Abstract
Objective
To evaluate whether cervicovaginal secretions inhibit HIV-1 infectivity in an in vitro model, and estimate concentration of immune mediators.
Study design
We enrolled mid-trimester pregnant and regularly menstruating (non-pregnant) women. Cervicovaginal lavage (CVL) was collected at 2 visits and incubated with HIV-1 and TZM-bl cells. Infectivity was compared to positive controls. Concentrations of immune mediators were compared between groups.
Results
At enrollment, CVL inhibited IIIB virus 88.2% and 82.4%, and BaL virus 72.8% and 77.9%, among pregnant (n=13) and non-pregnant women (n=9), respectively. At second visit, CVL inhibited IIIB 89.7% and 82.5%, and BaL 77.4% and 69.9% among pregnant (n=15) and non-pregnant women (n=8), respectively (all P ≤ 0.04). Adjusting for body mass index, race, and protein content of CVL, antimicrobials were suppressed but cytokines and chemokines were not markedly different in pregnancy.
Conclusion
Cervicovaginal secretions significantly suppress HIV-1 infectivity in this model. Concentrations of certain immune mediators are altered in pregnancy.
Keywords: cervicovaginal lavage, cervicovaginal secretions, HIV, pregnancy
Introduction
Women account for half of all people living with HIV-1 globally and for 60% of those in sub-Saharan Africa.1 This figure represents a significant overall increase in the number of adult women infected with HIV globally since 2001. The vast majority of incident HIV worldwide is caused by heterosexual intercourse. The lower genital tract is one of the most susceptible areas in the body for HIV acquisition. During vaginal intercourse, women are twice as likely to contract HIV from their male partner as men are from a female partner.2,3 As more women of reproductive age become HIV-infected, they may become pregnant and are at risk of transmitting HIV to their fetus. As a result of infection in women, there are now nearly 2 million children living with HIV, the vast majority of these perinatally infected.1 A large, rigorous study following over 10,000 women done in Rakai, Uganda found that women were at significantly increased risk of HIV acquisition during pregnancy. Data from this community cohort with longitudinal data were analyzed for the incidence rate of HIV during pregnancy, and compared to the incidence rate during periods of non-pregnancy. The incidence rate was 2.3 per 100 person years in pregnancy as compared to 1.1 per 100 person years in non-pregnant women. This difference in incidence rates resulted in an incident rate ratio of HIV acquisition in pregnancy of 2.16 (95% CI 1.39-3.37) after adjusting for age, marital status, education, number of sex partners, genital ulcer disease, and condom use.4 The data are not entirely consistent, however, as several smaller studies have not demonstrated such an association.4-7
The biologic reasons for a potentially increased risk of HIV acquisition during pregnancy have not been elucidated. It has been suggested that mucosal immunity in the genital tract is compromised during pregnancy. Concentrations or expression of certain antimicrobial peptides, cytokines, and chemokines have been shown to be altered under certain conditions in pregnancy, such as bacterial vaginosis8, trichomoniasis9, or premature rupture of membranes10.
A number of studies have examined the use of a TZM-bl indicator assay as an in vitro surrogate of HIV-1 infectivity. This infectivity assay is the World Health Organization (WHO) preferred infectivity assay and is commonly used in HIV vaccine research. It is considered to be more standardized than traditional peripheral blood mononuclear cell (PBMC) infectivity assays.11 The assay has been studied to measure the impact of genital tract secretions on prevention of HIV infectivity but its performance testing CVL from pregnant women has not been explored. 12-14 Our purpose in this study was three-fold. First, we sought to assess whether cervicovaginal lavage (CVL) fluid would suppress HIV-1 infection of target cells differentially in pregnant and non-pregnant women, second, to evaluate whether protective immune mediator concentrations were altered in pregnancy and third, determine whether cytokines, chemokines and anti-HIV molecules results vary when expressed per unit volume versus per unit protein.
Materials and Methods
We enrolled HIV-negative pregnant and non-pregnant women between the ages of 18 and 35 presenting for care at our tertiary care institution. Pregnant women were offered enrollment if they were between 14 and 26 weeks’ gestation as determined by best obstetrical estimate. Non-pregnant women were offered enrollment if they had regular menses for the previous three months. Exclusion criteria were pre-gestational diabetes mellitus, chronic hypertension requiring medications, antibiotic use within two weeks of specimen collection, use of hormonal contraception, current or planned cerclage, planned termination of pregnancy, known fetal anomalies, or symptomatic vaginal discharge requiring doctor visit within two weeks of enrollment.
All participants signed written, informed consent. The study was approved by the Women and Infant's Hospital Institutional Review Board on October 6, 2008, Protocol number 08-0115. At enrollment, baseline data were collected, including demographic information, basic medical and obstetric risks, and vaginal practices. All women underwent cervicovaginal lavage collection performed in a standard manner. 10 cc of normal saline was instilled into the vaginal cavity with the stream directed toward the external os of the cervix. The fluid was allowed to pool in the posterior fornix, and then aspirated. At second study visits, CVL was collected in the same manner. Pregnant women were in the third trimester at the time of follow-up. Non-pregnant women were enrolled during the proliferative phase of the menstrual cycle and follow-up was performed when they were peri-ovulatory. On the same day as collection, CVL was centrifuged at 1500g for 10 minutes and the supernatant was frozen at -80°C until used in the TZM-bl assay.
The HIV-1 strains used, IIIB (X4), a virus that infects via the CXCR4 co-receptor and BaL (R5), which infects via the CCR5 co-receptor, thought to be a more common viral co-receptor for sexual transmission were kindly provided by Dr P. Gupta (University of Pittsburgh, PA). Virus stocks were propagated in PHA-stimulated human PBMC and stored frozen at -80°C. Details on this assay have been previously described.15 The light intensity of each well was measured using a luminometer and expressed as Relative Light Units (RLU). Uninfected cells and cells incubated with CVL only were used to determine background luminescence. HIV-1 incubated in media alone prior to adding it to the TZM-bl cells was used as positive control. TZM-bl cells were incubated with secretions alone and media alone were used a negative controls and determination of background values. Viability of TZM-bl cells upon treatment with CVL was quantified using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega) according to manufacturer's instructions.
The relative light units were expressed as median values, percent inhibition as compared to virus-only positive control set at 100%, and after adjustment for background luminescence. Comparisons were made between pregnant and non-pregnant groups by Wilcoxon rank sum test, and each group was tested against the positive control. The van Elteren test was used to compare medians adjusting for body mass index and race. Statistical significance was considered p<0.05.
Antimicrobial peptides, cytokines, and chemokines that have been previously shown to have an impact on HIV infectivity were measured in the CVL of pregnant and non-pregnant women. Secretory leukocyte protease inhibitor (SLPI), macrophage inflammatory protein-3-alpha (MIP)-3α, and elafin were measured using ELISA kits from R&D Systems (Minneapolis, MN) according to manufacturer's instructions. Standards for each ELISA were re-suspended in phosphate buffered saline (PBS). Samples were diluted in 1xPBS. Antimicrobials were quantified based on standard curves obtained using an ELISA reader (Dynex, Chantilly, VA). Human beta defensin (HBD)2 was assayed using ELISA test kit from PeproTech (Rocky Hill, NJ) according to manufacturer's instructions. CVL were assayed for 14 different chemokines and cytokines (BioRad, Hercules, CA), using a multiplex bead assay (Luminex Corp., Austin, TX) as previously described.16 Total protein concentration in each CVL sample was determined using the BCA Protein Assay kit from Fisher Scientific, according to manufacturer's instructions. Concentration of each molecule was expressed as picograms/ml as well as per 10 micrograms of protein and compared between pregnant and non-pregnant women. The van Elteren test was used to compare concentrations between groups after controlling for BMI and race.
Results
A total of 31 subjects were enrolled in the study, 20 pregnant and 11 non-pregnant. Thus, we obtained 13 usable samples from women pregnant in the second trimester, 15 in the third trimester, and 17 samples from non-pregnant women at various stages of the menstrual cycle. In the latter group, sample numbers were too low to allow cycle dependent stratification. Pregnant women were slightly older, but of similar race, with the majority being Caucasian. Nearly all participants had completed high school. Pregnant women were more likely to be married (Table 1). Using the TZM-bl assay, we investigated whether anti-HIV activity in CVL is similar in pregnancy as we have shown in non-pregnant women15. As seen in Tables 2 and 3, when compared to non-pregnant samples, CVL from pregnant subjects collected at enrollment and second visits markedly suppressed infectivity of both X4 and R5 viral strains. These studies indicated further that inhibitory capacity was slightly lower, but not dramatically so, in the presence of IIIB (Figure 1A and C) and Bal (Figure 1B and D) virus in CVL collected at Enrollment or at Return visit. Within the limits of the sample size, no difference was detected in infectivity between visits for a given viral strain, or for viral inhibition between pregnant and non-pregnant women, (not shown).
Table 1.
Demographic characteristics of all enrolled participants
| Pregnant (n=13) | Non-pregnant (n=9) | |
|---|---|---|
| Age in years | 24 (18-33) | 21 (18-35) |
| Gestational age in weeks | 23.5 (15-26) | Not applicable |
| Race | ||
| Black/African-American | 2 (11.1) | 2 (18.2) |
| White | 12 (66.7) | 7 (63.6) |
| Other/More than one race | 4 (22.2) | 2 (18.2) |
| Insurance | ||
| Private | 6 (30.0) | 6 (54.6) |
| Medicaid | 8 (40.0) | 0 |
| Other | 4 (20.0) | 0 |
| Uninsured | 2 (10.0) | 5 (45.5) |
| Marital status | ||
| Single | 10 (50.0) | 9 (81.8) |
| Married | 10 (50.0) | 2 (18.2) |
| Education level | ||
| <High school | 2 (10.0) | 0 |
| High school/equivalent | 8 (40.0) | 1 (9.1) |
| Some college | 8 (40.0) | 8 (72.7) |
| College graduate | 2 (10.0) | 2 (18.2) |
| Employment | ||
| Unemployed | 8 (40.0) | 2 (18.2) |
| Employed full-time | 6 (30.0) | 4 (36.4) |
| Employed part-time | 5 (25.0) | 5 (45.5) |
| Other | 1 (5.0) | 0 |
| Income level | ||
| <$10,000 | 2 (11.1) | 4 (36.4) |
| $10,000-24,999 | 8 (44.4) | 1 (9.1) |
| $25,000-49,999 | 4 (22.2) | 3 (27.3) |
| $50,000+ | 4 (22.2) | 3 (27.3) |
| Body Mass Index (kg/m2) | 25.9 (20.1-40.5) | 24.4 (20.6-39.8) |
Data presented as median (range) or n(%)
Table 2.
HIV-1 infectivity in the presence of CVL as compared to positive control at enrollment
| Pregnant (n=13) Median (Range) |
P* | Non-pregnant (n=9) Median (Range) |
P* | |
|---|---|---|---|---|
| Illb % inhibition | 88.2% (21.3 – 99.1) | 0.0002 | 82.4% (-10.4 – 93.9) | 0.02 |
| BaL % inhibition | 72.8% (-1.7 – 95.7) | 0.0005 | 77.9% (-7.2 – 91.3) | 0.04 |
p value for comparison to no inhibition (positive control) by signed rank test
Table 3.
HIV-1 infectivity in the presence of CVL as compared to positive control at return visit
| Pregnant (n=15) Median (Range) |
P | Non-pregnant (n=8) Median (Range) |
P | |
|---|---|---|---|---|
| Illb % inhibition | 89.7% (-19.1 – 98.3) | 0.0002 | 82.5% (-1.8 – 91.1) | 0.02 |
| BaL % inhibition | 77.4% (-11.1 – 97.5) | 0.004 | 69.9% (-10.3 – 83.1) | 0.04 |
* p value for comparison to no inhibition (positive control) by signed rank test
Figure 1. Median percent inhibition by pregnancy status.
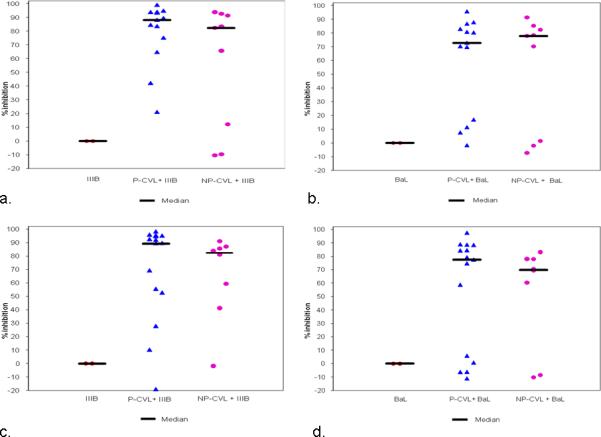
Each data point in the graph represents one individual patient sample. In the positive control (virus only) column, each point represents replicate wells and no inhibition of infectivity is noted. When CVL from pregnant and non-pregnant women is added, infectivity is inhibited.
a: Enrollment visit using IIIB virus
b: Enrollment visit using BaL virus
c: Second study visit using IIIB virus
d: Second study visit using BaL virus
P-CVL: pregnant women cervicovaginal lavage fluid, NP-CVL: non-pregnant women cervicovaginal lavage fluid
To understand whether immune parameters in CVL change with pregnancy, an analysis was carried out in which CVL from non-pregnant and pregnant women were analyzed for antimicrobial, cytokine and chemokine levels. As seen in Tables 4 and 5, data are expressed both as pg/ml (shaded boxes) and pg/10μg protein (clear boxes). Data are presented in both ways owing to our finding of an elevation in protein concentration that occurs in CVL during pregnancy. Overall protein concentration was significantly higher among pregnant women, median 139.8 μg/ml (range: 48.3-514.2) in CVL compared to 22.6 μg/ml(range: 1.0-1139.5) (p=0.03) in CVL from non-pregnant women after controlling for race and body mass index.
Table 4.
Immune mediators by pregnancy status at enrollment (n=22)
| Median (Range) | ||||
|---|---|---|---|---|
| Pregnant (n=13) | Non-pregnant (n=9) | P1 | P2 | |
| Gestational age in weeks | 23.5 (15-26) | |||
| RANTES | ||||
| (pg/ml) | 6.0 (4.2-92.2) | 5.1 (4.2-17.9) | 0.03 | 0.05 |
| (pg/10μg protein) | 0.8 (0.2-2.8) | 1.9 (0.2-50.6) | 0.1 | 0.1 |
| Eotaxin | ||||
| (pg/ml) | 28.4 (0-136.1) | 18.0 (0-92.3) | 0.2 | 0.1 |
| (pg/10μg protein) | 2.5 (0-6.7) | 8.1 (0-66.7) | 0.06 | 0.2 |
| Fractalkine | ||||
| (pg/ml) | 86.7 (44.2-1475.7) | 44.2 (44.2-351.1) | 0.1 | 0.09 |
| (pg/10μg protein) | 8.5 (1.6-37.4) | 29.7 (1.8-440.2) | 0.1 | 0.09 |
| G-CSF | ||||
| (pg/ml) | 12.1 (3.5-2795.6) | 6.7 (3.5-24447.5) | 0.1 | 0.1 |
| (pg/10μg protein) | 1.7 (0.5-73.9) | 7.2 (0.7-214.5) | 0.2 | 0.6 |
| IL-1α | ||||
| (pg/ml) | 56.2 (9.9-1812.2) | 6.5 (2.7-1838.5) | 0.02 | 0.03 |
| (pg/10μg protein) | 4.0 (0.8-35.2) | 4.4 (1.9-26.9) | 0.9 | 0.7 |
| IL-1RA | ||||
| (pg/ml) | 24588.8 (1991.5-84614.6) | 776.4 (0.1-57124.8) | 0.03 | 0.03 |
| (pg/10μg protein) | 1283.0 (289.3-5908.1) | 405.1 (0.3-2133.9) | 0.06 | 0.2 |
| IL-6 | ||||
| (pg/ml) | 6.3 (4.5-103.9) | 4.9 (4.0-751.6) | 0.2 | 0.2 |
| (pg/10μg protein) | 0.9 (0.3-3.5) | 4.4 (0.8-39.8) | 0.01 | 0.03 |
| IL-8 | ||||
| (pg/ml) | 243.4 (0-4443.1) | 32.9 (0-36052.4) | 0.3 | 0.3 |
| (pg/10μg protein) | 13.0 (0-143.5) | 6.3 (0-316.4) | 0.7 | 0.9 |
| IP-10 | ||||
| (pg/ml) | 165.0 (0-933.5) | 0 (0-2970.4) | 0.2 | 0.1 |
| (pg/10μg protein) | 10.1 (0-132.3) | 0 (0-27.0) | 0.1 | 0.1 |
| MCP-1 | ||||
| (pg/ml) | 18.2 (4.2-94.6) | 8.5 (4.2-890.5) | 0.8 | 0.5 |
| (pg/10μg protein) | 0.9 (0.6-2.6) | 7.4 (2.0-48.2) | 0.002 | 0.001 |
| MIP-1α | ||||
| (pg/ml) | 30.2 (13.4-108.2) | 19.6 (2.6-455.0) | 0.3 | 0.2 |
| (pg/10μg protein) | 2.2 (0.9-8.3) | 7.8 (1.2-176.0) | 0.04 | 0.09 |
| MIP-1β | ||||
| (pg/ml) | 20.4 (0-585.3) | 7.3 (0-660.8) | 0.3 | 0.1 |
| (pg/10μg protein) | 1.9 (0-11.4) | 2.4 (0-72.7) | 0.6 | 0.6 |
| TNF-α | ||||
| (pg/ml) | 0 (0-145.8) | 0 (0-13.0) | 0.3 | 0.1 |
| (pg/10μg protein) | 0 (0-2.8) | 0 (0-0.1) | 0.2 | 0.1 |
| GM-CSF | ||||
| (pg/ml) | 0.2 (0-91.8) | 0 (0-6.1) | 0.2 | 0.3 |
| (pg/10μg protein) | 0.1 (0-1.8) | 0 (0-1.2) | 0.1 | 0.1 |
| Elafin | ||||
| (pg/ml) | 32310 (7510-79160) | 35930 (12270-89800) | 0.7 | 0.5 |
| (pg/10μg protein) | 3749.1 (146.1-7503.0) | 25718.6 (162.5-181990.0) | 0.02 | 0.03 |
| SLPI | ||||
| (pg/ml) | 58426 (0-224000) | 18304 (3404-230000) | 0.4 | 0.4 |
| (pg/10μg protein) | 2790.8 (0-21060.9) | 9500.4 (1842.9-77655.4) | 0.06 | 0.07 |
| HBD2 | ||||
| (pg/ml) | 710 (100-8220) | 1110 (30-9650) | 0.6 | 0.3 |
| (pg/10μg protein) | 35.1 (8.8-1164.9) | 211.1 (39.3-4265.4) | 0.03 | 0.03 |
| MIP-3α | ||||
| (pg/ml) | 24 (4-660) | 40 (4-3420) | 1.0 | 0.8 |
| (pg/10μg protein) | 1.4 (0.1-24.5) | 22.5 (0.8-42.7) | 0.01 | 0.04 |
P-value by Wilcoxon rank-sum test for difference between groups.
P-value by van Elteren test for difference between groups, adjusting for overweight BMI & White race.
RANTES: Regulated on Activation, Normal T Expressed and Secreted; G-CSF: granulocyte-colony stimulating factor; IL: interleukin; RA: receptor antagonist; IP: interferon-inducible protein; MCP: monocyte chemoattractant protein; TNF: tumor necrosis factor; GM: granulocyte macrophage; SLPI: Secretory leukocyte protease inhibitor; HBD: human beta defensin; MIP: macrophage inflammatory protein
Table 5.
Immune mediators by pregnancy status at return visit 1 (n=23)*
| Median (Range) | ||||
|---|---|---|---|---|
| Pregnant (n=15) | Non-pregnant (n=8) | P1 | P2 | |
| Gestational age in weeks | 34 (28-38) | -- | -- | -- |
| RANTES | ||||
| (pg/ml) | 6.0 (4.2-47.2) | 5.1 (4.2-28.3) | 0.2 | 0.1 |
| (pg/10μg protein) | 0.9 (0.1-2.1) | 3.1 (1.3-42.3) | 0.004 | 0.01 |
| Eotaxin | ||||
| (pg/ml) | 20.9 (0-133.6) | 17.1 (0-48.9) | 0.4 | 0.3 |
| (pg/10μg protein) | 2.9 (0-12.6) | 6.0 (0-41.1) | 0.04 | 0.1 |
| Fractalkine | ||||
| (pg/ml) | 44.2 (0-625.2) | 44.2 (0-360.4) | 0.8 | 0.2 |
| (pg/10μg protein) | 9.0 (0-22.5) | 37.1 (0-451.4) | 0.02 | 0.1 |
| G-CSF | ||||
| (pg/ml) | 8.5 (0-14202.9) | 6.7 (3.5-54.2) | 0.1 | 0.3 |
| (pg/10μg protein) | 1.2 (0-205.3) | 4.5 (1.1-67.9) | 0.08 | 0.5 |
| IL-1α | ||||
| (pg/ml) | 49.1 (5.9-2193.1) | 6.9 (2.7-41.3) | 0.01 | 0.01 |
| (pg/10μg protein) | 2.7 (0.7-31.7) | 4.3 (0.7-33.8) | 0.7 | 0.3 |
| IL-1RA | ||||
| (pg/ml) | 40898.3 (408.4-85612.1) | 442.7 (0-14440.9) | 0.002 | 0.01 |
| (pg/10μg protein) | 3012.9 (161.5-7003.0) | 272 (0-5929.3) | 0.01 | 0.01 |
| IL-6 | ||||
| (pg/ml) | 4.5 (2.9-70.8) | 4.2 (3.5-29.9) | 0.5 | 0.3 |
| (pg/10μg protein) | 0.8 (0.1-2.2) | 3.3 (1.2-40.8) | 0.006 | 0.04 |
| IL-8 | ||||
| (pg/ml) | 118.0 (0-4100.3) | 25.8 (0-778.1) | 0.1 | 0.4 |
| (pg/10μg protein) | 11.4 (0-281.9) | 13.7 (0-104.3) | 0.8 | 0.7 |
| IP-10 | ||||
| (pg/ml) | 165.0 (0-2141.8) | 0 (0-144.3) | 0.06 | 0.01 |
| (pg/10μg protein) | 8.0 (0-82.9) | 0 (0-19.4) | 0.1 | 0.09 |
| MCP-1 | ||||
| (pg/ml) | 7.6 (2.8-103.0) | 6.5 (4.2-20.9) | 0.7 | 0.7 |
| (pg/10μg protein) | 0.8 (0.1-2.7) | 3.3 (1.3-42.4) | 0.006 | 0.008 |
| MIP-1α | ||||
| (pg/ml) | 30.2 (0-103.5) | 21.1 (0-66.6) | 0.2 | 0.2 |
| (pg/10μg protein) | 3.9 (0-17.3) | 8.7 (0-77.3) | 0.09 | 0.3 |
| MIP-1β | ||||
| (pg/ml) | 13.6 (0-267.8) | 10.5 (0-103.3) | 0.4 | 0.09 |
| (pg/10μg protein) | 2.4 (0.1-7.7) | 7.7 (0-13.8) | 0.2 | 0.6 |
| TNF-α | ||||
| (pg/ml) | 0 (0-59.9) | 0 (0-23.2) | 1.0 | 0.2 |
| (pg/10μg protein) | 0 (0-1.6) | 0 (0-3.1) | 0.8 | 0.8 |
| GM-CSF | ||||
| (pg/ml) | 1.2 (0-27.6) | 0 (0-5.6) | 0.2 | 0.04 |
| (pg/10μg protein) | 0.2 (0-1.0) | 0 (0-0.8) | 0.5 | 0.06 |
| Elafin | ||||
| (pg/ml) | 29390 (12270-81700) | 28405 (18240-54900) | 0.5 | 0.6 |
| (pg/10μg protein) | 3025.2 (186.9-12345.1) | 15375 (4184.5-186122.4) | 0.02 | 0.04 |
| SLPI | ||||
| (pg/ml) | 31616 (0-68672) | 22759 (6586-98452) | 1.0 | 0.5 |
| (pg/10μg protein) | 2551.5 (0-21898.6) | 11764.9 (2069.4-146571.4) | 0.02 | 0.1 |
| HBD2 | ||||
| (pg/ml) | 900 (10-8740) | 640 (80-5140) | 0.8 | 0.8 |
| (pg/10μg protein) | 36.8 (3.5-1239.4) | 451 (69.5-2110.4) | 0.03 | 0.004 |
| MIP-3α | ||||
| (pg/ml) | 4 (4-104) | 16 (4-5140) | 0.1 | 0.2 |
| (pg/10μg protein) | 1.6 (0.1-15.3) | 16.3 (1.1-2110.4) | 0.01 | 0.04 |
P-value by Wilcoxon rank-sum test for difference between groups.
P-value by van Elteren test for difference between groups, adjusting for overweight BMI & White race.
RANTES: Regulated on Activation, Normal T Expressed and Secreted; G-CSF: granulocyte-colony stimulating factor; IL: interleukin; RA: receptor antagonist; IP: interferon-inducible protein; MCP: monocyte chemoattractant protein; TNF: tumor necrosis factor; GM: granulocyte macrophage; SLPI: Secretory leukocyte protease inhibitor; HBD: human beta defensin; MIP: macrophage inflammatory protein
As seen in Tables 4 and 5, when cytokines and chemokines are expressed as pg/ml, and analyzed by Wilcoxon Rank-sum test and van Elteren test, Regulated on Activation, Normal T Expressed and Secreted (RANTES), Interleukin (IL)-1α, and IL-1 receptor antagonist (RA) are significantly higher in CVL from pregnant when compared to non-pregnant women. The remainder of cytokines and chemokines were unchanged with the exception of granulocyte macrophage colony stimulating factor (GM-CSF), which increased in return visit samples (Table 5). As a part of these studies key antimicrobials were analyzed. When expressed as pg/ml, no differences were seen in the concentrations of Elafin, SLPI, HBD2 or MIP3α.
In contrast to our CVL findings expressed per unit volume (pg/ml), we found that when results were expressed based on CVL protein content that marked changes were seen in a number of compounds. For example, as shown in Table 4, IL-6, monocyte chemoattractant protein (MCP)-1, MIP-1α, Elafin, HBD-2 and MIP-3α in CVL from pregnant women were significantly lower than that seen in non-pregnant women. In contrast, as seen in Table 5, RANTES, Eotaxin, Fractalkine, IL-6, MCP-1, Elafin, SLPI, HBD2 and MIP-3α in CVL from pregnant women were lower when compared to non-pregnant CVL. Overall, unlike Elafin, HBD2, and MIP-3α, which were not decreased when expressed as pg/ml, all three were significantly decreased in pregnant women after controlling for protein content.
Comment
In this study, we found that genital secretions collected from both pregnant and non-pregnant women suppress HIV-1 infectivity in an in vitro model and to a similar degree. To our knowledge, this is the first comparison of intrinsic anti-HIV activity and concentrations of anti-HIV antimicrobials in the CVL of second and third trimester pregnant and non-pregnant women. Our data suggest that genital secretions contain anti-viral factors including SLPI, Elafin, MIP-3α and HBD2 that inhibit HIV infection and/or replication
Our approach to studying HIV-1 infection among pregnant women is novel because it focuses on the point-of-entry into the body, the genital tract. Given that the majority of incident HIV occurs through heterosexual contact, recent studies have begun focusing on ways to understand and prevent infection at the level of the genital tract. Vaginal microbicides seem like a logical choice for prevention but thus far, their effectiveness has not been proven. Research such as ours highlights the potential to develop preventive therapies using natural immune mediators, possibly in the form of microbicides. The recent publication of tenofovir vaginal gel as an effective means of HIV-1 prevention is highly promising.17 However, use of this product in pregnant women has yet to be established and would mean exposing a fetus to a medication without a current medical indication for use. The recent cessation of a large clinical trial of tenofovir gel has made this area even more controversial18.
Our study did not demonstrate that anti-HIV activity in the CVL of pregnant women during the second and third trimester is different from that seen in CVL from non-pregnant women. There are a number of reasons that could account for this. For example, since the epidemiologic finding of increased risk of HIV acquisition in pregnancy controlled for behavioral factors4, it suggests that the underlying mechanism may be biological rather than behavioral. Alternatively, while past studies included women throughout pregnancy, our study analyzed CVL exclusively from women between 14 and 38 weeks (2nd and 3rd trimester). Whether susceptibility to HIV infection and other sexually transmitted infections is elevated early in pregnancy remains to be determined and is under investigation by our group.
Another contributing factor to increased risk of HIV infection is the bacterial content in the lower genital tract of pregnant women. While much needs to be learned about the effect of sex hormones and pregnancy on the vaginal ecosystem, it has been shown that early disruption of the immune milieu in pregnancy is associated with infectious complications of pregnancy later in gestation.19 Our study excluded women with symptomatic vaginal discharge. In doing so, we may have eliminated that portion of the population at risk, and who might have compromised lower genital tract immune protection. Further studies are needed to determine whether baseline bacterial populations differ between women in our study and the Rakai cohort and whether subsequent infectious risk is the same in different study populations.
An alternative explanation is that we used two laboratory-adapted viral strains, one that is a CXCR4-tropic virus and one that is CCR5-tropic. Therefore, we cannot exclude the possibility that other viral strains, especially primary or transmitted/founder viruses, may infect differently depending on the biological conditions during pregnancy that differ from that in the non-pregnant lower genital tract.20 In previous studies, our group found that innate anti-HIV activity in CVL of healthy non-pregnant HIV-infected and un-infected women varied with the viral strain analyzed.15 Further studies are needed to define the extent to which CVL from pregnant women inhibit different viral strains. Our study wasn't designed to test this but it is possible that certain viral strains may be more infectious during pregnancy than in the non-pregnant milieu.
Antimicrobial peptides, cytokines, and chemokines are important effectors of innate immune protection within the female lower genital tract. Some recruit immune cells and others directly target microbes. Many of these molecules are known to have potent anti-HIV activity.21-25 Previous work by our group showed that CVL from healthy HIV-infected and uninfected non-pregnant women inhibit infection of HIV target cells.15 We reported a positive correlation with certain antimicrobials including MIP-3α and HBD2 in CVL and anti-HIV-1 activity. We suspect that similar antimicrobial peptides function to inhibit infectivity in the CVL of pregnant women as well. In the present study, we found that concentrations of MIP-3α and HBD2 were the same as that measured in CVL from non-pregnant women when expressed per unit volume. Whether these molecules are responsible for the anti-HIV activity seen in CVL from pregnant women remains to be determined given that more than 20 antimicrobials have been identified in CVL .16 Previously, we found that MIP-3α and HBD2 levels in CVL vary with stage of the menstrual cycle, thereby contributing to a window of vulnerability when women are more likely to be at risk of infection by HIV and other STI. We reported that levels of HBD2 range from a low of 600 pg/ml at midcycle to 1600 pg/ml during the early proliferative and late secretory phase of the menstrual cycle. 26 Our finding that these values are comparable to those measured in our samples during pregnancy (640-1100pg/ml) suggests that pregnancy levels are comparable to that seen during the menstrual cycle when innate protection against HIV and other STI is elevated. Further studies are needed to identify the key immune factors responsible for protection during pregnancy.
An unexpected finding in the present study was the pronounced increase in protein in the CVL from pregnant women. As seen in Tables 4 and 5, when results are expressed in terms of protein content, antimicrobials as well as a number of cytokine and chemokine concentrations are diminished during pregnancy relative to that seen in CVL from non-pregnant women. Our results are presented in both ways for completeness. What remains incompletely elucidated is why protein levels increase sharply with pregnancy. Whether these proteins alter immune function is an unanswered question. One source of protein in CVL during pregnancy may be due to altered genital tract secretion resulting from hormone levels that are distinct from that seen in the non-pregnant women. Alternatively, the protein content of the cervical mucous plug may be high in protein content. Whatever the reason, further studies are needed to understand the underlying mechanisms of elevated protein levels in CVL during pregnancy and whether immune protection is altered as a result of these changes.
Because the body needs to tolerate the foreign paternal antigens in a developing fetus, maternal immunity is altered during pregnancy. This has been shown in previous studies of genital immunity in pregnancy and could be a plausible explanation for the epidemiologic phenomena demonstrating an increased risk of HIV acquisition in pregnancy.27,28 This is an area of controversy because there are a number of elements of maternal immunity that remain intact throughout gestation so while maternal immunity may be altered, it is not likely to be suppressed per se.29 The difference in concentration in these antimicrobial peptides between people more or less susceptible to infection in this in vitro model is the topic of planned future work. To the best of our knowledge, this is the first study to demonstrate that secretions from the lower genital tract of pregnant women are capable of potent anti-HIV activity. It further suggests that immune mediators play a role in protecting pregnant and non-pregnant women. Determining which factor or factors are responsible for preventing HIV infection could lead to therapeutic approaches in the form of a vaginal microbicide utilizing a woman's own immunity. Future work will examine the differences between pregnant and non-pregnant women with this goal in mind.
Acknowledgments
Funding: 1K23HD062340-01 (Anderson-PI), AI071761 (Wira-PI), and K24 AI066884 (Cu-Uvin-PI) does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: None of the authors has any relevant financial conflicts of interest
Presentation information: Portions of this work presented in abstract form at: 30th Annual Meeting of the Society for Maternal Fetal Medicine, February, 2010, Chicago, IL and the 37th Annual Meeting for the Infectious Disease Society for Obstetrics and Gynecology, Santa Fe, NM, August, 2010.
References
- 1.AIDS Epidemic Update. 2008 (Accessed at http://data.unaids.org/pub/GlobalReport/2008/jc1510_2008_global_report_pp29_62_en.pdf.)
- 2.Padian NS, Shiboski SC, Jewell NP. Female-to-male transmission of human immunodeficiency virus. JAMA. 1991;266:1664–7. [PubMed] [Google Scholar]
- 3.European Study Group on Heterosexual Transmission of HIV Comparison of female to male and male to female transmission of HIV in 563 stable couples. British Medical Journal. 1992;304:809–13. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 5.Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1027–34. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 6.Patterson KB, Leone PA, Fiscus SA, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007;21:2303–8. doi: 10.1097/QAD.0b013e3282f155da. [DOI] [PubMed] [Google Scholar]
- 7.Reid SE, Dai JY, Wang J, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2010;53:606–13. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock SJ, Duthie L, Tremaine T, Calder AA, Kelly RW, Riley SC. Elafin (SKALP/Trappin-2/proteinase inhibitor-3) Is Produced by the Cervix in Pregnancy and Cervicovaginal Levels Are Diminished in Bacterial Vaginosis. Reproductive Sciences. 2009;16:1125–34. doi: 10.1177/1933719109341998. [DOI] [PubMed] [Google Scholar]
- 9.Draper DL, Landers DV, Krohn MA, Hillier SL, Wiesenfeld HC, Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol. 2000;183:1243–8. doi: 10.1067/mob.2000.107383. [DOI] [PubMed] [Google Scholar]
- 10.Tromp G, Kuivaniemi H, Romero R, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. American Journal of Obstetrics & Gynecology. 2004;191:1331–8. doi: 10.1016/j.ajog.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Polonis VR, Brown BK, Borges AR, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375:315–20. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh M, Shen Z, Schaefer T,M, Fahey J,V, Gupta P, Wira C,R. CCL20/MIP3alpha is a Novel Anti-HIV-1 Molecule of the Human Female Reproductive Tract. American Journal of Reproductive Immunology. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller MJ, Mesquita PMM, Torres NM, et al. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS ONE [Electronic Resource] 2010;5:e8781. doi: 10.1371/journal.pone.0008781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mselle TF, Howell AL, Ghosh M, Wira CR, Sentman CL. Human Uterine Natural Killer Cells but Not Blood Natural Killer Cells Inhibit Human Immunodeficiency Virus Type 1 Infection by Secretion of CXCL12. J Virol. 2009;83:11188–95. doi: 10.1128/JVI.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh M, Fahey JV, Shen Z, et al. Anti-HIV Activity in Cervical-Vaginal Secretions from HIV-Positive and -Negative Women Correlate with Innate Antimicrobial Levels and IgG Antibodies. PLoS ONE. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wira CR, Fahey JV, Ghosh M, et al. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. American Journal of Reproductive Immunology. 2010;63:544–65. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Straten Aa, van Damme Lb, Haberer JEc, Bangsberg DRd. How well does PREP work? Unraveling the divergent results of PrEP trials for HIV prevention. AIDS. 2012 doi: 10.1097/QAD.0b013e3283522272. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Simhan HN, Caritis SN, Krohn MA, Martinez de Tejada B, Landers DV, Hillier SL. Decreased cervical proinflammatory cytokines permit subsequent upper genital tract infection during pregnancy. Am J Obstet Gynecol. 2003;189:560–7. doi: 10.1067/s0002-9378(03)00518-0. [DOI] [PubMed] [Google Scholar]
- 20.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–17. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau T, Baranger K, Dadé S, Dallet-Choisy S, Guyot N, Zani M-L. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie. 2008;90:284–95. doi: 10.1016/j.biochi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Cole AM. Innate host defense of human vaginal and cervical mucosae. Current Topics in Microbiology & Immunology. 2006;306:199–230. [PubMed] [Google Scholar]
- 23.Cole AM, Cole AL. Antimicrobial Polypeptides are Key Anti-HIV-1 Effector Molecules of Cervicovaginal Host Defense. American Journal of Reproductive Immunology. 2008;59:27–34. doi: 10.1111/j.1600-0897.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3α is a Novel Anti-HIV-1 Molecule of the Human Female Reproductive Tract. American Journal of Reproductive Immunology. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2009;129:207–19. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keller MJ, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–76. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 27.Beigi RH, Yudin MH, Cosentino L, Meyn LA, Hillier SL. Cytokines, pregnancy, and bacterial vaginosis: comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis. Journal of Infectious Diseases. 2007;196:1355–60. doi: 10.1086/521628. [DOI] [PubMed] [Google Scholar]
- 28.Donders GG, Vereecken A, Bosmans E, Spitz B. Vaginal cytokines in normal pregnancy. American Journal of Obstetrics & Gynecology. 2003;189:1433–8. doi: 10.1067/s0002-9378(03)00653-7. [DOI] [PubMed] [Google Scholar]
- 29.Mor G, Abrahams VM. The Immunology of Pregnancy. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, editors. Creasy & Resnik's Maternal-Fetal Medicine. Saunders Elsevier; Philadelphia, PA: 2009. pp. 87–99. [Google Scholar]


