Abstract
Here we demonstrate that the dual leucine zipper kinase (DLK) promotes robust regeneration of peripheral axons following nerve injury in mice. Peripheral axon regeneration is accelerated by prior injury, however DLK KO neurons do not respond to a preconditioning lesion with enhanced regeneration in vivo or in vitro. Assays for activation of transcription factors in injury-induced pro-regenerative pathways reveal that loss of DLK abolishes upregulation of p-STAT3 and p-cJun in the cell body following axonal injury. DLK is not required for the phosphorylation of STAT3 at the site of nerve injury, but is necessary for retrograde transport of p-STAT3 to the cell body. These data demonstrate that DLK enhances regeneration by promoting a retrograde injury signal that is required for the activation of the neuronal pro-regenerative program.
Introduction
Axons in the peripheral nervous system (PNS) can regenerate after injury. However, axon regenerative capacity declines in the adult nervous system as the neuronal intrinsic factors for axon growth diminish in mature neurons. Moreover, inhibition of these intrinsic pathways likely contributes to the poor regenerative capacity in the adult central nervous system (CNS) (Liu et al., 2011; Park et al., 2008; Smith et al., 2009). Therefore, defining how these regenerative pathways are regulated may suggest novel therapeutic approaches to improve neuronal recovery after axonal injury.
Following injury to a peripheral nerve, there is a rapid and local regenerative response involving the formation of a growth cone and cytoskeletal changes promoting regrowth (Bradke et al., 2012). This local response is subsequently augmented by the activation of intrinsic pro-regenerative transcriptional pathways (Abe and Cavalli, 2008; Smith and Skene, 1997). Within hours of a peripheral nerve injury, the levels of phosphorylated transcription factors that promote axon regeneration increase in the cell body (Lindwall et al., 2004; Qiu et al., 2005; Zou et al., 2009). These transcription factors activate a program that increases expression of axonal growth-associated proteins and enhances the rate of regrowth in the days after injury (Hoffman, 2010). In addition, this injury-induced pro-regenerative signaling leads to a preconditioning injury effect, in which a neuron exposed to a prior lesion exhibits a dramatic improvement in axonal regeneration compared to that of a naïve neuron (Neumann and Woolf, 1999). The preconditioning injury effect is a powerful paradigm for defining mechanisms that promote axon regeneration. For example, a peripheral nerve injury induces cAMP, Janus kinase (JAK)-STAT3, and mTOR-S6 pathways, and activation of these pathways is sufficient to improve CNS axon regeneration (Abe et al., 2010; Park et al., 2008; Qiu et al., 2005; Qiu et al., 2002). While many pro-regenerative pathways are known, the mechanism by which injury activates these pathways is less well understood.
Dual leucine zipper kinase (DLK) is a mitogen-activated protein kinase kinase kinase (MAPKKK) that can activate cJun N-terminal kinases (JNK) and p38 MAPK (Fan et al., 1996). In addition to its role for neural development (Bloom et al., 2007; Hirai et al., 2006; Itoh et al., 2011), DLK is involved in injury responses such as axon degeneration and neuronal apoptosis (Chen et al., 2008; Ghosh et al., 2011; Miller et al., 2009; Xiong and Collins, 2012). Moreover, recent studies in C. elegans and Drosophila have demonstrated that DLK is required for the regenerative response after axotomy: in the absence of DLK, reformation of a growth cone from the severed stump is disrupted (Hammarlund et al., 2009; Xiong et al., 2010; Yan et al., 2009), while in juvenile DLK gene trap mice there is less regrowth of axons from dissected and cultured DRG explants (Itoh et al., 2009). Here we demonstrate that in the absence of DLK, in vivo regeneration of mammalian motor and sensory axons is impaired. DLK is not required for the initial outgrowth of injured axons, but is necessary for the retrograde transport of injury signals that activate the intrinsic regenerative program to mediate the preconditioning effect. This study thus identifies DLK as a key intermediate required for axonal injury to activate the regenerative program.
Results
Mouse peripheral axon regeneration requires DLK in vivo
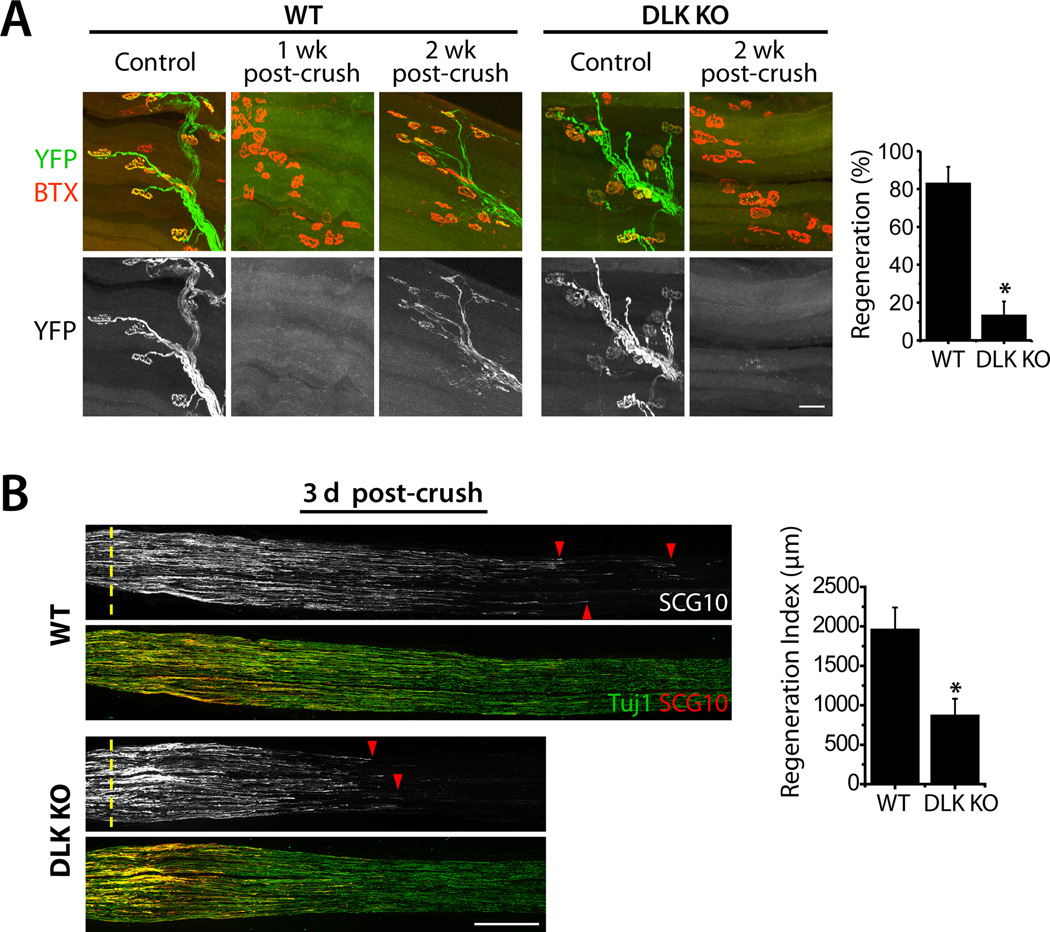
To test whether DLK is required for axonal regrowth in vivo, we first examined motor axon regeneration in DLK conditional knockout (KO) mice. To delete DLK expression in motor neurons, we mated floxed DLK mice (Miller et al., 2009) to HB9-Cre line and labeled Cre-expressing motor neurons with Thy-STOP-YFP15 (see Supplemental Information). We crushed sciatic nerves of wild-type (WT) and DLK conditional KO animals unilaterally, and assessed retargeting of YFP-positive motor axons to the neuromuscular junctions (NMJs) on the extensor hallucis longus (EHL) muscle in the hindlimb. The muscles were stained with α-bungarotoxin (BTX) to label acetylcholine receptors at the endplates. On the un-lesioned side, EHL muscles from both WT and DLK KO mice display apposition of the axon terminals and the endplates, showing that the developmental targeting of DLK KO axons is largely normal (Fig.1A). When the WT muscles were observed 1 week after the crush injury, they were completely devoid of YFP-positive axons (n = 3) (Fig. 1A), demonstrating that motor axons degenerate and are cleared by 1 week. Hence, axons detected after this point are regenerating fibers. Indeed, 2 weeks after the crush, WT axons exhibit robust re-targeting to the NMJs as described previously (Magill et al., 2007). We assessed the re-targeting by counting the number of postsynaptic endplates colocalized with axonal YFP fluorescence, and find that ~80% of the YFP-positive WT axons occupy endplates when normalized to the un-lesioned contralateral muscle. However in DLK KO littermates, the motor axon regeneration is greatly attenuated, with an approximately 8-fold reduction in the number of re-targeted axons (p < 0.001) (Fig. 1A). At 3 week post-injury we observed an improvement in re-targeting of DLK KO axons, however the regeneration is still impaired compared to that in WT (Fig. S1A). These data demonstrate that DLK is essential for efficient axon regeneration to the target muscle in mouse motor neurons.
Figure 1. Peripheral axon regeneration is inhibited in DLK KO mice.
A, Confocal images of whole-mounted EHL muscles after crush injury of the sciatic nerve. In WT, YFP (green)-labeled axons degenerate by 1 week after injury, and robustly regenerate to the endplates labeled with α-bungarotoxin (BTX; red) by 2 weeks after injury. The axon re-targeting is significantly attenuated in HB9-Cre conditional DLK KO mice. WT (DLKF/WT; HB9-Cre; Thy-STOP-YFP15), n = 5; DLK KO (DLKF/F; HB9-Cre; Thy-STOP-YFP15), n = 4; p* < 0.001. Scale bar, 50 µm.
B, Longitudinal sections of sciatic nerves from WT or Wnt1-Cre conditional DLK KO mice were immunolabeled for SCG10 and neuron-specific β3 tubulin (Tuj1) 3 days after crush injury. Axon regeneration from the crush site (dotted line) is significantly reduced in the DLK KO mice. SCG10 labels regenerating axons (red arrowheads). WT (DLKF/F), n = 4; DLK KO (DLKF/F; Wnt1-Cre), n = 3; p* < 0.05. Scale bar, 500 µm.
Data are presented as means ± SEM. See also Fig. S1 and S2.
We also examined the requirement of DLK in sensory axons that are the major component of the sciatic nerve. We deleted DLK expression in sensory neurons with Wnt1-Cre (Danielian et al., 1998) (Fig. S1B), crushed sciatic nerves of KO and littermate controls, and assessed sensory axon regeneration by measuring the length of axons growing past the crush site. To label regenerating axons, we stained longitudinal nerve sections with a growth-associated neuronal protein, superior cervical ganglion 10 (SCG10), which is highly expressed in developing and regenerating axons (Mason et al., 2002). SCG10 levels in uninjured sciatic nerves were not significantly different between WT and DLK KO though there was a tendency for higher levels of SCG10 in the DLK KO (26 ± 10 % increase [mean ± s.e.m.]; n = 5; p = 0.07). When WT sensory axons were allowed to re-grow for 3 days after injury, they robustly regenerated beyond the site of lesion to a distance of approximately 4 mm, consistent with previous findings (La Fleur et al., 1996). However, the length of regenerating axons is reduced in the absence of DLK (Fig. 1B). Immunolabeling with another marker of regenerating axons, GAP43 (Abe et al., 2010), shows similar results (Fig. S2). To quantify the regenerative deficit, we measured the distance from the crush site to the location where the SCG10 level is reduced to half of its level at the crush site, and defined that as regeneration index (Abe et al., 2010). Loss of DLK results in 2-fold reduction in the regeneration index (p < 0.05) (Fig. 1B), demonstrating that DLK promotes sensory axon regeneration in vivo.
In addition to sensory neurons, the Wnt1-Cre driver line is also active in other neural crest lineages including Schwann cells (Danielian et al., 1998). Since changes in myelination and the reaction of Schwann cells to injury may indirectly affect axonal structure and growth (Jessen and Mirsky, 2008), we examined whether myelin is normally formed in Wnt1-Cre conditional DLK KO mice. No obvious abnormalities were noted in sciatic nerve axons from DLK KO mice by light and electron microscopic analysis (Fig. S1C). Additionally, we assessed a battery of myelination parameters such as cumulative g-ratio and fiber-width distribution by semi-automated nerve histomorphometry (see supplemental methods) and found no alterations in the absence of DLK (Fig. S1C). This quantitative analysis also demonstrates that axon caliber distributions in DLK deficient nerves are indistinguishable from control preparations. In addition, we studied the cellular reactions three days following nerve injury, when Schwann cells lose their myelin sheaths and dedifferentiate. Ultrastructural assessment shows normal dedifferentiation features of Schwann cells after sciatic nerve transection in DLK KO mice (Fig. S1D). Immunolabeling for markers of dedifferentiated Schwann cells, cJun (Fig. S1E) (Jessen and Mirsky, 2008) and Oct6 (data not shown) (Kawasaki et al., 2003), demonstrates that injury-induced Schwann cell dedifferentiation is normal in Wnt1-Cre conditional DLK KO. This analysis of Schwann cell function, the retarded motor fiber regrowth in HB9-Cre conditional DLK KO mice, and the in vitro work described below are all consistent with a cell-autonomous neuronal role for DLK in promoting regeneration.
DLK is dispensable for early axonal regrowth after injury
Axon regeneration can be promoted at multiple steps including growth cone formation, axonal extension, and injury-induced acceleration (preconditioning effect). We investigated which of these processes require DLK. We first assessed the early phase of axonal regrowth in vivo using the Wnt1-Cre conditional DLK KOs. The sciatic nerve axons of WT or DLK KO mice were subjected to a crush lesion, and allowed to re-grow for 1 day. The initial growth of SCG10-positive axons is comparable between the WT and DLK KO mice (Fig. 2A and C), demonstrating that DLK is not required for this early stage of axonal regrowth. In addition, we directly tested whether DLK is necessary for the formation of a growth cone from the severed axon stump. We assessed growth cone formation after axotomy in cultured embryonic DRG neurons from DLK constitutive KO and littermate control mice. When these neurons are plated in a confined area on a culture dish, axons extend away from the cell body area so that many axons can be severed and assessed simultaneously. We find that the ratio of severed axons that form a growth cone within 2 hours after axotomy is not significantly different between WT and DLK KO neurons (Fig. S3). Hence, DLK is not essential in the early stage of axon regrowth that involves local regulation of the growth cone and axon.
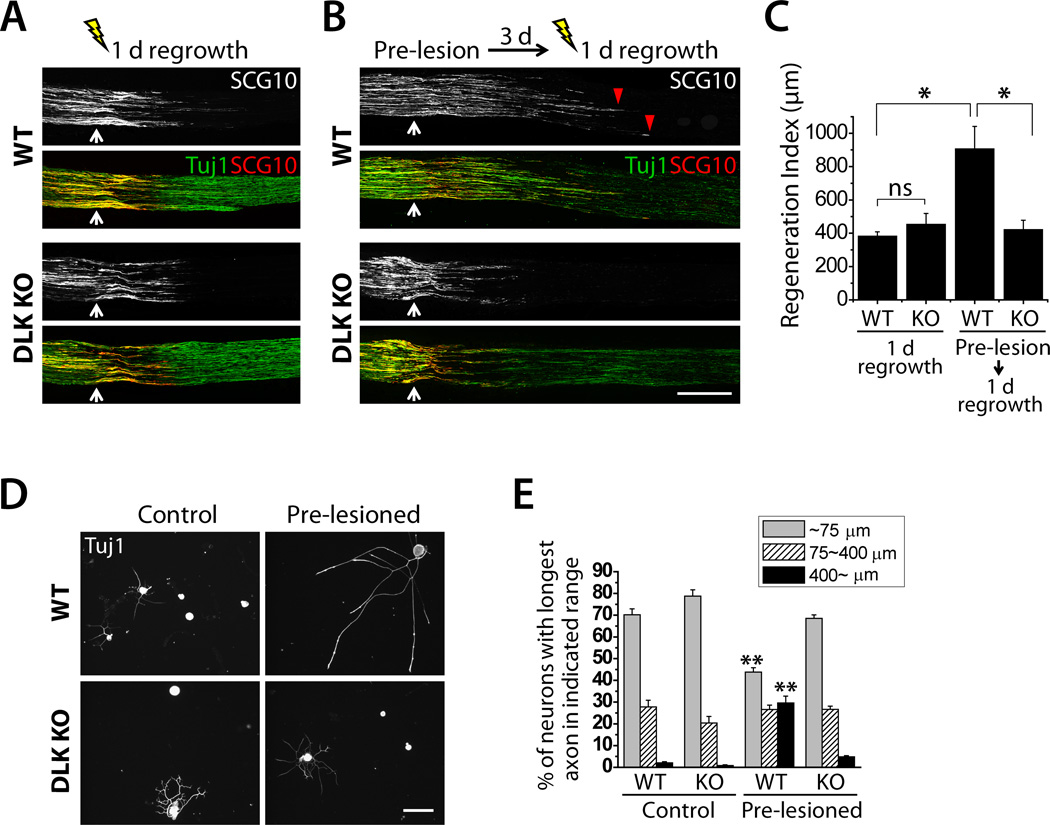
Figure 2. DLK is required for the accelerated axon regeneration induced by a preconditioning injury.
A and B, Longitudinal sections of sciatic nerves of WT or Wnt1-Cre conditional DLK KO mice were immunolabeled for SCG10 and β3 tubulin (Tuj1) 1 day after either single crush injury (A) or double crush injury (B). Axon regeneration 1 day after crush injury is comparable between WT and DLK KO (A). Double crushed mice were given a preconditioning crush injury (Pre-lesion) for 3 days, subsequently subjected to another crush injury, and allowed to re-grow for 1 day (B). A pre-lesion significantly potentiates axon regeneration in WT (red arrowhead), but this effect is abolished in DLK KO. White arrow indicates the crush site. Scale bar, 500 µm.
C, Results from (A) and (B) were quantified to generate a regeneration index. WT (DLKF/F), n = 4; DLK KO (DLKF/F; Wnt1-Cre), n = 4 for single crush, n = 5 for double crush; p* < 0.005.
D, Cultured DRG neurons from WT or DLK KO were immunostained for β3 tubulin (Tuj1). A prior lesion of the sciatic nerve promotes axon regeneration in WT DRG neurons cultured for 16 hours, however this effect is abolished in DLK KO. Scale bar, 100 µm.
E, Axon regrowth shown in (D) was quantified by percentage of neurons with longest axon in each range: ~75 µm, 75~400 µm, or 400~ µm. WT (DLKF/F or DLKF/WT; Wnt1-Cre); DLK KO (DLKF/F; Wnt1-Cre); n = 5; p** < 0.001 vs. WT, control and DLK KO, pre-lesioned.
Data are presented as means ± SEM. See also Fig. S3.
DLK is required for accelerated regeneration induced by a conditioning injury
Hours after injury to the sciatic nerve, DRG neurons respond by activating pro-regenerative factors in the cell body that induce a pro-regenerative program that accelerates axonal regrowth and mediates the preconditioning effect (Abe and Cavalli, 2008). Because axon regeneration in DLK deficient mice is normal 1 day after injury (Fig. 2A) but is reduced 3 days after injury (Fig. 1B), we hypothesized that DLK may be required for this later neuronal injury response. To test this injury effect, we performed preconditioning injury experiments. We crushed the sciatic nerve, waited three days to allow for induction of the pro-regenerative program, and then applied a second crush just proximal to the first crush and then allowed the axons to re-grow for 1 day. In WT, axon regeneration is markedly accelerated—preconditioning leads to a 2-fold increase in the regeneration index (p < 0.005) (Fig. 2B and C). However, this conditioning injury effect was completely abolished in the absence of DLK (p < 0.005) (Fig. 2B and C), demonstrating that DLK is necessary for the injury-induced acceleration of axon regeneration.
As an independent test for the role of DLK in the conditioning lesion response, we performed an in vitro axon regrowth assay with DRG neurons. We crushed the sciatic nerve, waited three days, and then cultured adult DRG neurons for 16 hours in order to evaluate their regeneration capacity. We assessed axon regrowth by measuring the length of the longest axon from each neuron. Prior nerve injury markedly potentiates axon regrowth in WT DRG culture as previously demonstrated (Smith and Skene, 1997), leading to a significant increase in the ratio of neurons bearing long (> 400 µm) axons (p < 0.001) and a significant decrease in the ratio of neurons with short (< 75 µm) axons (p < 0.001) (Fig. 2D and E). However, this accelerated axonal growth was blocked in DLK KO neurons (p < 0.001) (Fig. 2D and E), demonstrating the requirement of DLK for the preconditioning effect. Importantly, these in vitro results highlight that the reduced in vivo regeneration in DLK KOs is unlikely to be secondary to the delayed degeneration of the distal stump in the absence of DLK (Miller et al., 2009). Instead, our data shows that DLK directly promotes the preconditioning effect in injured neurons.
DLK is required for activation of injury-induced pro-regenerative signals in cell bodies
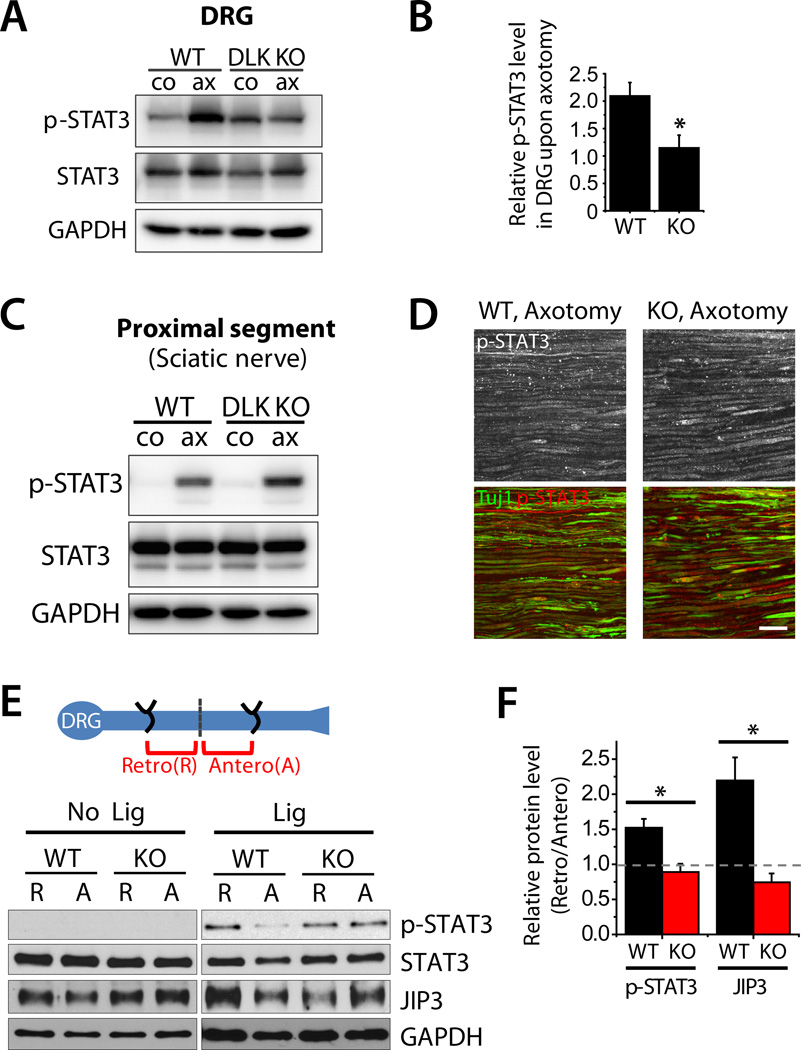
We next investigated mechanisms by which DLK promotes neuronal response to injury. Nerve injury activates molecular pathways that contribute positively to axonal regeneration. We hypothesized that DLK is required for these injury-induced signals, and so assayed markers of these injury-induced pathways. Of these the most likely candidate is the transcription factor cJun, a downstream target of the DLK/JNK pathway that is phosphorylated upon axonal injury and promotes axon regeneration in the mouse peripheral nervous system (Raivich et al., 2004). We used immunofluorescence to detect p-cJun in the nuclei of DRG neurons from WT and Wnt1-Cre conditional DLK KO animals 3 days after sciatic nerve lesion and found that the injury-induced increase in the number of cells expressing p-cJun is blocked in DLK KO mice (p < 0.001) (Fig. S4). These data are consistent with the previous report by Itoh et al. (Itoh et al., 2009) using DLK gene-trap mice. To our surprise, however, this was not the only injury signaling pathway blocked by the loss of DLK. Upon injury, the transcription factor STAT3 is phosphorylated and accumulates in DRG cell bodies, where it promotes axonal regeneration (Bareyre et al., 2011; Qiu et al., 2005). In the absence of DLK, however, this accumulation of p-STAT3 is blocked. In WT DRGs, there is a 2-fold increase in the p-STAT3 levels in DRG cell bodies upon nerve injury, however there is no significant increase in p-STAT3 in DLK KO DRGs (p < 0.05) (Fig. 3A and B). Hence, DLK is required for the activation of two pro-regenerative pathways in the cell bodies of injured neurons.
Figure 3. DLK is required for retrograde transport of an injury-induced pro-regenerative signal.
A, Immunoblot analysis of p-STAT3 in cell bodies following axon injury. Sciatic nerves of WT or DLK KO mice were unilaterally injured for 3 days and DRGs from either control (co) or injured (ax) side were subjected to immunoblot for p-STAT3. p-STAT3 levels are significantly elevated by axotomy (ax) in WT DRG but this increase is abolished in DLK KO DRG. Total STAT3 levels are comparable between conditions. GAPDH is shown as a loading control.
B, Quantification of results shown in (A). p-STAT3 levels were quantified by normalizing the levels in the DRGs on the injured side to those in the uninjured contralateral DRGs. WT (DLKF/F); DLK KO (DLKF/F; Wnt1-Cre); n = 4; p* < 0.05.
C, Sciatic nerves of WT or DLK KO mice were unilaterally injured for 1 day and the proximal nerve segments from either control (co) or injured (ax) side were subjected to immunoblot analysis of p-STAT3. p-STAT3 levels are significantly elevated in injured nerve (ax) and this increase is similar between WT and DLK KO mice, showing that DLK is not required for the local activation of STAT3. Total STAT3 levels are comparable between conditions. GAPDH is shown as a loading control. WT (DLKF/F); DLK KO (DLKF/F; Wnt1-Cre); n = 3.
D, Longitudinal sections of the sciatic nerves of WT or DLK KO mice were immunostained for p-STAT3 1 day after crush injury. Confocal images visualize the proximal segments of the injured nerve and show that p-STAT3 localizes in axons. β3 tubulin (Tuj1) labels axons. WT (DLKF/F); DLK KO (DLKF/F; Wnt1-Cre). Scale bar, 50 µm.
E, Schematic diagram of double ligation experiment shows that double ligation injury induces concentration of retrogradely transported cargoes (R) in the proximal segment and anterogradely transported cargoes (A) in the distal segment. In WT, p-STAT3 and JIP3 accumulate in the retrograde pool 6 hours after ligation (Lig). The accumulation of both proteins is blocked in the absence of DLK, showing that DLK is required for injury-induced retrograde transport of p-STAT3 and JIP3. Total STAT3 and JIP3 are similarly distributed in the proximal and distal segments in uninjured control nerve. GAPDH is shown as a loading control.
F, Quantification of the results shown in (E). Retrograde protein accumulation is quantified as ratio of the protein levels in retrograde pool to the levels in anterograde pool. WT (DLKF/F); DLK KO (DLKF/F; Wnt1-Cre); n = 3; p* < 0.05.
Data are presented as means ± SEM. See also Fig. S4.
DLK promotes retrograde transport of an injury signal
STAT3 is phosphorylated by JAK kinase (Qiu et al., 2005), so the decrease in p-STAT3 in the DLK KO was surprising. Although STAT3 is a transcription factor, it is present in axons, locally phosphorylated following a nerve injury, and retrogradely transported after injury (Ben-Yaakov et al., 2012). To determine the mechanism by which DLK promotes the accumulation of p-STAT3 in the cell body, we first examined whether DLK is directly required for the phosphorylation of STAT3 at the site of injury. We analyzed levels of p-STAT3 in the proximal nerve stump 1 day after sciatic nerve lesion. In WT, p-STAT3 is barely detectable in the un-lesioned contralateral nerve but is dramatically upregulated by injury (Fig. 3C). p-STAT3 is localized in neuronal axons as shown by immunostaining (Fig. 3D). In the absence of DLK, STAT3 is still phosphorylated in the injured axons, and the levels are similar to WT (Fig. 3C and D; n = 3). These data show that the local activation of STAT3 does not require DLK. Instead, these findings suggest that DLK may be necessary for translocation of the injury signal to the cell body.
We next examined whether DLK is indeed required for the transport of p-STAT3 to the cell body. To track the movement of the phosphorylated protein upon injury, we performed a double nerve ligation in which the sciatic nerve is sutured at two locations (Fig. 3E). The nerve ligation injures axons and blocks axonal transport, so that transported cargoes accumulate near the knots. Retrograde cargoes accumulate in the proximal segment of the nerve while anterograde cargoes concentrate in the distal segment, so the ratio of protein present in the proximal/distal segment is a measure of retrograde transport (Cavalli et al., 2005). Upon double ligation of WT sciatic nerves for 6 h, p-STAT3 levels are 1.5-fold higher in the proximal segment, consistent with the retrograde transport of p-STAT3 after injury. However, this accumulation is blocked in DLK KOs (p < 0.05) (Fig. 3E and F). We also analyzed transport of JIP3, a scaffolding protein that links DLK and JNK to the axon transport machinery (Cavalli et al., 2005; Ghosh et al., 2011). Injury facilitates the association of JIP3 with the retrograde transport machinery and increases the retrograde transport of both JIP3 and phosphorylated JNK (Cavalli et al., 2005). In the double ligation assay, injury-induced accumulation of JIP3 in the proximal stump is abolished in the absence of DLK (p < 0.05) (Fig. 3E and F). Therefore, DLK is necessary for the retrograde transport of both p-STAT3 and JIP3 upon axon injury. Collectively, these results demonstrate that DLK plays an essential role for the axonal transport of injury signaling components to the cell body.
Taken together, these data demonstrate that DLK is required for robust axon regeneration in the vertebrate PNS in vivo, that DLK promotes retrograde transport of injury signals that enhance axonal regenerative capacity, and that injury-induced potentiation of axonal regeneration requires DLK.
Discussion
Trauma, neurotoxins, and neurological disease can all trigger axonal damage and the loss of neuronal connections. The capacity of a neuron to regenerate an injured axon is crucial for the recovery of neural function. In an injured peripheral nerve, there are two phases to the regenerative response. There is a rapid, local modification to the cytoskeleton that promotes growth cone formation and axonal outgrowth (Bradke et al., 2012). Later, retrograde injury signals activate transcription factors in the cell body that turn on pro-regenerative programs (Liu et al., 2011). These programs accelerate axonal outgrowth, which is likely important since rapid peripheral regeneration improves functional outcomes (Gordon et al., 2011). In addition, these transcriptional programs mediate the preconditioning effect, in which injured neurons regenerate more robustly following exposure to a prior axon injury. Indeed, a preconditioning injury can even stimulate the normally refractory central axons to re-grow (Neumann and Woolf, 1999). Hence, identifying the mechanisms that activate this injury signal may allow for novel interventions to stimulate axon regeneration.
We tested whether DLK regulates axon regeneration mechanisms in vertebrates using a mouse model. In worms and flies, DLK is required for the formation of the regenerative growth cone response after axotomy (Hammarlund et al., 2009; Xiong et al., 2010; Yan et al., 2009). In mice, we find that DLK is dispensable for the early, local response of axon regeneration. In vitro, growth cone formation after axotomy is not altered, and in vivo outgrowth of injured axons is normal in the first 24 hours after injury. However, by 3 days after injury axonal outgrowth is reduced in the DLK KO, and, most significantly, regeneration to functional targets is impaired. These findings demonstrate that, in the mouse, DLK is selectively required for the second phase of the regenerative response. Although loss of DLK significantly delays regeneration, it does not completely block axonal regrowth, likely because the local regenerative response is maintained. By genetically separating these phases, this mutant demonstrates the physiological importance of activation of the pro-regenerative cell body program for the timely re-innervation of postsynaptic targets.
DLK is necessary for the pro-regenerative program that promotes axonal growth after a single injury and that mediates the preconditioning effect of a prior injury. To identify the mechanism of action of DLK, we assayed activation of markers for known injury-activated pro-regenerative signals and found significant differences for cJun and STAT3—the upregulation of p-cJun and p-STAT3 in DRGs following axonal injury is abolished in DLK KO mice. The levels of p-CREB and p-S6, the markers for cAMP pathway and mTOR signaling, respectively, were not significantly different between WT and DLK KO. cJun is a known target of DLK-JNK MAPK pathway and the role of DLK for injury-induced cJun activation has been previously reported in nerve growth factor (NGF)-deprivation in embryonic mouse culture and a sciatic nerve lesion in DLK gene-trap mice (Ghosh et al., 2011; Itoh et al., 2009). However, the requirement of DLK for injury-induced STAT3 activation in the cell body was unexpected. STAT3 phosphorylation is dependent on Janus kinase 2 (Qiu et al., 2005), which may be activated by neuropoietic cytokine released upon injury. Indeed, STAT3 is present within the axon and phosphorylated upon injury, after which it is retrogradely transported to the cell body (Ben-Yaakov et al., 2012). In DLK KO neurons, STAT3 is still robustly phosphorylated in the injured axon, so DLK is not required for the activation of STAT3. Instead, DLK is necessary for the retrograde transport of the p-STAT3 injury signal to the cell body. It will be of interest to determine whether DLK is also required for the nuclear accumulation of p-STAT3 within the DRG cell bodies.
Retrograde axonal transport is necessary for normal axonal regeneration (Abe and Cavalli, 2008; Hanz et al., 2003; Xiong et al., 2010). JIP3 is a central player in the retrograde injury signal—it is a scaffolding protein for the MAP kinase JNK and preferentially associates with the retrograde motor complex after nerve injury (Cavalli et al., 2005). Upon NGF deprivation, JIP3 promotes neuronal apoptosis by mediating formation of a DLK-JNK signaling module (Ghosh et al., 2011). Here we demonstrate that upon axonal injury, DLK is required for the injury-induced retrograde transport of JIP3. Hence JIP3 not only serves as a scaffold for JNK pathway kinases (Kelkar et al., 2000), but is itself regulated by the function of those kinases. This is consistent with our findings in Drosophila, where the ortholog of DLK regulates the association of JIP1, a structurally unrelated JNK scaffolding protein, with the transport machinery (Horiuchi et al., 2007). Since JIP3 associates with JNK, the absence of its retrograde transport is likely responsible for the failure to phosphorylate the JNK-target cJun in the DLK KO. Moreover, since JIP3 links motor proteins to a variety of cargoes (Abe et al., 2009), promoting JIP3 retrograde transport may be central to the role of DLK in the injury response. We suggest that JIP3 could facilitate the retrograde transport of p-STAT3, although our data are also consistent with the model that DLK independently regulates the retrograde transport of JIP3 and p-STAT3.
Here we demonstrate that after axonal injury DLK enhances the regeneration of the proximal axon. Previously, we showed that DLK also functions in the distal axon to promote Wallerian degeneration (Miller et al., 2009). A dramatic delay in clearance of these distal fibers due to the expression of the Wallerian degeneration slow (Wlds) protein physically inhibits regeneration (Brown et al., 1992), however this is unlikely to be the explanation for the defects in regeneration in the DLK KO. First, the DLK KO leads to a much shorter delay in degeneration than does Wlds. Second, the absence of DLK blocks retrograde injury signal transport and accumulation of activated pro-regenerative signals in the DRG cell bodies, demonstrating a direct signaling role for DLK in the proximal axon. Third, the defects in in vitro regeneration in cultured DLK KO neurons occur in the absence of distal axons. Hence, these data support the model that DLK independently promotes axonal regeneration in the proximal axon while facilitating degeneration in the distal axon. Since DLK also promotes neuronal apoptosis (Ghosh et al., 2011; Itoh et al., 2011), it functions as a key component of the neuronal injury response, regulating cell survival, axon regeneration, and axon degeneration.
Our data demonstrate that DLK promotes axonal regeneration by regulating transport of injury-derived signals. These data emphasize that injury signals and their regulators are crucial factors controlling the efficacy of in vivo axonal regeneration to functional targets. Retrograde transport-dependent injury signals including STAT3 fail to be activated upon a CNS axonal lesion (Qiu et al., 2005). We speculate that methods to promote DLK function may spur retrograde transport in CNS axons, mimicking a preconditioning injury and enhancing CNS axon regeneration.
Materials and Methods
Mice
We used 3 month or older adult mice for analysis. Mouse lines are described in Supplemental Information.
Surgical procedures
Animals were anesthetized, a small incision was made unilaterally to expose the sciatic nerve at thigh level, and the sciatic nerve was lesioned by crush, ligation, or transection. The incision was closed with nylon suture and the animals were then housed until they were euthanized and samples taken for analysis.
Immunofluorescence
Dissected tissues were fixed in 4% paraformaldehyde for 2 h, and incubated in 30% sucrose. DRGs and sciatic nerve tissues were then embedded in OCT compound (Tissue-Tek), cryopreserved, and sectioned at 10 µm thickness. Tissue sections were then blocked in blocking solution (10% normal goat serum in PBS with 0.1% Triton X-100 (PBS-T)) at room temperature and subsequently incubated with primary antibodies diluted in blocking solution overnight at 4°C. Samples were then treated with two washes of PBS-T, incubation with secondary antibodies in blocking solution for 2 h at room temperature, three washes in PBS-T, and mounting in VectaShield (Vector Laboratories). Antibodies are listed in Supplementary Information.
Confocal imaging
Samples were imaged with a Nikon D-Eclipse C1 confocal microscope using 10X air or 20X oil objective. Images shown are z projections of confocal stacks acquired from serial laser scanning.
In vitro axon regeneration assay
Adult DRG cultures were prepared as described in Supplementary Information. After overnight (16 h) incubation, cultures were fixed in 4% paraformaldehyde for 20 min and subjected to immunostaining following the same procedure as described in “Immunofluorescence” section. Antibodies to β3 tubulin (Tuj1; Covance) were used to label neurites. Samples were imaged with a light microscope (Nikon eclipse 80i) using a 10× air objective. To assess axon growth, at least 75 neurons were quantified per experimental set. Tuj1-positive axons were traced and their length was measured with the NeuronJ plug-in of ImageJ.
Further Experimental Procedures are available as Supplementary Information.
Supplementary Material
Highlights.
DLK promotes peripheral axon regeneration in mice
DLK is required for accelerated axon growth induced by a preconditioning injury
DLK is required for retrograde transport of axon injury signals
Acknowledgments
We are grateful to Dr. Joshua Sanes and Dr. Lawrence B. Holzman for sharing reagents. We thank the members of the DiAntonio, Cavalli and Milbrandt laboratories for helpful discussions. We also appreciate Dr. Namiko Abe, Dr. Santosh Kale, Alice Tong and Dennis Oakley for their advice, and Sylvia Johnson for her technical assistance. The work was supported by the NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University, the HOPE Center for Neurological Disorders, European Molecular Biology Organization (EMBO) long-term fellowship (B.B.), Edward Mallinckrodt Jr. Foundation (V.C.), NIH grants NS060709 (V.C.), AG13730 (J.M.), and NS070053 and NS065053 (J.M. and A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: A.D., J.E.S., and Washington University may receive income based on a license by the University to Novus Biologicals.
References
- Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LS, Cavalli V. Sunday driver interacts with two distinct classes of axonal organelles. The Journal of biological chemistry. 2009;284:34628–34639. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. The Journal of biological chemistry. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Cavalli V. Nerve injury signaling. Current opinion in neurobiology. 2008;18:276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6282–6287. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. The EMBO journal. 2012 doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Miller BR, Sanes JR, DiAntonio A. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes & development. 2007;21:2593–2606. doi: 10.1101/gad.1592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nature reviews. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. Journal of neurobiology. 1992;23:521–536. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. The Journal of cell biology. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Rzhetskaya M, Kareva T, Bland R, During MJ, Tank AW, Kholodilov N, Burke RE. Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci. 2008;28:672–680. doi: 10.1523/JNEUROSCI.2132-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Fan G, Merritt SE, Kortenjann M, Shaw PE, Holzman LB. Dual leucine zipper-bearing kinase (DLK) activates p46SAPK and p38mapk but not ERK2. The Journal of biological chemistry. 1996;271:24788–24793. doi: 10.1074/jbc.271.40.24788. [DOI] [PubMed] [Google Scholar]
- Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. The Journal of cell biology. 2011;194:751–764. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31:5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science (New York, N.Y. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Hirai S, Cui de F, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banba Y, Ohno S. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci. 2006;26:11992–12002. doi: 10.1523/JNEUROSCI.2272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PN. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Experimental neurology. 2010;223:11–18. doi: 10.1016/j.expneurol.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Horiuchi D, Collins CA, Bhat P, Barkus RV, DiAntonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–1317. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochemical and biophysical research communications. 2009;383:258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Itoh A, Horiuchi M, Wakayama K, Xu J, Bannerman P, Pleasure D, Itoh T. ZPK/DLK, a mitogen-activated protein kinase kinase kinase, is a critical mediator of programmed cell death of motoneurons. J Neurosci. 2011;31:7223–7228. doi: 10.1523/JNEUROSCI.5947-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Oka N, Tachibana H, Akiguchi I, Shibasaki H. Oct6, a transcription factor controlling myelination, is a marker for active nerve regeneration in peripheral neuropathies. Acta neuropathologica. 2003;105:203–208. doi: 10.1007/s00401-002-0630-9. [DOI] [PubMed] [Google Scholar]
- Kelkar N, Gupta S, Dickens M, Davis RJ. Interaction of a mitogen-activated protein kinase signaling module with the neuronal protein JIP3. Molecular and cellular biology. 2000;20:1030–1043. doi: 10.1128/mcb.20.3.1030-1043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. The Journal of experimental medicine. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Dahlin L, Lundborg G, Kanje M. Inhibition of c-Jun phosphorylation reduces axonal outgrowth of adult rat nodose ganglia and dorsal root ganglia sensory neurons. Molecular and cellular neurosciences. 2004;27:267–279. doi: 10.1016/j.mcn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annual review of neuroscience. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Magill CK, Tong A, Kawamura D, Hayashi A, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM. Reinnervation of the tibialis anterior following sciatic nerve crush injury: a confocal microscopic study in transgenic mice. Experimental neurology. 2007;207:64–74. doi: 10.1016/j.expneurol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Lieberman AR, Grenningloh G, Anderson PN. Transcriptional upregulation of SCG10 and CAP-23 is correlated with regeneration of the axons of peripheral and central neurons in vivo. Molecular and cellular neurosciences. 2002;20:595–615. doi: 10.1006/mcne.2002.1140. [DOI] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nature neuroscience. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science (New York, N.Y. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Collins CA. A Conditioning Lesion Protects Axons from Degeneration via the Wallenda/DLK MAP Kinase Signaling Cascade. J Neurosci. 2012;32:610–615. doi: 10.1523/JNEUROSCI.3586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Wang X, Ewanek R, Bhat P, DiAntonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. The Journal of cell biology. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.