Abstract
STUDY DESIGN
Prospective cohort study
OBJECTIVES
To characterize knee cartilage change in individuals with knee osteoarthritis (KOA) who have completed a therapeutic exercise program.
BACKGROUND
While therapeutic exercise is frequently used successfully to improve pain and function in individuals with KOA, no studies have reported the volume of cartilage change, or individual factors that may impact volume of cartilage change, in those completing an exercise program for KOA.
METHODS
13 individuals with KOA underwent magnetic resonance imaging (MR) to quantify cartilage volume change for the weight-bearing regions of the medial and lateral femoral condyles and the entire surface of the tibial plateaus from baseline to 1-year follow-up. Measurements of body structure and function and activity levels/limitations such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Physical Activity Scale for the Elderly (PASE) were performed prior to the therapeutic exercise program. At 6 months from baseline follow-up clinical measurements of knee strength and motion were performed. At 1-year from baseline, imaging of the knee cartilage, knee alignment, and the WOMAC and PASE questionnaires were completed.
RESULTS
The central region of the medial femoral condyle (cMF) had a median volume of cartilage loss of 3.8%. The other 3 knee tibiofemoral articular surfaces had minimal median cartilage volume change. Individuals were dichotomized into progressors (n=6) and non-progressors (n=7) based on the standard error of measurement (SEM) of cartilage volume change for the cMF. Progressors were younger, had a larger body mass index, had a higher Kellgren-Lawrence grade in the medial compartment of the knee, and had a greater increase in knee varus alignment from baseline to 1-year follow-up. The progressors also had frontal plane hip and knee kinetics during baseline gait analysis that potentially increase medial knee joint loading.
CONCLUSION
The loss of cMF cartilage volume was highly variable and the median loss of cartilage was within the range previously reported. Seven of the 13 individuals did not have cMF cartilage volume loss greater than the SEM. Change in cartilage volume of the cMF may be influenced to a greater extent by personal factors than by completion of a therapeutic exercise program. Additional research is needed to decipher the interactions among therapeutic exercise and personal characteristics that impact knee cartilage loss.
Keywords: arthritis, biomechanics, magnetic resonance imaging, MRI
BACKGROUND
The prevalence of knee osteoarthritis (KOA) has been reported to be between 19% to 28% in adults older than 45 years of age, and as high as 37% in adults over the age of 60.39 In total, over 9 million adults in the United States are believed to have symptomatic KOA.39 A myriad of negative sequelae, including impairments of knee strength and motion, knee pain, knee instability, limited functional abilities, and increased disability have been well documented in individuals with KOA.22,28,31,32,43,52,53 In addition to impairments and functional limitations associated with KOA, an increased rate of articular cartilage loss at the knee, as measured by quantitative magnetic resonance imaging (MR), has been shown to be a predictor of subsequent total knee arthroplasty.15 Therefore, treatments that slow the rate of knee cartilage loss may delay or potentially prevent knee arthroplasty. Therapeutic exercise may be one such option to positively change the rate of knee cartilage loss in individuals with KOA.
To treat both the impairments and functional limitations that result from KOA, therapeutic exercise is often recommended. Therapeutic exercise has been shown to be effective in reducing pain and improving function in patients with KOA and is recommended by the American College of Rheumatology (ACR) in their clinical guideline for treatment of individuals with KOA.31,35,35,40,55 Although therapeutic exercise improves the functional abilities and symptomatology of those with KOA,25 whether therapeutic exercise affects the rate of articular cartilage loss at the knee in persons with KOA is currently unknown.
While basic science research and animal studies have shown that cartilage synthesis responds favorably to loading,26,27,44 human MR imaging studies investigating loss of knee cartilage in individuals with KOA have reported an increased loss of tibiofemoral cartilage with factors that increase loading.20,49,50 Individuals with KOA have been shown to have an increased knee adduction moment during gait compared to those without KOA, even when gait speed is standardized.5 While a predominantly weight-bearing therapeutic exercise program increases loading through the knee, it has also been shown to improve quadriceps strength, knee range of motion, and joint proprioception.35,36 Improved quadriceps strength, increased knee joint motion, and heightened joint proprioception resulting from a therapeutic exercise program may have a protective effect on the cartilage of the knee and reduce the rate of cartilage loss. To determine if therapeutic exercise has a protective effect on knee cartilage, the changes in knee cartilage found in individuals with KOA completing a therapeutic exercise program must be compared to the natural rate of cartilage loss in individuals with KOA.14,19,45–47
Loss of knee joint cartilage may be influenced by many factors. Evidence of gender, age, body mass index (BMI), medial meniscal damage, and knee alignment as factors impacting the rate of cartilage loss has previously been reported.19,20,45–47,49 Abnormal variations in gait pattern and the resulting hip and knee kinetics may also serve as risk factors for knee cartilage loss in those with KOA. Gait analysis studies have shown that individuals with KOA exhibit biomechanical risk factors for knee cartilage loss such as increased knee adduction moments, decreased hip adduction moments, and increased rates of loading across the knee joint.5,10,13,33 Knee adduction moments are used as a surrogate measurement for loading of the medial compartment of the knee, with increased knee adduction moments equating to increased medial compartment loading.34 At the hip, external hip adduction moments are maintained in equilibrium by contraction of the hip abductor musculature. In a prior study measuring hip adductor moments during gait, stronger hip abductor muscles were shown to slow the progression of medial joint space narrowing in individuals with medial KOA.13 To our knowledge, there are currently no longitudinal studies that have examined both lower extremity gait kinetics and the rate of knee cartilage loss simultaneously in patients with KOA.
Despite the common prescription of exercise programs to individuals with KOA, there is a paucity of research investigating how completion of a therapeutic exercise program may affect knee cartilage volume loss. The main purpose of this study is to measure knee cartilage change in individuals with KOA who have completed a therapeutic exercise program and compare their rate of cartilage loss to the currently known rates for individuals with KOA. We will also explore the differences in those individuals who had a loss of cartilage volume greater than measurement error to those subjects who had a cartilage volume loss within the SEM.
METHODS
Subjects who were to participate in a larger randomized trial investigating the effectiveness of therapeutic exercise for knee OA21 were invited to have imaging of both tibiofemoral joints prior to beginning the randomized trial and follow-up imaging 1-year after the baseline imaging. Consecutive subjects were invited to participate in the imaging arm of the study until 15 subjects were enrolled. One subject did not return for the follow-up imaging and 1 subject was determined to not meet the inclusion criteria. This resulted in a sample size of 13 subjects for the imaging arm of the study. Inclusion criteria for the randomized trial required: 1. Kellgren and Lawrence grade of II or greater in at least 1 compartment of the tibio-femoral joint 2. diagnosis of KOA made using the criteria established by the ACR, which are 3 or more of the following: morning stiffness less than 30 minutes, crepitus with active motion of the knee such as when squatting while weight bearing, tenderness on palpation of the bony margins of the joint, bony enlargement, and/or no palpable warmth.1 Exclusion criteria included: less than 40 years of age, history of myocardial infarction, cerebral vascular accident, or other neurological disorder, lower extremity joint arthroplasty; and the inability to walk without an assistive device. When both knees of a subject met the criteria for inclusion in the study, only the knee indicated by the subject as most affected by pain was used for analysis.
The group characteristics are presented in Table 1 and each individual characteristics as well as treatment group assignment are presented in Table 3. All subjects signed an informed consent approved by the University of Pittsburgh Institutional Review Board and their rights were protected at all times.
TABLE 1.
Group characteristics at baseline
| Mean | SD | |
|---|---|---|
| Age (years) | 63.5 | 11.4 |
| Sex | ||
| Female | n = 3 | |
| Male | n = 10 | |
| Body Mass Index (kg/m2) | 28.0 | 4.0 |
| Kellgren Lawrence Grade of medial compartment | ||
| Grade 1 | n = 2 | |
| Grade 2 | n = 4 | |
| Grade 3 | n = 5 | |
| Grade 4 | n = 2 | |
| Knee Axial Alignment(°) | 175.8° | 3.5° |
| WOMAC total score (0 to 96) | 17.9 | 9.8 |
| PASE (0 to 400) | 137.5 | 76.9 |
| Quadriceps MVIC (Nm/kg) | 2.0 | .4 |
Abbreviations: WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; PASE, Physical Activity Scale for the Elderly; MVIC, Maximum voluntary isometric contraction.
TABLE 3.
Subject baseline measurements and 1-year change in cMF cartilage volume
| ID | Sex | Age (Yrs) | BMI (Kg/m2) | Treatment Group | KL Med MA | KL Lat MA | Initial cMF volume (mm3) | cMF VC change (mm3) | Percent cMF VC change | |
|---|---|---|---|---|---|---|---|---|---|---|
| Progressors | 136 | M | 67 | 30.9 | Agility | 4 | 1 | 846 | −422 | −49.9 |
| 130 | M | 54 | 30.8 | Agility | 2 | 3 | 1841 | −420 | −22.8 | |
| 149 | F | 49 | 29.4 | Standard | 3 | 0 | 1633 | −335 | −20.5 | |
| 142 | M | 66 | 34.2 | Agility | 3 | 2 | 1056 | −240 | −22.7 | |
| 148 | M | 59 | 25.7 | Standard | 3 | 4 | 2628 | −153 | −5.8 | |
| 145 | M | 50 | 27.2 | Standard | 1 | 3 | 3975 | −121 | −3.1 | |
|
| ||||||||||
| Non-progressors | 150 | M | 82 | 26.5 | Agility | 2 | 3 | 746 | −86 | −11.5 |
| 151 | M | 67 | 25.9 | Standard | 2 | 0 | 2073 | −79 | −3.8 | |
| 135 | M | 71 | 33.0 | Agility | 3 | 1 | 1828 | −53 | −2.9 | |
| 138 | F | 63 | 19.3 | Standard | 2 | 0 | 1857 | −6 | −0.3 | |
| 153 | F | 83 | 24.1 | Standard | 1 | 2 | 1955 | 49 | 2.5 | |
| 147 | M | 48 | 25.8 | Agility | 3 | 2 | 1269 | 67 | 5.2 | |
| 131 | M | 66 | 30.6 | Agility | 4 | 2 | 1246 | 113 | 9.1 | |
|
| ||||||||||
| Median Values by Group | Progressors | 5 M | 57 | 30 | 3 Agility | 3 | 2.5 | 1737 | −287 | −22 |
| 1 F | 3 Standard | |||||||||
| Non-progressors | 5 M | 67 | 26 | 4 Agility | 2 | 2 | 1823 | −6 | 0 | |
| 2 F | 3 Standard | |||||||||
Abbreviations: Yrs, years; KL, Kellgren Lawrence Grade (0 to 4); MVIC, maximum voluntary isometric contraction; BMI, body mass index; VC, volume of cartilage; M, male; F, female; Med, medial tibiofemoral compartment; Lat, lateral tibiofemoral compartment; MA, most affected side
Tests and Measures
Subjects underwent baseline testing prior to initiating the therapeutic exercise treatments. The baseline testing consisted of: MR imaging of both knee joints, radiographs to measure varus/valgus knee alignment, gait analysis, testing of quadriceps strength, goniometric measurement of knee motion and the completion of self-report questionnaires to measure physical activity and functional limitations. Following 12 clinical treatment sessions over 2 months which was immediately followed by a 4 month home exercise program, clinical measurements of knee strength and motion were reassessed at the 6 month follow-up visit which coincided with the end of the monitored home exercise program. MR imaging, knee alignment radiographs, and self-report questionnaires were repeated 1 year after baseline which coincided to a timeframe of 6 months after completion of the home exercise program.
MR Imaging Assessments
A 3.0 Tesla scanner (3.0T Trio, Siemens Medical Solutions USA, Inc) was used to acquire a Sagital 3D double-echo steady state with water excitation (SAG 3D DESS WE) scan of both knee joints. Coronal multiplanar reformats were performed from the SAG 3D DESS WE scans and used for segmentation of the femoral and tibial cartilage. The SAG 3D DESS WE MR scan parameters were: in-lane resolution: 0.365mm × 0.456mm, repetition time: 16.3 ms, echo time: 2.8 ms, flip angle: 25°, matrix: 384 × 384, field of view: 14 × 14 cm, slice thickness: 0.7 mm, scan time: 10 minute 36 seconds.
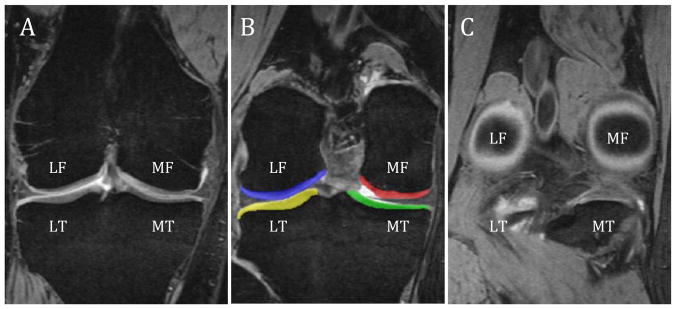
Using a commercial software package (Sliceomatic, Tomovision, Montreal, Quebec), the coronal MR images of the knee were manually segmented to distinguish the cartilage from the surrounding bone and soft tissues (Figure 1-B). Four regions of knee cartilage were segmented to quantify their cartilage volume. The cartilage coverings of the medial and lateral tibial plateaus were segmented in their entirety while the medial and lateral femoral condyles were segmented into a central region for each condyle (central region of the medial femoral condyle (cMF) and central region of the lateral femoral condyle (cLF). By calculating the volume of only the central region of the condyle, the advantage is to measure only the subregion of the condyle that is most subject to weight-bearing loads during ambulation.38 To ensure volumetric measurements were performed on the same region of the femoral condyles, baseline and 1-year follow-up image analyses for the cMF and cLF were performed on the same number of image slices constituting the anterior 60% distance between the image starting with the divergence of the trochlea into the femoral condyles (Figure 1-A) and the last image showing the circular structure of the posterior femoral condyles (Figure 1-C).18 Measurement of this region has previously been shown to be reliable and closely matches the region of the femoral condyle determined to undergo the greatest amount of loading during normal walking.38 Volumetric measurements were subsequently performed by multiplying the segmented surface areas on each image by the slice thickness (1.5 mm after multiplanar reformatting). All MR images were segmented by 1 of the investigators (SF) who was trained in the methods used to quantify cartilage morphology. In our laboratory, the SEM for quantifying cMF cartilage volume was calculated to be 120mm3. The coefficient of variation for intratester reliability of cMF cartilage volume quantification was 2.4%. The coefficient of variation for the cMF surface area digitized was calculated to be 6.9%. Both of these coefficients of variation are within acceptable limits.18
FIGURE 1.
(A) anterior landmark used to determine the central medial and lateral femoral regions of interests, (B) color maps displaying the tagged regions of interest over the femur and tibia cartilage, (C) posterior landmark used to determine the central medial and lateral femoral regions of interest (60% criterion between A/C). Abbreviations: LF, lateral femoral condyle; MF, medial femoral condyle; LT, lateral tibial plateau; MT, medial tibial plateau
Knee Alignment and Kellgren-Lawrence Grade
Weight-bearing anterior-posterior long cassette radiographs were taken and used to calculate the axial alignment of the tibiofemoral joint as described by Moreland et al.41 An axial alignment of 180° indicated a neutral alignment. Values less than 178° indicated a varus alignment of the knee while values greater than 182° indicated a valgus alignment.
A rheumatologist assigned a Kellgren-Lawrence grade to each subject’s baseline knee radiographs according to the guidelines established by Kellgren and Lawrence.37
Gait Analysis
Subjects ambulated on an 8.5m long vinyl-tiled walkway. An 8 M2-camera Vicon® (Vicon Peak–UK) 612 motion measurement system recorded 3-dimensional motion data at a sampling rate of 120 Hz from the Plug-In-Gait maker set. Ground reaction forces were measured on 2 Bertec® (Bertec Corporation, OH, USA) force plates embedded into the walkway. The force data were recorded at a sampling rate of 1080 Hz and synchronized with the motion data. Five walking trials were collected and averaged, where subjects contacted the force platforms without targeting. Marker trajectories and ground reaction force data were low-pass filtered (Butterworth fourth order, phase lag) at 6 and 40 Hz, respectively. Data were analyzed using MatLab™ version 7.0 (The Mathworks, Inc, Natick, MA, USA). External joint moments were derived using inverse dynamics and were normalized to body mass (kg). Peak moments were calculated during the loading phase (i.e., from heel strike to the first peak of vertical ground reaction force).
Quadriceps Muscle Strength
Maximum voluntary isometric torque output for knee extension was measured using a Biodex System 3 dynamometer (Biodex Medical Systems, Inc. Shirley, NY). The subject was seated with the knee in 60° of flexion and was instructed to extend the knee against the dynamometer with maximal effort for 5 seconds. A minimum of 3 trials and maximum of 6 trials were performed. After 3 trials, when a trial had a maximum torque output less than the previous trial, the strength testing was concluded. The highest maximum torque output from all trials was normalized to body weight (kg) and used in the analysis. This procedure has been shown to yield reliable quadriceps femoris torque measurements in our laboratory (Intraclass correlation coefficient (ICC2,1) = 0.96).
Self-report Questionnaires
The Physical Activity Scale for the Elderly (PASE) was used to provide an estimate of each subject’s level of physical activity. The PASE is a self-administered questionnaire that queries an individual on their level of leisure, occupational, and household activities and it has been shown to be reliable and valid in a sample of community dwelling, older adults.56 PASE scores can range from 0 to greater than 400 with higher PASE scores indicative of higher physical activity levels. Past studies have shown the average PASE score to be between 118 and 128 for older adults with arthritic or musculoskeletal conditions.11,58 The Likert version of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was used to quantify self-reported functional limitations. The WOMAC is a disease specific measure of pain, stiffness, and physical function for individuals with knee OA. The WOMAC includes 5 items related to pain, 2 items related to stiffness, and 17 items related to physical function. Each item is scored on a 5-point Likert scale (each scored from 0 to 4), with a total score of up to 96 points. Higher scores indicate greater functional limitations. Reliability and validity of the WOMAC has been established.6,7,29
Treatment Interventions
The exercise protocol used in this report has been published previously and is provided in Appendix A.48 All subjects completed a 6-week, 12-session outpatient physical therapy program that included aerobic treadmill walking and lower extremity strengthening and flexibility exercises. The subjects randomized to the experimental treatment group also completed dynamic agility and perturbation training. Following completion of outpatient physical therapy, a home exercise program (HEP) was completed 2 times per week for 4 months.
The 7 subjects who were randomized to the standard treatment group completed an exercise program that consisted of muscle stretching (quadriceps, hamstrings, and calf musculature); muscle strengthening (single-leg leg presses, seated isometric knee extensions, standing hamstring curls, standing heel raises, prone hip extensions, supine straight leg raises, and quad sets); and treadmill walking. The 6 subjects who were randomized to the agility and perturbation treatment group received the same exercises as the standard treatment group as well as the following agility exercises: side stepping, braiding (lateral stepping combined with forward and backward cross-over steps), forward cross-over steps during forward ambulation, forward/backward shuttle walking, change in direction drill (the therapist provided hand signals that would require the subject to combine random forward/backward walking with lateral stepping and diagonal stepping). The perturbation techniques were done using foam surfaces, tilt boards, and rollerboards. The subjects attempted to maintain balance while experiencing the destabilizing perturbations.
To account for a potential confounding effect of contact/treatment time between the 2 treatment groups, the subjects in the standard exercise group completed 10 to 15 minutes of upper extremity exercise on an arm-bike. This additional treatment time approximated the time required for the subjects in the agility and perturbation group subjects to complete their agility and perturbation activities.
For the HEP, the content was similar to the outpatient sessions with modifications for the exercises that were done clinically using exercise machines. Wall squat exercises were substituted for the leg press and gold theraband was given to the patients in order for them to perform isometric quadriceps exercises similar to those exercises completed clinically on the knee extension machine. All subjects were given a cuff weight that was adjustable in weight from 1 to 10 lbs (0.45 to 4.54 kg) that was used for the straight leg raises, hip extensions, and hamstring curls.
Subjects in the agility treatment group performed all exercises completed by the standard group. All agility activities were completed at home with the exception of the change in direction during walking activity and the rollerboard and tilt board exercises due to safety concerns and inability to provide this equipment. Therefore, single leg balance on level surfaces and carpeted surfaces were included in the agility groups HEP to provide a balance/perturbation aspect to their program. Subjects in both groups were also encouraged to continue a walking program of 30 minutes/day for at least 3 days a week. The trial coordinator contacted each subject monthly to remind the subjects to complete their HEP and to discuss any barriers to the subject completing the HEP as instructed. At the midpoint of the HEP duration, each subject had a face-to-face visit with the trial coordinator to review their HEP and insure the exercises were being completed correctly and to encourage compliance with the HEP.
RESULTS
Because this study has a relatively small sample of subjects, descriptive statistics were used to examine the data and inferential statistics were not performed. The annual percentage change in cartilage volume for the medial and lateral tibial and femoral articular surfaces were calculated by dividing the difference in cartilage volume from baseline to follow-up by the baseline volume. To explore the differences in those subjects who had a loss of cartilage volume greater than measurement error to those subjects who had a cartilage volume loss within the SEM, the 13 subjects were dichotomized into Progressors and Non-progressors using the standard error of the measure (SEM) for the cMF. The median values for each baseline factor were then calculated for the individuals that had a cartilage loss greater than the SEM, the Progressors, and for those individuals that had a change within the SEM, the Non-progressors. The decision to use change at the cMF region to examine factors that may impact cartilage volume loss was based on the recent work of Eckstein et al,19 who found the cMF to be the most responsive region of the tibiofemoral joint with regards to change in cartilage volume. In addition, prior studies have found the central region of the medial femoral condyle to be the area of greatest cartilage loss over time in patients with KOA3,8
The median percent changes in cartilage volume for the 4 articular surfaces of the tibiofemoral joint are shown in Table 2. The cMF had a median loss of 3.8% while the other 3 articular surfaces had median changes in cartilage volume of less than 1%. When the SEM was used to dichotomize the loss of cMF cartilage volume, the Progressors had a median cMF cartilage volume loss of 22% while the Non-progressors had a median change of 0%.
TABLE 2.
Median (minimum, maximum) percentage change in cartilage volume over 1 year by tibiofemoral articular surface
| Articular Surface of the tibiofemoral joint | Median Cartilage Volume Change |
|
|---|---|---|
| Femur | cMF | −3.8% (−50 to +9) |
| cLF | 0.0% (−17 to +20) | |
|
| ||
| Tibia | Medial plateau | 0.8% (−47 to +19) |
| Lateral plateau | 0.1% (−25 to +18) | |
Abbreviations: cMF, central region of medial femoral condyle; cLF, central region of lateral femoral condyle.
Table 3 presents the individual subject characteristics, treatment group assignment, KL grade of the most affected knee as well as the baseline, absolute change and percentage change in cartilage volume for the cMF. In the Progressor group, 3 subjects were assigned to the standard treatment group while 3 were assigned to the agility treatment group. In the Non-progressors, 3 subjects were in the standard group while 4 were in the agility group. Comparing the median values of the Progressor group to the Non-progressor group, the Progressors had greater BMI (30 versus 26 kg/m2), a lower age (57 versus 67 years), and a higher KL grade for the medial compartment (3 versus 2). The initial cMF cartilage volumes for the groups were similar at baseline (median of 1737mm3 for the Progressors and 1823mm3 for the Non-progressors).
Table 4 presents the baseline and 1-year scores for the WOMAC and its 3 subscales, the PASE, and the worst pain level reported using the numerical pain rating scale.
TABLE 4.
WOMAC, PASE and self-reported pain levels at initial visit and 12-month follow-up*
| ID | WOMAC Physical Function subscale | WOMAC Pain subscale | WOMAC Knee Stiffness subscale | WOMAC Total score | PASE | Worst pain in past 24h | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Initial | 12M | Initial | 12M | Initial | 12M | Initial | 12M | Initial | 12M | Initial | 12M | ||
| Progressors | 136 | 12 | 12 | 4 | 4 | 3 | 2 | 19 | 18 | 138 | 184 | 4 | 4 |
| 130 | 10 | 5 | 3 | 3 | 1 | 0 | 14 | 8 | 347 | 295 | 4 | 3 | |
| 149 | 29 | 5 | 4 | 1 | 6 | 4 | 39 | 10 | 181 | 174 | 4 | 4 | |
| 142 | 10 | 8 | 0 | 3 | 3 | 0 | 13 | 11 | 109 | 120 | 1 | 2 | |
| 148 | 12 | 5 | 3 | 2 | 2 | 0 | 17 | 7 | 202 | 211 | 2 | 1 | |
| 145 | 2 | 0 | 1 | 0 | 2 | 2 | 5 | 2 | 104 | 94 | 1 | 1 | |
|
| |||||||||||||
| Non-progressors | 150 | 12 | 4 | 1 | 1 | 1 | 0 | 14 | 5 | 78 | 138 | 2 | 2 |
| 151 | 3 | 3 | 3 | 0 | 1 | 1 | 7 | 4 | 124 | 31 | 1 | 1 | |
| 135 | 16 | 13 | 5 | 6 | 2 | 3 | 23 | 22 | 34 | 267 | 3 | 5 | |
| 138 | 8 | 5 | 1 | 2 | 2 | 3 | 11 | 10 | 123 | 166 | 2 | 3 | |
| 153 | 11 | 13 | 4 | 3 | 3 | 2 | 18 | 18 | 115 | 140 | 3 | 5 | |
| 147 | 21 | 9 | 6 | 2 | 3 | 3 | 30 | 14 | 155 | 192 | 8 | 3 | |
| 131 | 12 | 36 | 3 | 10 | 1 | 5 | 16 | 51 | 77 | 116 | 4 | 5 | |
|
| |||||||||||||
| Median Values by Group | Progressors | 11 | 5 | 3 | 3 | 3 | 1 | 16 | 9 | 160 | 179 | 3 | 3 |
| Non-progressors | 12 | 9 | 3 | 2 | 2 | 3 | 16 | 14 | 115 | 140 | 3 | 3 | |
Abbreviations: WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; PASE, Physical Activity Scale for the Elderly; 12M, 12 month follow-up visit.
Ranges for the WOMAC: 0–68 physical function subscale, 0–20 pain subscale, 0–8 stiffness subscale, 0–96 total score. Higher values indicate greater functional deficits, pain and stiffness. Range for PASE: 0 to greater than 400 with higher scores indicating increased physical activity
The baseline and follow-up values for knee range of motion, quadriceps strength, and knee axis alignment are presented in Table 5. Both groups showed a modest improvement in knee flexion and a slight worsening in knee extension range of motion. Change in quadriceps maximum voluntary isometric contraction was minimal in both groups from baseline to 6 month testing. There was a 5° increase in the knee varus angle of the Progressors while the Non-progressors had no change in alignment.
TABLE 5.
Knee range of motion, quadriceps strength and knee axis alignment at baseline and follow-up
| ID | Passive Knee range of motion (°) | Quadriceps MVIC (Nm/kg) | Knee Axis Alignment (°) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Initial | 6M | Initial | 6M | Initial | 12M | ||
| Progressors | 136 | 14–114 | 20–117 | 1.8 | 1.7 | 170 varus | 170 varus |
| 130 | 4-0-118 | 0–126 | 2.2 | 2.4 | 173 varus | 171 varus | |
| 149 | 8–124 | 12–131 | 1.9 | 1.8 | 176 varus | 172 varus | |
| 142 | 3–139 | 6–135 | 2.1 | 2.1 | 177 varus | 170 varus | |
| 148 | 8-0-112 | 10-0-117 | 2.5 | 2.6 | 179 neutral | 182 neutral | |
| 145 | 8–127 | 9–130 | 2.2 | 2.2 | 180 neutral | 182 neutral | |
|
| |||||||
| Non-progressors | 150 | 5–150 | 5–140 | 1.5 | 1.1 | 174 varus | 175 varus |
| 151 | 5–146 | 6–149 | 1.9 | 1.7 | 176 varus | 176 varus | |
| 135 | 5–130 | 6-0-132 | 1.8 | 1.7 | 176 varus | 178 neutral | |
| 138 | 2-0-146 | 1–146 | 2.4 | 2.4 | 179 neutral | 180 neutral | |
| 153 | 4–130 | 12–140 | 1.4 | 0.7 | 181 neutral | 183 valgus | |
| 147 | 4–128 | 8–136 | 1.7 | 1.6 | 174 varus | 175 varus | |
| 131 | 13–130 | 17–135 | 2.3 | 2.1 | 170 varus | 168 varus | |
|
| |||||||
| Median Values by Group | Progressors | 121 flex/5 loss of extension | 128 flex/7 loss of extension | 2.1 | 2.2 | 177 | 172 |
| Non-progressors | 130 flex/5 loss of extension | 140 flex/6 loss of extension | 1.8 | 1.7 | 176 | 176 | |
Abbreviations: 6M, 6 month follow-up visit; 12M, 12 month follow-up visit; MVIC, maximum voluntary isometric contraction; TKE, terminal knee extension.
Table 6 presents frontal plane kinetic data collected at baseline for the hip and knee joints during the loading phase of gait. The Progressors had a 13% greater median peak knee adduction moment than the Non-progressors (0.61 Nm/kg versus 0.54 Nm/kg). At the hip joint, the Non-progressors had a 12% greater median peak hip adduction moment than the Progressors (1.0 Nm/kg versus 0.89 Nm/kg).
TABLE 6.
Individual values and group median values (range) for baseline hip and knee frontal plane gait kinetics
| Subject ID | Peak Hip Adduction moment during loading Nm/kg | Peak Knee Adduction moment during loading Nm/kg | |
|---|---|---|---|
| Progressors | 136 | .91 | .70 |
| 130 | .46 | .88 | |
| 149 | .87 | .30 | |
| 142 | 1.14 | .73 | |
| 148 | .78 | .52 | |
| 145 | 1.16 | .30 | |
|
| |||
| Non-progressors | 150 | .62 | .40 |
| 151 | 1.10 | .98 | |
| 135 | .76 | 1.10 | |
| 138 | 1.13 | .54 | |
| 153 | 1.00 | .31 | |
| 147 | 1.03 | .46 | |
| 131 | .80 | .62 | |
|
| |||
| Median Values by group | Progressors | 0.89 (0.46 to 1.16) | 0.61 (0.30 to .88) |
| Non-progressors | 1.0 (0.62 to 1.13) | 0.54 (0.31 to 1.10) | |
Compliance for the treatment sessions completed in the clinic and the home exercise program is listed in Table 7. All 13 subjects completed all 12 treatment sessions that occurred in the clinic. The Progressors completed 94% of their home exercise program sessions while the Non-progressors completed 66%.
TABLE 7.
Compliance with clinic sessions and home exercise program
| ID | Compliance with Clinic Sessions (% of clinic session completed) | Compliance with HEP (% of HEP sessions completed) | |
|---|---|---|---|
| Progressors | 136 | 100 | 100 |
| 130 | 100 | 34 | |
| 149 | 100 | 100 | |
| 142 | 100 | 88 | |
| 148 | 100 | 100 | |
| 145 | 100 | 100 | |
|
| |||
| Non-progressors | 150 | 100 | 100 |
| 151 | 100 | 100 | |
| 135 | 100 | 56 | |
| 138 | 100 | 44 | |
| 153 | 100 | 97 | |
| 147 | 100 | 66 | |
| 131 | 100 | 56 | |
|
| |||
| Median values by group | Progressors | 100 | 94 |
| Non-progressors | 100 | 66 | |
Abbreviations: HEP, home exercise program
DISCUSSION
To our knowledge, this is the first report to present the rate of change of tibiofemoral cartilage volume in subjects enrolled in a therapeutic exercise program for treatment of KOA. The 3.8% median loss of cMF cartilage volume is within the range of 2% to 7.5%/year reported in prior studies.17,19,45–47 But, it must be noted that some earlier studies measured all cartilage of the femoral condyle, while the current study, and 2 previously published studies, measured only the weight-bearing central aspect of the femoral condyles. Quantification of cartilage volume changes in the weight-bearing region of the condyle has been shown to experience greater changes than non-weight bearing regions.3,9 Therefore, it is most appropriate to compare the data from this study to those studies that measured cartilage change in the cMF rather than in the entire femoral condyle. The 2 studies that quantified change in the weight-bearing cMF reported annual cartilage volume losses of 1.5% and 6%.19,45 The lateral femoral condyle and both tibial plateaus experienced cartilage loss at a decreased rate compared to prior studies.17 The median cMF cartilage volume loss was within previously reported ranges in spite of the increased severity of radiographic KOA as measured using the KL scale. An increasing rate of cMF cartilage volume loss with a higher KL grade has been reported.19 In the current study, 7 out of 13 (54%) subjects had a KL grade of 3 or 4 in the medial compartment of the knee. In the study by Eckstein et al, only 34% of the knees had a KL grade of 3 or 4.19 The study by Pelletier et al45, excluded those with a KL grade of 4 and 53% and 47% of their subjects had KL grades of 2 and 3, respectively. Previously, it has been shown that a higher KL grade is indicative of more extensive cartilage loss and a smaller baseline cartilage volume.16 Mathematically, as the baseline cartilage volume decreases, a given absolute amount of cartilage loss results in a higher percentage cartilage volume loss.
The large variability of cMF cartilage loss found in this report (range of 50% loss to 9% gain) and by other researchers underscores the importance of understanding mediating factors that impact the rate at which articular cartilage is lost. For instance, Raynauld et al46 determined KOA subgroups by rate of cartilage loss and found a fast progressor group that lost 21.5% of their medial tibiofemoral articular cartilage volume over 2 years and a slow progressor group that lost just 3.2%. Likewise, our study had a Progressors group that lost a median cMF cartilage volume of 22% while the Non-progressors had a median cMF cartilage volume change of zero percent. Although both groups improved their total WOMAC scores from baseline to 1-year follow-up, the Progressors actually had a greater improvement than did the Non-progressors. Both groups demonstrated the greatest change in the physical function subscale of the WOMAC while the pain and stiffness subscales showed little overall change. As expected, given the minimal change in the WOMAC pain subscale, the NPRS did not indicate any significant change from baseline to 1-year follow-up in the subjects’ worst level of knee pain regardless of whether or not they had cMF cartilage volume loss.
As for physical activity, the PASE scores showed the Progressors to have a slightly higher baseline and 1-year PASE score than the Non-progressors. This was expected given the younger age of the Progressors as compared to the Non-progressors. Prior research has shown that PASE scores decrease with increasing age and the differences in activity levels seen between our two groups could have been the result of the age difference.11 In addition, both groups underwent approximately a 20 point increase in the PASE scores from baseline to 1-year. Physical activity levels maybe an important mediator of cartilage volume loss, but to our knowledge, there are currently no longitudinal MR studies considering this relationship in individuals with KOA. Amin et al2 conducted a cross-sectional study that reported an increased risk of worse patellofemoral Whole Organ MRI cartilage scores (WORMS) in workers with self-reported occupational exposure to kneeling/squatting and heavy lifting. Their research demonstrates the potentially deleterious relationship of repeated, stressful activities on the articular cartilage of the knee. Foley et al23, in a sample of healthy subjects, found greater quadriceps strength and higher levels of fitness to be associated with less knee cartilage loss. However, their findings of a positive relationship between activity and knee cartilage response cannot be assumed to occur in individuals with KOA. In fact, Andriacchi et al4 have shown that healthy knees appear to respond favorably to loading by increasing the thickness of articular cartilage in response to increased loading while the opposite is true of arthritic knees. The lack of studies examining the impact of physical activity levels on knee cartilage loss in individuals with KOA makes it difficult to prescribe the proper therapeutic dosage of physical activity and knee joint loading. Making it even more difficult to assess the impact of physical activity on cartilage loss are the limitations present in self-report measures of physical activity, such as the PASE.24 Additional research is indeed required to more precisely capture activity levels given the potentially large role of physical activity in affecting outcomes in a myriad of disease processes, including KOA.
Paralleling the similar changes seen between the Progressors and Non-progressors in self-reported pain and activity levels were the impairment measures of knee motion and knee strength. Both groups had a modest improvement in passive knee flexion and a slight worsening in the loss of terminal knee extension. Despite the inclusion of stretching exercises to improve knee extension, both groups failed to improve terminal knee extension. Similarly, despite a thorough strengthening program, both groups showed minimal change in their quadriceps strength.
The strengthening program used in this study utilized 1-repetition maximum strength testing in order to prescribe at least 70% of the 1-repetition maximum as dosage for single limb leg press. Knee extension exercises were performed isometrically at maximum effort. This dosage for leg press and knee extension strengthening exercises should have provided adequate stimulus for strengthening of the hip and knee extensor musculature. While the individuals in this study showed no significant change in their maximum quadriceps strength, the larger sample from the full randomized trial did demonstrate an improvement in knee extension strength.48 Therefore, the minimal change in strength was unexpected. Potential explanations for a lack of strength gain in individuals with KOA are that pain during exercise may limit the ability of the treating therapist to increase dosage of the exercises and individuals may limit their force output in response to pain. Future studies should consider collecting pain data during exercises to determine if pain during exercise can affect lower extremity strength changes.
Although the self-reports and measures of knee impairment demonstrated similar changes between the groups with the exception of the Progressors having a greater improvement in the physical function subscale of the WOMAC, there were several factors that differed between the Progressors and Non-progressors. Age, BMI, severity of KOA, progression of knee varus alignment, and gait kinetics all differed between the Progressors and Non-progressors. These factors, with the exception of age, have been previously shown to impact the rate of knee cartilage loss in KOA.
As to the role of age in cMF cartilage volume loss, 2 previous studies failed to find a statistically significant association between cMF cartilage volume loss and age.19,45 In the current report, the Progressors had a median age of 57 years and the Non-progressors had a median age of 67 years. Despite this difference, age may not greatly impact cMF cartilage loss once other factors that more directly affect loading are taken into account.
In the current study, Progressors had a BMI of 30 kg/m2 while Non-progressors had a BMI of 26 kg/m2. BMI has been shown to have a significant association (r=0.21, p=0.03) with cMF cartilage volume loss.45 In addition, Eckstein et al19 reported a trend towards subjects with BMI greater than 30 kg/m2 having a faster rate of cartilage loss in the cMF than those with a BMI of less than 30 kg/m2. These findings all support a deleterious effect of increasing BMI on cMF cartilage loss in those with KOA.
Progressors also had more severe radiographic KOA as well as a greater progression towards varus alignment of the knee than the Non-progressors. The Progressors had a median KL grade of 3 in the medial compartment of the knee while the Non-progressors had a median of 2. Eckstein et al19 have shown that knees with a KL grade of 3 tend to have a higher rate of cMF cartilage volume loss than knees with a KL grade of 2. Increasing severity of KOA has also been shown to increase the knee adduction moment during gait.42 An increased knee adduction moment is believed to result in greater loading across the medial compartment of the knee.
While the groups had nearly identical alignment of the knee at baseline, at 1-year, the Progressor group had a median knee alignment of 172° while the Non-progressors had a median of 176°. Therefore, the Progressor group median alignment became more varus while the Non-progressors median alignment remained 176°. The deleterious effect of varus alignment on the cartilage of the medial compartment of the tibiofemoral joint has been well established by previous research.20,49
From the gait analysis data, the Progressors had a 13% higher median external knee adduction moment and 12% lower median hip adduction moment compared to the Non-progressors. While higher than normal knee adduction moments, similar to the magnitude found between our Progressors and Non-progressors, have been shown to be common in those with medial KOA,5,42,54,59 there are currently no studies that have examined the relationship of the external knee adduction moment during gait to the rate of cMF cartilage volume loss. However, the differences between our groups are small and the ranges for both knee and hip adduction moments are quite similar between the Progressors and Non-progressors. Therefore, these results suggest larger studies to investigate these relationships are warranted.
The slightly larger external hip adduction moment found in the Non-progressors appears to be in agreement with the results reported by Chang et al.13 In fact, in their study, the 12% difference in the frontal plane hip moment between those who lost knee joint space and those who did not, matches closely the difference seen between the 2 groups in the current report. Using inverse dynamics to equate internal hip abduction moments created by the gluteus medius with the external hip adduction moment, Chang et al13 suggested that higher internal hip abduction moments during loading of the lower extremity should decrease the load through the medial compartment of the knee. Strong hip abductors that could prevent contralateral rotation of the pelvis and a shift of the center of mass away from the hip joint axis during stance would accomplish a lower external knee adduction moment.10,12
In support of Chang’s13 findings, Mundermann et al42 found smaller external hip adduction moments in subjects with more severe KOA when compared to those with less severe KOA. From these findings, the authors suggested hip abductor strengthening may reduce the knee adduction moment. However, a recent trial examining the impact of a hip abductor strengthening on the knee adduction moment during gait found no change in the knee adduction moment after 8 weeks of hip abductor strengthening despite a 33% gain in hip abductor strength.51 Also calling into question the role of hip abductor strength in reducing the knee adduction moment, Henriksen et al30 reported experimentally induced hip abductor weakness did not result in an increased knee adduction moment. However this study was completed in a small sample of young, healthy individuals. The lack of agreement as to whether or not hip abductor strength deficits impact the external adduction moment at the knee requires additional research to not only examine the role of strength but also of hip muscle activation patterns. The potentially beneficial effects of hip abductor strength may be mitigated if muscle activation patterns are not properly coordinated during the stance phase of gait. Finally, actual hip abductor strength may have a weaker association with hip and knee moments compared to other factors such as gait compensations, hip muscle activation patterns, and alignment of the lower extremity.
The fact that all subjects completed the therapeutic exercise program but cartilage loss varied greatly suggests that the exercise program itself may have not altered the rate of cartilage loss. Undeniably, therapeutic exercise has shown itself to be an effective treatment for improving pain and function in those with KOA.25 In the current study, both groups demonstrated improved WOMAC total scores, WOMAC physical functioning scores, and PASE scores. By better understanding how individual factors impact cartilage loss, researchers will be able to refine and assess therapeutic exercise programs for those at risk for rapid cMF cartilage loss. Programs that limit loading, such as non-weight-bearing exercise and aquatic therapy, may be better suited to improve function and pain and limit cartilage loss in a subset of individuals with KOA who are at greatest risk for rapid cartilage loss.
Limitations
This research report includes only a a small number of subjects and therefore serves to provide preliminary evidence that larger trials are needed to more accurately determine the effects exercise, physical activity, gait kinetics, and anthropometrics have on knee cartilage loss in individuals with KOA. Although we performed MR scans, our MR scans did not examine the condition of the medial meniscus, which has previously been shown to be a strong predictor of change in knee cartilage morphology.46,49 Physical activity was quantified using the PASE. While this self-report questionnaire has undergone psychometric testing to demonstrate its reliability and validity, it is unable to capture temporal changes in one’s level of physical activity over the course of a year or the amount of impact one imparts to the knee cartilage during their activities. It has also correlated only modestly with accelerometer data used to measure physical activity levels.57 Additionally, a recent systematic review of self-administered physical activity scales concluded that additional high-quality validation studies are needed to support the use of these instruments.24 Due to the potentially large, but currently unproven, influence of physical activity on cartilage loss in those with KOA, we advocate that future studies examine the interaction between physical activity levels and knee cartilage loss in this population.
CONCLUSION
While 6 subjects had a loss of cMF cartilage volume greater than the SEM, 7 subjects had a loss within the SEM, indicating no loss of cMF cartilage volume beyond measurement error. The median absolute volume loss for the cMF of 86 mm3 was within the SEM. Expressed as a percent change, median loss of 3.8% at the cMF cartilage volume was within the range published by previous authors who have quantified volume at the weight-bearing region of the medial femoral condyle. The large variability in cMF cartilage change may potentially be mediated by individual factors, especially those that have the potential to increase loading at the medial compartment of the knee, rather than the common factor of completing a therapeutic exercise program. In spite of differences in the amount of cMF cartilage volume loss between the groups, changes in physical activity levels as well as impairments of knee motion and strength were similar between the Progressors and Non-progressors. The scores on the physical functioning subscale of the WOMAC improved for both groups with the Progressors having a slightly more robust improvement in that subscale.
KEY POINTS.
FINDINGS
In individuals with KOA, the completion of a therapeutic exercise program resulted in a rate of cMF cartilage loss within ranges previously reported. Factors that increase loading across the knee joint may increase the rate of cMF cartilage loss.
IMPLICATION
Since knee cartilage loss is a predictor of eventual knee arthroplasty, rehabilitation programs that improve symptoms and function while limiting cartilage loss would be optimal. This study is to serve as an impetus for studies that will compare rehabilitation protocols and their effects on symptoms, function and cartilage loss in individuals with KOA.
CAUTION
Due to the nature of this study design and the small sample size, future studies are required to elucidate optimal rehabilitation strategies for preserving cartilage while also improving symptoms and function.
Acknowledgments
Source of Support: Supported in part by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases, Grant # 1-R01-AR048760
Footnotes
The Institutional Review Board at the University of Pittsburgh approved this study’s research protocol.
Financial Disclosure and Conflict of Interest. I affirm that I have no financial affiliation (including research funding) or involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript, except as disclosed in an attachment and cited in the manuscript. Any other conflict of interest (ie, personal associations or involvement as a director, officer, or expert witness) is also disclosed in an attachment.
Reference List
- 1.Altman RD, Bloch DA, Bole GG, Jr, Brandt KD, Cooke DV, Greenwald RA, et al. Development of clinical criteria for osteoarthritis. J Rheumatol. 1987;14(Spec No):3–6. [PubMed] [Google Scholar]
- 2.Amin S, Goggins J, Niu J, Guermazi A, Grigoryan M, Hunter DJ, et al. Occupation-related squatting, kneeling, and heavy lifting and the knee joint: a magnetic resonance imaging-based study in men. J Rheumatol. 2008;35(8):1645–1649. [PMC free article] [PubMed] [Google Scholar]
- 3.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52(10):3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 5.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(7):573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy N, Kean WF, Buchanan WW, Gerecz-Simon E, Campbell J. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren):post validation reapplication of the WOMAC osteoarthritis index. Journal of Rheumatology. 1992;19:153–159. [PubMed] [Google Scholar]
- 7.Bellamy N, Watson-Buchanan W, Goldsmith CH, Campbell J. Validation study of WOMAC: A health status instrument for measuring clincically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 8.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46(11):2884–2892. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 9.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46(11):2884–2892. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 10.Briem K, Snyder-Mackler L. Proximal gait adaptations in medial knee OA. J Orthop Res. 2009;27(1):78–83. doi: 10.1002/jor.20718. [DOI] [PubMed] [Google Scholar]
- 11.Chad KE, Reeder BA, Harrison EL, Ashworth NL, Sheppard SM, Schultz SL, et al. Profile of physical activity levels in community-dwelling older adults. Med Sci Sports Exerc. 2005;37(10):1774–1784. doi: 10.1249/01.mss.0000181303.51937.9c. [DOI] [PubMed] [Google Scholar]
- 12.Chang A, Hayes K, Dunlop D, Hurwitz D, Song J, Cahue S, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50(12):3897–3903. doi: 10.1002/art.20657. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Hayes K, Dunlop D, Song J, Hurwitz D, Cahue S, et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52(11):3515–3519. doi: 10.1002/art.21406. [DOI] [PubMed] [Google Scholar]
- 14.Cicuttini F, Wluka A, Hankin J, Wang Y. Longitudinal study of the relationship between knee angle and tibiofemoral cartilage volume in subjects with knee osteoarthritis. Rheumatology (Oxford) 2004;43(3):321–324. doi: 10.1093/rheumatology/keh017. [DOI] [PubMed] [Google Scholar]
- 15.Cicuttini FM, Jones G, Forbes A, Wluka AE. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study. Ann Rheum Dis. 2004;63(9):1124–1127. doi: 10.1136/ard.2004.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicuttini FM, Wluka AE, Forbes A, Wolfe R. Comparison of tibial cartilage volume and radiologic grade of the tibiofemoral joint. Arthritis Rheum. 2003;48(3):682–688. doi: 10.1002/art.10840. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14 (Suppl A):A46–A75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65(4):433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68(5):674–679. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59(11):1563–1570. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald GK, Piva SR, Gil AB, Wisniewski SR, Oddis CV, Irrgang JJ. Agility and Perturbation Training Techniques in Exercise Therapy for Reducing Pain and Improving Function in People With Knee Osteoarthritis: A Randomized Clinical Trial. Phys Ther. 2011;91(4):452–469. doi: 10.2522/ptj.20100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald GK, Piva SR, Irrgang JJ, Bouzubar F, Starz TW. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51(1):40–48. doi: 10.1002/art.20084. [DOI] [PubMed] [Google Scholar]
- 23.Foley S, Ding C, Cicuttini F, Jones G. Physical activity and knee structural change: a longitudinal study using MRI. Med Sci Sports Exerc. 2007;39(3):426–434. doi: 10.1249/mss.0b013e31802d97c6. [DOI] [PubMed] [Google Scholar]
- 24.Forsen L, Loland NW, Vuillemin A, Chinapaw MJ, van Poppel MN, Mokkink LB, et al. Self-administered physical activity questionnaires for the elderly: a systematic review of measurement properties. Sports Med. 2010;40(7):601–623. doi: 10.2165/11531350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36(6):1109–1117. doi: 10.3899/jrheum.090058. [DOI] [PubMed] [Google Scholar]
- 26.Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem. 1999;125(5):966–975. doi: 10.1093/oxfordjournals.jbchem.a022376. [DOI] [PubMed] [Google Scholar]
- 27.Galois L, Etienne S, Grossin L, Cournil C, Pinzano A, Netter P, et al. Moderate-impact exercise is associated with decreased severity of experimental osteoarthritis in rats. Rheumatology (Oxford) 2003;42(5):692–693. doi: 10.1093/rheumatology/keg094. [DOI] [PubMed] [Google Scholar]
- 28.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawker G, Melfi C, Paul J, Green R, Bombardier C. Comparison of a generic (SF-36) and a disease specific (WOMAC) (Western Ontario and McMaster Universities Osteoarthritis Index) instrument in the measurement of outcomes after knee replacement surgery. J Rheumatol. 1995;22(6):1193–1196. [PubMed] [Google Scholar]
- 30.Henriksen M, Aaboe J, Simonsen EB, Alkjaer T, Bliddal H. Experimentally reduced hip abductor function during walking: Implications for knee joint loads. J Biomech. 2009;42(9):1236–1240. doi: 10.1016/j.jbiomech.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38(11):1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 32.Hochberg MC, Kasper J, Williamson J, Skinner A, Fried LP. The contribution of osteoarthritis to disability: preliminary data from the Women’s Health and Aging Study. J Rheumatol Suppl. 1995;43:16–18. [PubMed] [Google Scholar]
- 33.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20(1):101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31(5):423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 35.Jan MH, Lin CH, Lin YF, Lin JJ, Lin DH. Effects of weight-bearing versus nonweight-bearing exercise on function, walking speed, and position sense in participants with knee osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(6):897–904. doi: 10.1016/j.apmr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Jan MH, Lin JJ, Liau JJ, Lin YF, Lin DH. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2008;88(4):427–436. doi: 10.2522/ptj.20060300. [DOI] [PubMed] [Google Scholar]
- 37.KELLGREN JH, LAWRENCE JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage. 2005;13(9):782–789. doi: 10.1016/j.joca.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin DH, Lin CH, Lin YF, Jan MH. Efficacy of 2 non-weight-bearing interventions, proprioception training versus strength training, for patients with knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther. 2009;39(6):450–457. doi: 10.2519/jospt.2009.2923. [DOI] [PubMed] [Google Scholar]
- 41.Moreland JR, Bassett LW, Hanker GJ. Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987;69(5):745–749. [PubMed] [Google Scholar]
- 42.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52(9):2835–2844. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 43.Nunez M, Nunez E, Sastre S, Del Val JL, Segur JM, Macule F. Prevalence of knee osteoarthritis and analysis of pain, rigidity, and functional incapacity. Orthopedics. 2008;31(8):753. [PubMed] [Google Scholar]
- 44.Otterness IG, Eskra JD, Bliven ML, Shay AK, Pelletier JP, Milici AJ. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41(11):2068–2076. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 45.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8(1):R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50(2):476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 48.Scopaz KA, Piva SR, Gil AB, Woollard JD, Oddis CV, Fitzgerald GK. Effect of baseline quadriceps activation on changes in quadriceps strength after exercise therapy in subjects with knee osteoarthritis. Arthritis Rheum. 2009;61(7):951–957. doi: 10.1002/art.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716–1726. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 50.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41(7):1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Sled EA, Khoja L, Deluzio KJ, Olney SJ, Culham EG. Effect of a Home Program of Hip Abductor Exercises on Knee Joint Loading, Strength, Function, and Pain in People With Knee Osteoarthritis: A Clinical Trial. Phys Ther. 2010;90(6):895–904. doi: 10.2522/ptj.20090294. [DOI] [PubMed] [Google Scholar]
- 52.Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW. Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology (Oxford) 2000;39(9):955–961. doi: 10.1093/rheumatology/39.9.955. [DOI] [PubMed] [Google Scholar]
- 53.Tan J, Balci N, Sepici V, Gener FA. Isokinetic and isometric strength in osteoarthrosis of the knee. A comparative study with healthy women. Am J Phys Med Rehabil. 1995;74(5):364–369. [PubMed] [Google Scholar]
- 54.Thorp LE, Sumner DR, Block JA, Moisio KC, Shott S, Wimmer MA. Knee joint loading differs in individuals with mild compared with moderate medial knee osteoarthritis. Arthritis Rheum. 2006;54(12):3842–3849. doi: 10.1002/art.22247. [DOI] [PubMed] [Google Scholar]
- 55.Topp R, Woolley S, Hornyak J, III, Khuder S, Kahaleh B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Arch Phys Med Rehabil. 2002;83(9):1187–1195. doi: 10.1053/apmr.2002.33988. [DOI] [PubMed] [Google Scholar]
- 56.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 57.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39(4):336–340. [PubMed] [Google Scholar]
- 58.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 59.Weidow J, Tranberg R, Saari T, Karrholm J. Hip and knee joint rotations differ between patients with medial and lateral knee osteoarthritis: gait analysis of 30 patients and 15 controls. J Orthop Res. 2006;24(9):1890–1899. doi: 10.1002/jor.20194. [DOI] [PubMed] [Google Scholar]