (GIRK) channels (Lee and Sherman, 2009a). Such relatively long time-course changes to membrane excitability elicited by Group II mGluR activation likely modify membrane conductance to attenuate thalamocortical EPSPs, although such effects have not yet been demonstrated.
Interestingly, our previous finding of postsynaptic Group II mGluRs in layer 4 neurons is somewhat novel; as such a postsynaptic localization has only been found in a few other nervous structures (Cox and Sherman, 1999; Dutar et al., 2000; Sekizawa et al., 2009). In fact, presynaptically localized Group II mGluRs are more common, and may be present on auditory thalamocortical terminals (Cartmell and Schoepp, 2000; Mateo and Porter, 2007; Ngomba et al., 2011; Petralia et al., 1996), potentially acting as autoreceptors to reduce the efficacy of thalamocortical synaptic transmission during periods of high activity. Alternatively, presynaptic Group II mGluRs could be activated by nearby synapses, such as those arising from intracortical or corticocortical sources (Covic and Sherman, 2011; Thomson, 2010) that affect thalamocortical synaptic release probability directly. Thus, potential pre- and post-synaptic mechanisms exist by which activation of Group II mGluRs might attenuate thalamocortical transmission.
Consequently, we investigated whether the activation of Group II mGluRs in layer 4 could modify the efficacy of thalamocortical synaptic transmission using in vitro slice preparations of the thalamocortical pathways in the mouse (Agmon and Connors, 1991; Cruikshank et al., 2002). In this study, we combined electrical stimulation of the thalamocortical inputs to layer 4 with activation of Group II mGluRs, either pharmacologically or via photostimulation of intracortical projections originating in layer 6. Our results demonstrate that the activation of Group II mGluRs reduces thalamocortical synaptic transmission via both pre- and post-synaptic mechanisms.
2. Materials and Methods
2.1. Slice preparation and recording
BALB/c mice (ages 11–18d) were used in these experiments and the Institutional Animal Care and Use Committee approved all procedures. After deeply anesthetizing with isofluorane followed by decapitation, the brains were submerged in cool, oxygenated, artificial cerebral spinal fluid (ACSF: 125 mM NaCl, 25 mM HCO3, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, and 25 mM glucose). Whole brains were then blocked to preserve the thalamocortical pathway from the ventral division of the medial geniculate body (MGBv) to the primary auditory cortex (AI) (Cruikshank et al., 2002), or from the ventrobasal complex (VB) to the primary somatosensory cortex (SI)(Agmon and Connors, 1991). The blocked surface was then affixed to a vibratome stage, 500 µm thick sections collected and quickly transferred to a holding chamber containing physiological ACSF to recover for 1 h at 32°C, then returned to room temperature for the remainder of the experiment.
A recording chamber perfused with ACSF was used for whole cell recordings of the slice preparation under DIC optics using recording pipettes with tip resistances of 4–8MΩ filled with intracellular solution (135mM KGluconate, 7mM NaCl, 10mM HEPES, 1–2mM Na2ATP, 0.3mM GTP, 2mM MgCl2 at a pH of 7.3 obtained with KOH and osmolality of 290 mosm obtained with distilled water). This solution results in ~10mV junction potentials that is uncorrected for in voltage measurements. Current or voltage clamp (c-SEVC) recordings were made using the Axoclamp 2A amplifier and pCLAMP software (Axon Instruments). Voltage errors were minimized with ~60–70% series resistance compensation. Cells with shifting resting membrane potential were discarded, as were cells with input resistance <100 MΩ and cells with access resistance >30 MΩ. The acquired data was digitized using a Digidata 1200 board and then stored in a computer for later analysis.
2.2. Pharmacology
Stock solutions of receptor agonists and antagonists were prepared in distilled water, 0.1M NaOH, or DMSO. The drugs are first prepared in a separate container to the reported concentrations and then injected to the input line at a separate port. The initial bath concentration is thus somewhat diluted relative to the container concentration, but equilibrates over time. In the case of the recirculating bath for caged glutamate, the bath volume dilutes the drug concentrations in the container by roughly one-fourth. Initial concentrations of agonists and antagonists (TOCRIS, Ellisville, MO) were as follows: APDC (100 µM) agonist for Group II mGluRs; SR 95531 (20 µM) antagonist for GABAARs; CGP 46381 (40 µM) antagonist for GABABRs: MK-801 (40 µM) antagonist for NMDARs; LY341495 (1 mM) antagonist for Group II mGluRs. In order to block G protein-coupled activity, GDPβS (100 µM) was added to the intracellular solution.
2.3. Stimulation
In order to identify the thalamic and intracortical layer 6 regions connected to layer 4, excitatory input maps were produced using laser-scanning photostimulation with nitroindolinylcaged glutamate (0.37 mM) (Sigma-RBI, St Louis, MO) (Lam and Sherman, 2010; Papageorgiou et al., 1999; Shepherd et al., 2003). Caged glutamate was photolysed with an average beam intensity of 5 mW to give a 1-ms, 100-pulse light stimulus by Q-switching the pulsed UV laser (355 nm wavelength, frequency-tripled Nd: YVO4, 100-kHz pulse repetition rate; DPSS Lasers, Inc., Santa Clara, CA). Responses were analyzed using custom software written in Matlab (MathWorks, Inc., Natick, MA), and the traces were superimposed on a photomicrograph corresponding to the stimulation sites. Bipolar concentric electrodes (Frederick Haer) were used to deliver electrical stimulation in the thalamus (MGB or VPm), consisting of pulses of 0.1–0.2ms (Gibson et al., 1999; Gil et al., 1999). Responses were considered monosynaptic if the latency jitter was less than 1 ms and had similar rise times between trials (Rose and Metherate, 2005). A paired pulse protocol was used to deliver pulses (0.1–0.2ms) separated by 10–100ms.
2.4. Histology
To reveal the distribution of mGluRs in relation to thalamocortical terminals, mice were transcardially perfused with 4% paraformaldehyde/50% saturated picric acid, cryoprotected overnight in 4% paraformaldehyde/30% sucrose and then frozen sectioned at 40 µm. The sections were pretreated for 30 min in 0.5% H2O2, rinsed in PBS, then incubated for 1 h in PBS containing 0.3% Triton X-100, followed by normal rabbit serum for 1 h (Vector Labs). Sections were then incubated at 4°C for 24 h in a 1:1000 dilution of primary antibody for VGluT2 (abcam 101760: lot GR33200-2) (Abcam Inc., Cambridge, MA), followed by 1 h with a 1:100 dilution of FITC-labeled secondary antibody solution (abcam 6737: lot 912388) in PBS. Sections were rinsed in PBS, then incubated in 0.15% normal mouse serum for 1h followed by 24 h incubation in a 1:1000 dilution of primary antibody solution for mGluR2 (abcam 15672: lot GR14718-1), followed by 1 h incubation in a 1:100 dilution of Texas Red-labeled secondary antibody (abcam 6787: lot GR9811-2). Confocal images were acquired with a 3I Marianas Yokogawa-type spinning disk confocal microscope at the Integrated Microscopy Core Facility of the University of Chicago.
3. Results
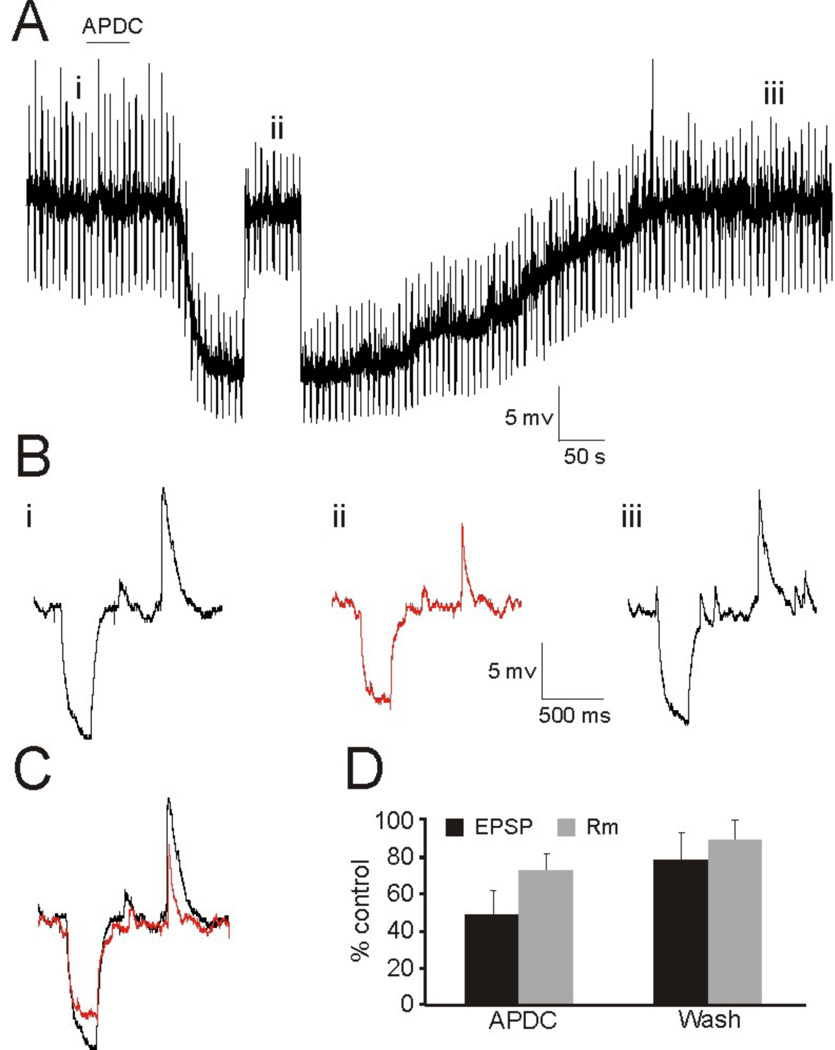
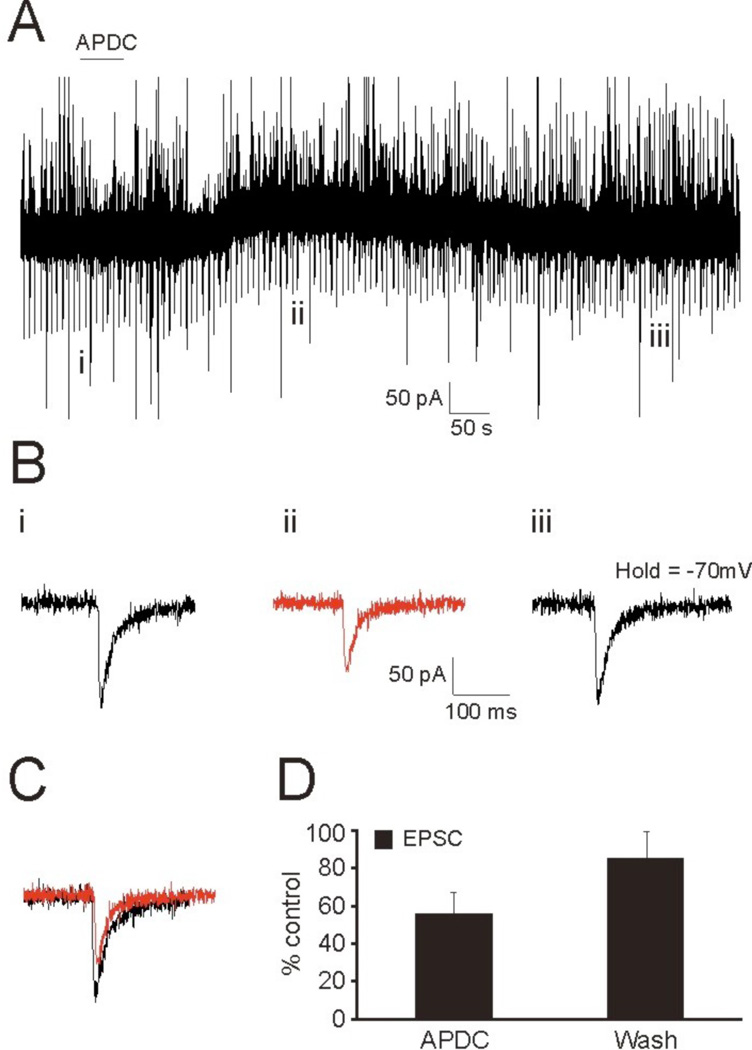
In order to assess whether the activation of Group II mGluRs affect the efficacy of auditory thalamocortical synaptic transmission, we initially employed a pharmacological approach. Using whole-cell patch clamp, we recorded from layer 4 neurons in the primary auditory cortex (A1) (n=13) and the primary somatosensory cortex (S1) (n=9) while bath applying the Group II mGluR agonist, APDC, to examine its effect on elicited thalamocortical EPSP/Cs. Recordings were performed in both current clamp (Fig. 1) and voltage clamp (Fig. 2) in response to APDC application and thalamic stimulation at regular intervals (5s ISI), and the elicited EPSP/Cs were compared before, during, and after application of the agonist (Fig. 1Bi–iii, 2Bi–iii). In current clamp, hyperpolarizing current injections preceded thalamic stimulation to test changes in input resistance during the experiment (Fig. 1, downward deflections). Using this approach, we found that the bath application of APDC resulted in a marked reduction of thalamocortical EPSP/Cs (Fig. 1C, 2C), which was recovered following a wash (Figs. 1Biii, 2Biii). However, the decrease in the amplitude of the elicited thalamocortical EPSPs (54 ± 16% control) could not be accounted for by the induced changes to input resistance (76 ± 10% control, p<0.05, t-test) (Fig. 1D). This suggested that the postsynaptic change to membrane conductance elicited by APDC could only account partially, if at all, for the attenuation of thalamocortical EPSP/Cs. This is supported by the voltage clamp data (Fig. 2), since the elicited thalamocortical EPSCs likely originate from proximal synapses (Richardson et al., 2009) that could be electrotonically controlled by the clamp (Spruston et al., 1993; Williams and Mitchell, 2008) and thus would not significantly reflect changes in input resistance.
Figure 1.
Pharmacological activation of Group II mGluRs via bath application of APDC results in attenuation of thalamocortical EPSPs. (A) Current clamp recording from a layer 4 RS neuron in response to APDC application (solid line) and thalamic stimulation. The membrane potential was briefly stepped up to the pre-agonist levels in order to compare changes in EPSP amplitude and input resistance. (B) Elicited EPSPs from the time points indicated in (A) before (i), during (ii), and after (iii) application of APDC. Hyperpolarizing current injections precede thalamic stimulation to test input resistance during the experiment. (C) Overlay of responses before (i) and after (ii) application of Group II mGluR agonist. (D) Average change in EPSP amplitude and Rm during and after washout of APDC.
Figure 2.
Voltage clamp recording from an RS neuron in layer 4 during bath application of APDC results in the attenuation of thalamocortical EPSCs. (A) Voltage clamp recording in response to APDC application (solid line) and thalamic stimulation. (B) Elicited EPSCs from the time points indicated in (A) before (i), during (ii), and after (iii) application of APDC. (C) Overlay of EPSC responses before (i) and after (ii) application of Group II mGluR agonist. (D) Average change in EPSC amplitude during and after washout of APDC.
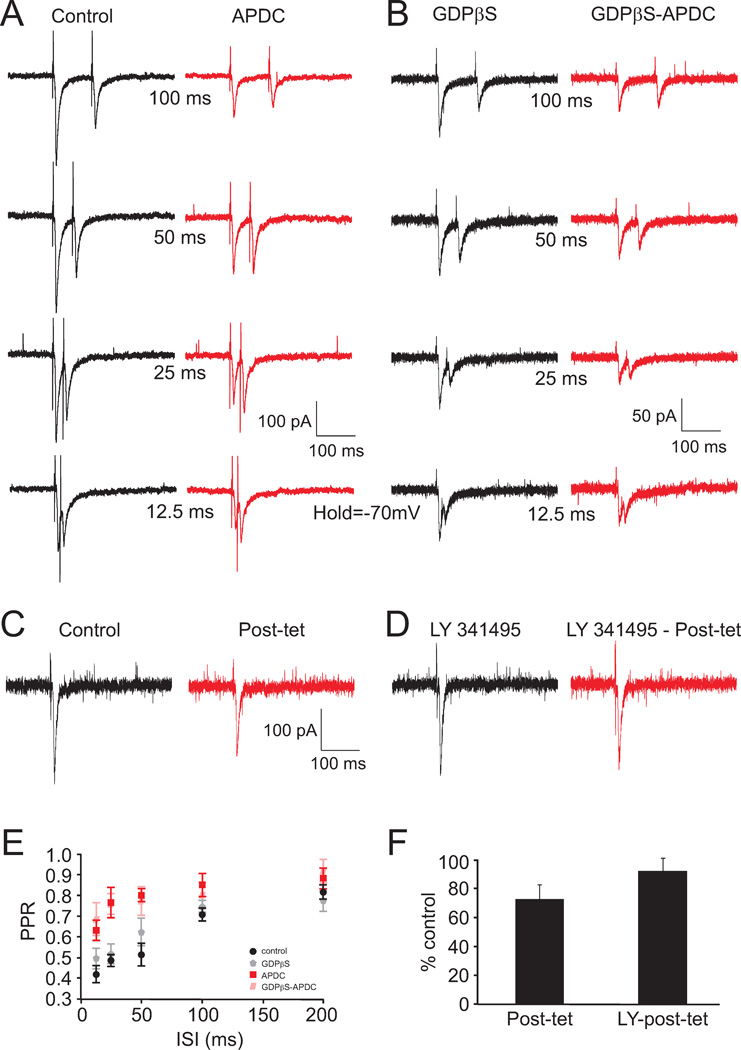
We then examined whether presynaptic mechanisms might be involved in this APDC mediated attenuation of thalamocortical EPSCs. Paired-pulse ratios (PPRs; each of which was assessed as the ratio of the 2nd evoked EPSC amplitude in a train divided by the 1st) to thalamic stimulation were assessed in the presence or absence of APDC and/or intracellular GDPβS (100 µM), which inhibits G-protein activity postsynaptically. Voltage clamp recordings to paired-pulse stimulation were made at varying interstimulus intervals (ISIs; 12.5–200 ms). In all instances (A1: n=11; S1: n=6), thalamocortical PPRs exhibited depression (<1.0), which was most pronounced at shorter ISIs (Fig. 3A, B, E). Bath application of APDC decreased the amplitude of the first EPSC (57 ± 8% control) and increased the PPRs at all ISIs (Fig. 3A, E: red). Under separate conditions with GDPβS added to the intracellular electrode (A1: n=5; S1: n=3), APDC decreased the amplitude of the first EPSC, but less than that observed without its inclusion (71 ± 10% control; p<0.05, t-test) (Fig. 3B). Thalamocortical PPRs also increased at all ISIs, but most significantly at ISIs <50 ms, in the presence of intracellular GDPβS and bath applied APDC (Fig. 3B, E: pink). In the absence of APDC, GDPβS alone had no significant effect on the PPRs (Fig. 3B, E: gray). Since the inclusion of GDPβS blocks postsynaptic Group II mGluR effects and since the deviations in mean PPRs are indicative of presynaptic changes in release probability, these results suggest an involvement of presynaptic Group II mGluRs in the reduction of thalamocortical responses.
Figure 3.
Paired-pulse responses to thalamic stimulation in the presence of APDC and GDPβS and responses to high-frequency tetanus in the presence of mGluR2 antagonist. (A) Voltage clamp recording from a layer 4 RS neuron in response to paired-pulse stimulation at different interstimulus intervals in the absence (black traces) or presence (red traces) of APDC. (B) Paired-pulse responses in the absence (black traces) or presence (red traces) of APDC with GDPβS added to the intracellular solution to block G-protein activity. (C) Responses to thalamic stimulation prior to (black trace) and 500ms after (red trace) high frequency tetanus. (D) Response to thalamic stimulation in the presence of mGluR2 antagonist prior to (black trace) and 500ms after (red trace) high frequency tetanus. (E) Paired pulse ratios (PPRs) for each of the conditions in (A) and (B). Each symbol depicts the mean ± standard deviation under each condition. Black symbols: control; Grey symbols, GDPβS alone; Red Symbols: APDC; Pink symbols: GDPβS and APDC. (F) Relative amplitude of post-tetanus responses compared with control in the absence or presence of mGluR2 antagonist.
Additionally, we explored the involvement of Group II mGluRs by examining the elicited thalamocortical EPSP shortly (500 ms) after a brief tetanus (40 Hz train for 250 ms). The rationale here was that mGluRs affecting EPSC amplitude could be activated by the transiently elevated presence of glutamate in the synaptic cleft (Bandrowski et al., 2002; Cartmell and Schoepp, 2000; Conn and Pin, 1997). We found that the average thalamocortical EPSCs (A1: n=5; S1: n=4) were reduced by this brief preceding tetanus (Fig. 3C, F; 73 ± 11% control) and this reduction was significantly blocked by the Group II mGluR antagonist, LY341495 (Fig. 3D,F: 91 ± 6% control, p<0.05, t-test).
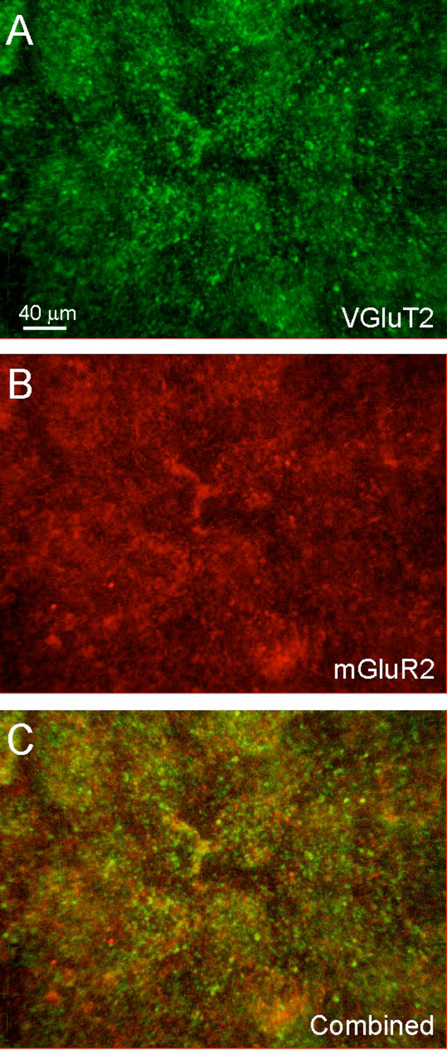
Given these physiological results, we sought anatomical evidence for a presynaptic localization of Group II mGluRs on thalamocortical synaptic terminals. We utilized the specific expression of the type 2 vesicular glutamate transporter (VGluT2) in thalamocortical synaptic terminals (Hackett et al., 2011; Nahmani and Erisir, 2005) in order to assay the location of Group II mGluRs. After immunostaining for both Group II mGluRs and VGluT2, we found extensive labeling for Group II mGluRs on cell membranes and distinct puncta in layer 4 (Fig. 4B,C: red) that was less pronounced in layer 5 and nearly absent in other layers. This pattern of staining was similar to that which we previously reported (Lee and Sherman, 2009a) and was evident in all sensory neocortical areas, suggesting that the effects mediated by Group II mGluRs are a general feature of neocortical synapses in layer 4. Labeling for VGluT2 was also heavily localized in layer 4 as distinct puncta (Fig. 4A,C: green), but labeled puncta were also found in layers 1 and 6, though were less densely distributed. Double-labeled puncta for both Group II mGluRs and VGluT2 were present throughout layer 4 (Fig 4C: yellow), indicating that Group II mGluRs were indeed localized on presynaptic thalamocortical terminals expressing VGluT2.
Figure 4.
Colocalization of Group II mGluRs and VGluT2 on terminals in cortex. Confocal image stack at 63X depicts labeling with fluorescent secondary antibodies against primary antibodies directed against (A) VGluT2 (green) and (B) mGluR2 (red). (C) Double labeled puncta for both VGluT2 and mGluR2 (yellow) are observed throughout layer 4.
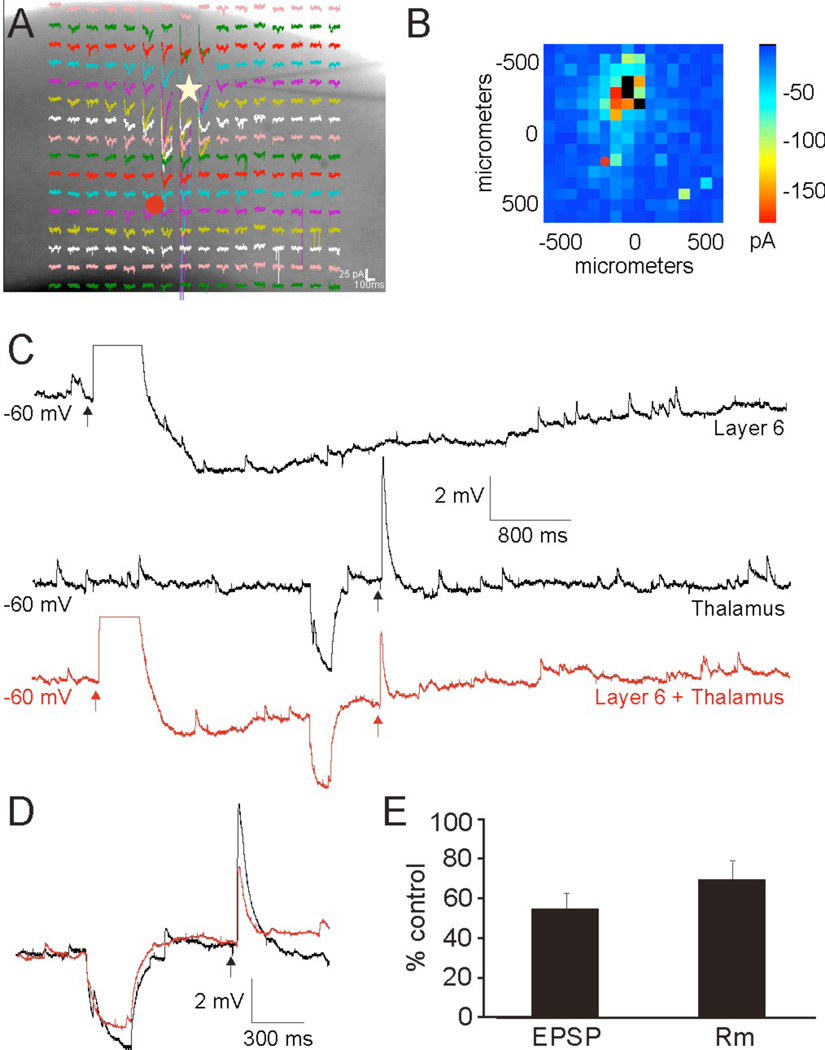
We compared the results from the pharmacological activation of Group II mGluRs with that observed following layer 6 induced activation (Lee and Sherman, 2009a). These experiments were similar to those described above, except that we activated the Group II metabotropic glutamate receptors from layer 6 activation instead of with bath applied APCD; we stimulated layer 6 neurons by photouncaging of glutamate in the presence of antagonists to NMDA glutamate receptors (MK-801), Group I (type 1) mGluRs (LY367385; MPEP), and GABARs (SR 95531; CGP 46381). We recorded from layer 4 neurons (AI: n=6; S1: n=4), while measuring the changes to thalamocortical EPSPs following the layer 6 induced activation of Group II mGluRs (Fig. 5). Hyperpolarizing current injections preceded thalamocortical stimulation to test changes to input resistance (Fig. 5C,D: downward deflections). Similar to our previous results (Lee and Sherman, 2009a), photostimulation of layer 6 intracortical projections resulted in a robust AMPA mediated depolarization followed by a prolonged hyperpolarization mediated by Group II mGluRs (3.7 ± 1.1 mV) (Fig. 6B: top). During this Group II mediated hyperpolarization, a concomitant reduction of thalamocortical EPSPs was observed that was similar in magnitude (Fig. 5C–E: 58 ± 11% control) to that induced by APDC, but this reduction could also not be completely accounted for, if at all, by the changes to input resistance (Fig. 5C–E: 69 ± 13% control), suggesting that stimulation of layer 6 neurons results in activation of Group II mGluRs postsynaptically on layer 4 thalamorecipient cells as well as presynaptically on thalamocortical terminals onto these cells.
Figure 5.
Conjoint activation of Group II mGluRs via photostimulation in layer 6 and electrical thalamic stimulation results in attenuation of the thalamocortical EPSPs. (A) Voltage clamp recording from a layer 4 RS neuron (star) in response to uncaging of glutamate. (B) Mean elicited EPSCs. (C) Current clamp recordings in response to photostimulation of layer 6 (upper trace), thalamic electrical stimulation (middle trace), or layer 6 photostimulation followed by thalamic electrical stimulation (lower red trace) under conditions necessary to elicit Group II mGluR responses. Hyperpolarizing current injections precede thalamic stimulation to test input resistance. (D) Expanded overlay of middle and lower trace in (C). (E) Average change in EPSP amplitude and Rm.
4. Discussion
In general, we found that the pharmacological and synaptic activation of Group II mGluRs in layer 4 of the primary auditory cortex reduced the efficacy of thalamocortical synaptic transmission by both pre- and postsynaptic mechanisms. These results extend our earlier findings (Lee and Sherman, 2009a), which demonstrated that Group II mGluRs mediate a prolonged postsynaptic hyperpolarization of layer 4 neurons through the downstream activation of GIRK channels. These induced postsynaptic changes to membrane conductance elicited by the direct activation of Group II mGluRs may partially result in the attenuation of responses to thalamic stimulation. The activation of Group II mGluRs localized on the presynaptic terminals of thalamocortical fibers also contributes by reducing the efficacy of neurotransmitter release at these synapses. Thus, complementary pre- and post-synaptic mechanisms mediated by Group II mGluRs result in the profound attenuation of thalamocortical synaptic transmission.
Our results are consistent with previous studies on the role of Group II mGluRs at other synapses in the brain. In general, Group II mGluRs are localized on presynaptic terminals (Cartmell and Schoepp, 2000; Neki et al., 1996), where they reduce neurotransmitter release, acting as autoreceptors during periods of intense firing (Bandrowski et al., 2002; Niswender and Conn, 2010). Such presynaptic autoreceptor effects have been observed in several brain structures (Cartmell and Schoepp, 2000; Farazifard and Wu, 2010), including the visual and somatosensory cortices (Flavin et al., 2000; Mateo and Porter, 2007), where their pharmacological activation in vivo appears to reduce cortical responses to external sensory stimuli (Beaver et al., 1999; Cahusac, 1994). The present results confirm these synaptic effects in the auditory and somatosensory cortices (Bandrowski et al., 2002), but also extend them by demonstrating their activation by stimulation of layer 6 neurons.
As we have remarked previously (Lee and Sherman, 2009a), the postsynaptic activation of Group II mGluRs may enable synaptic gain control in order to expand the dynamic range of these synapses. In addition, the finding of presynaptic Group II mGluRs provides a potential added mechanism for intracortical projections originating in layer 6 to inhibit thalamocortical activity, either directly or indirectly, although the recruitment, onset and duration of pre- and post-synaptic inhibitory effects in layer 4 thalamorecipient neurons may differ (Cartmell and Schoepp, 2000; Conn and Pin, 1997; Ngomba et al., 2011).
Our results suggest a model of suppressing auditory thalamocortical transmission via two distinct mechanisms: suppression of thalamorecipient layer 4 cells by activating Group II mGluRs both postsynaptically (leading to hyperpolarization) and presynaptically (leading to reduced transmitter release). These effects may be engaged during high firing periods in the thalamocortical cells, in which presynaptic Group II mGluRs autoregulate transmitter release. In addition, periods of high activity in layer 6 could activate pre- and postsynaptic Group II mGluRs resulting in a complementary attenuation of transmission. Interestingly, these intracortical layer 6 neurons also branch to innervate thalamic relay neurons directly and indirectly via the thalamic reticular nucleus (TRN) (Thomson, 2010), where the inhibitory effects from the TRN are generally stronger (Lam and Sherman, 2010). Thus, beyond gain control, these circuits could act as a gating mechanism, providing important modulatory control by reducing thalamocortical transmission when sufficiently active.
Highlights.
Group II mGluRs in layer 4 mediate a reduction in thalamocortical (TC) EPSP/Cs
Pre- and post-synaptic mechanisms are involved in the attenuation of TC EPSP/Cs
Group II mGluRs may autoregulate TC transmission via gating or gain control
Acknowledgements
We thank Dr. Vytas Byndokas for his valuable assistance with the confocal microscope and image acquisition. This work was supported by NIH/NIDCD grants R03 DC 011361 (CCL) and R01 DC 008794 (SMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Bandrowski AE, Moore SL, Ashe JH. Activation of metabotropic glutamate receptors by repetitive stimulation in auditory cortex. Synapse. 2002;44:146–157. doi: 10.1002/syn.10058. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience. 2002;113:957–974. doi: 10.1016/s0306-4522(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Daw NW. Effect of the group II metabotropic glutamate agonist, 2R,4R-APDC, varies with age, layer, and visual experience in the visual cortex. J Neurophysiol. 1999;82:86–93. doi: 10.1152/jn.1999.82.1.86. [DOI] [PubMed] [Google Scholar]
- Cahusac PM. Cortical layer specific effects of metabotropic glutamate receptor agonist 1S,3R-ACPD in rat primary somatosensory cortex in vivo. Eur J Neurosci. 1994;6 doi: 10.1111/j.1460-9568.1994.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex. 2011;21:2425–2441. doi: 10.1093/cercor/bhr029. 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Glutamate inhibits thalamic reticular neurons. J Neurosci. 1999;19:6694–6699. doi: 10.1523/JNEUROSCI.19-15-06694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Dutar P, Petrozzino JJ, Vu HM, Schmidt MF, Perkel DJ. Slow synaptic inhibition mediated by metabotropic glutamate receptor activation of GIRK channels. J Neurophysiol. 2000;84:2284–2290. doi: 10.1152/jn.2000.84.5.2284. [DOI] [PubMed] [Google Scholar]
- Farazifard R, Wu SH. Metabotropic glutamate receptors modulate glutamatergic and GABAergic synaptic transmission in the central nucleus of the inferior colliculus. Brain Res. 2010;1325:28–40. doi: 10.1016/j.brainres.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Flavin HJ, Jin XT, Daw NW. 2R,4R-4-Aminopyrrolidine-2,4-dicarboxylate (APDC) attenuates cortical EPSPs. Brain Res. 2000;873:212–217. doi: 10.1016/s0006-8993(00)02429-x. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically couple inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical synaptic connections: quanta, innervation and reliability. Neuron. 1999;23:385–397. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Takahata T, Balaram P. VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway. Hear Res. 2011;274:129–141. doi: 10.1016/j.heares.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb Cortex. 2010;20:13–24. doi: 10.1093/cercor/bhp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol. 2008;507:1879–1900. doi: 10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical intputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009a;19:2281–2289. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Modulator property of the intrinsic cortical projections from layer 6 to layer 4. Front Syst Neurosci. 2009b;3:3. doi: 10.3389/neuro.06.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Topography and physiology of ascending streams in the auditory tectothalamic pathway. Proc Natl Acad Sci U S A. 2010;107:372–377. doi: 10.1073/pnas.0907873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Z, Porter JT. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience. 2007;146:1062–1072. doi: 10.1016/j.neuroscience.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484:458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci Lett. 1996;202:197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, Santolini I, Salt TE, Ferraguti F, Battaglia G, Nicoletti F, van Luijtelaar G. Metabotropic glutamate receptors in the thalamocortical network: Strategic targets for the treatment of absence epilepsy. Epilepsia. 2011;52:1211–1222. doi: 10.1111/j.1528-1167.2011.03082.x. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou G, Odgen DC, Barth A, Corrie JET. Photorelease of carboxylic acids from 1-acyl-7-nitroindolines in aqeuous solution: rapid and efficient photorelease of L-glutamate. J Am Chem Soc. 1999;121:6503–6504. [Google Scholar]
- Petralia RS, Wanga Y-X, Niedzielskia AS, Wentholda RJ. The metabotropic glutamate receptors, MGLUR2 and MGLUR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS. Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci. 2009;29:6406–6417. doi: 10.1523/JNEUROSCI.0258-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2005;94:2019–2030. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Sekizawa S, Bechtold AG, Tham RC, Bonham AC. A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J Neurosci. 2009;29:11807–11816. doi: 10.1523/JNEUROSCI.2617-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing "drivers" from "modulators". Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2nd ed. London: MIT Press; 2006. [Google Scholar]
- Spruston N, Jaffe DB, Williams SH, Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol. 1993;70:781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010;4:13. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB. An overview of mammalian auditory pathways with an emphasis on humans. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: neuroanatomy. New York: Springer; 1992. pp. 1–22. [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci. 2008;11:790–798. doi: 10.1038/nn.2137. [DOI] [PubMed] [Google Scholar]