Abstract
Many observational epidemiologic studies suggest an association between exercise and breast cancer risk. However, the lack of controlled experimental studies that examine this relationship and the mechanisms involved weaken the basis for inferring a causal relationship. Inflammation plays a role in breast cancer progression and exercise has been reported to reduce inflammation; however, the inflammatory effects of exercise in breast cancer have yet to be established. We examined the relationship between exercise training and systemic inflammation in relation to breast cancer progression in C3(1)SV40Tag mice. Female C3(1)SV40Tag mice were assigned to either exercise (Ex) or sedentary (Sed) treatment (n=12-14/group). Beginning at 4wks of age mice (Ex) were run on a treadmill for 60 min/d (20 m/min and 5% grade), 6 d/wk for a period of 20wks. Mice were examined weekly for palpable tumors, and tumor number and volume were recorded. At 24wks of age mice were sacrificed and a more direct measure of tumor number and volume, and spleen weight was recorded. Plasma was analyzed for MCP-1 and IL-6 concentration using ELISA. Ex reduced palpable tumor number at sacrifice (24wks) by approximately 70% (P<0.05). Tumor volume was also reduced in Ex at 21 - 23wks (P<0.05). This reduction in tumor progression by Ex was associated with a reduction in plasma concentration of MCP-1 and IL-6, and spleen weight (P<0.05). These data provide strong support for a beneficial effect of exercise training on tumor progression in the C3(1)SV40Tag mouse model of breast cancer that may be partly mediated by its anti-inflammatory potential.
Keywords: physical activity, mammary tumorigenesis, mouse models, cytokines
1. Introduction
Accumulating epidemiological evidence suggests a beneficial relationship between exercise and reduced breast cancer risk [1-3], but little is known about the mechanisms involved. Animal models provide a useful tool to examine these potential mechanisms; however, the availability of studies using animal models in which experimentation is feasible, is limited and those that are available often report inconsistent findings [4]. To date, the most common model used in animal studies of breast cancer prevention has been chemically-induced mammary tumorigenesis in mice or rats (for example, 7,12-dimethylbenzanthracene, N-nitrosomethylurea) [5-8]. While several studies have reported a beneficial effect of exercise on tumor number, growth, and incidence using chemically-induced mammary tumorigenesis models, there have been quite a few studies that have reported negative findings [9-11]. Only one study has examined the effects of exercise on breast cancer using a genetically engineered mouse model [9]. Colbert et al. using a p53-deficient mouse model of breast cancer reported a harmful effect of exercise on mammary tumorigenesis [9]. The limited available studies and inconsistencies among findings can be explained, at least in part, by the lack of appropriate rodent models to study the relationship between exercise and breast cancer risk.
The mechanisms for a beneficial effect of exercise on breast cancer progression are potentially very complex and multifaceted. Recent epidemiological evidence suggests that an exercise-induced reduction in inflammation may play a role [12, 13]. Inflammation has been linked to various steps involved in tumorigenesis [14] and exercise has been reported to reduce inflammation [15]. For example, monocyte chemoattractant protein (MCP-1) (also know as chemokine ligand 2 (CCL2)) that has been implicated in breast cancer progression [16, 17] has been shown to be reduced by exercise [18, 19]. Similarly, interleukin 6 (IL-6), which has been associated with poor outcome in breast cancer patients [16, 20, 21], has been reported to be reduced following exercise training [22]. We have shown that exercise training can reduce plasma IL-6 concentration in the ApcMin/+ mouse model of intestinal tumorigenesis [23]. However, there have been no controlled experimental studies that have examined the effects of exercise on reducing systemic levels of MCP-1, IL-6, or any other inflammatory mediators in a mouse model of breast cancer.
The purpose of this study was to examine the relationship between exercise and systemic inflammation in relation to breast cancer progression in the triple negative C3(1)SV40Tag mouse model of mammary tumorigenesis. These mice lack expression for estrogen receptor (ER), progesterone receptor (PR) and ERBB2 (Her2, Neu), and the absence of these has been associated with poor prognosis [24]. The overexpression of the early region of SV40 in the mammary epithelium induces mammary tumors in this model is due, at least in part, to Tag inactivation of the tumor suppressors p53 and Rb [25]. The expression of this transgene results in the progressive development of mammary lesions that lead to invasive carcinoma formation [26]. C3(1)SV40Tag mice develop mammary epithelial atypia at 8 wks of age that progresses to mammary intraepithelial neoplasia (MIN) at 12 wks and which is histologically similar to human ductal carcinoma in situ (DCIS). Invasive carcinomas usually develop at approximately 16 wks of age [27, 28]. All of the chemopreventive agents examined in the C3(1)SV40Tag mouse model of breast cancer, including anti-inflammatory agents, appear to reduce the promotion of tumorigenesis [29]. However, there have been no studies that have evaluated exercise-induced prevention of breast cancer in this model. We hypothesized that exercise training would reduce tumor progression in C3(1)SV40tag mice and that this would be associated with a reduction in markers of inflammation (MCP-1, IL-6 and spleen weight).
2. Materials and Methods
2.1 Animals
Female FVB wildtype mice were purchased from (Harlan Spague-Dawley Laboratories) and bred with male homozygous C3(1)SV40Tag mice (a gift from Dr. Jeffrey Green, Chief, Transgenic Oncogenesis and Genomics Section, Laboratory of Cancer Biology and Genetics, National Cancer Institute) in the animal research facility at the University of South Carolina. Mice were maintained on a 12:12 h light-dark cycle in a low-stress environment (22°C, 50% humidity and low noise) and provided food and water ad libitum. All animal experimentation was approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
2.2 Exercise Treatment
At four weeks of age C3(1)SV40Tag mice were randomly assigned to either exercise (Ex) (n=12) or sedentary (Sed) (n=14) treatment. Mice in the exercise group ran on a treadmill for 60 min at a speed of 20m/min and 5% grade 6 days a wk for 20 wks (age 4 wks to 24 wks) that is estimated to be approximately 70% of VO2max. Training regimens similar to this routinely cause cardiovascular and muscular adaptations, which as we have reported is also the case in terms of muscle citrate synthase activity in an animal model of cancer [23]. Gentle hand prodding on the tail of the mouse was used to encourage them to maintain pace with the treadmill. Mice in the sedentary groups remained in their cages in the treadmill room throughout the exercise bouts. These mice were exposed to similar noise in an attempt to control for extraneous stresses that may be associated with treadmill running and were handled daily similar to the exercise mice. All exercise training was performed at the beginning of the active dark cycle. An FVB wildtype sedentary group (n=10) was also included as a comparison for inflammatory measures. We did not include a wildtype exercise group in our investigation; wildtype mice do not develop mammary tumors and it is unlikely that they would display significant inflammation.
2.3 Body weight and food intake
Body weight and food intake were measured weekly throughout the treatment period. C3(1)SV40Tag mice develop large mammary tumors that account for a substantial percentage of the animal’s total body weight, therefore at sacrifice body weight minus tumor weight also was recorded to obtain a more accurate measure of body weight.
2.4 Tumor Progression
Mice were examined weekly for external tumor growth, using calipers, by an investigator blinded to the treatments. C3(1)SV40Tag mice begin to develop mammary tumors at approximately 12 wks of age. Length and width of the tumors were recorded and volume calculated as L x W2 (L, length; W width) based on the external measurements and were summed to obtain total volume as previously described [30]. The length and width were consistently measured based on the orientation of the mouse (i.e. measurements made in the vertical plane were classified as length and in the lateral plane as width).
2.5 Sacrifice and Tissue Collection
All mice were sacrificed at 24 wks of age and 24 h following the last exercise bout using isoflurane inhalation. All noticeable tumors were removed from the mouse after which each tumor was measured as previously described to determine tumor volume. Spleens were removed and weight was recorded as an indirect measure of inflammation. At the time of sacrifice, blood was collected from the inferior vena cava in a heparinized syringe, and centrifuged at 4,000rpm for 10 minutes at 4°C. Plasma was aliquoted and stored at −80°C until analysis.
2.6 Concentration of inflammatory markers
Plasma concentration of MCP-1 and IL-6 were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN). The assay was performed according to the manufacturer’s instructions.
2.7 Statistical Analysis
All data were analyzed using commercial software (SigmaStat, SPSS, Chicago, IL). Food intake, body weight, tumor number, and tumor volume over time were analyzed using repeated measures ANOVA with Student-Newman-Keuls post-hoc analysis where appropriate. End point data were analyzed using a one-way ANOVA with Student-Newman-Keuls post-hoc analysis. Correlations were performed between inflammatory cytokines (MCP-1 and IL-6) and tumor number and volume. Statistical significance was set with an alpha value of P<0.05 (two tailed). Data are presented as mean (±SEM).
3. Results
3.1 Body Weight and Food Intake
Body weight and food intake were measured weekly during the treatment period (4 wks to 24 wks of age). Mice in the exercise group consumed significantly greater amounts of food at age 6-24 wks (P<0.05) (data not shown) and showed a reduction in body weight at 8 wks and 23wks (P<0.05) (data not shown). However, because C3(1)SV40Tag mice develop large mammary tumors that may account for a substantial percentage of the animal’s body weight, the weekly data on body weight should be interpreted with caution. At sacrifice tumor weight was subtracted from total body weight to get a direct measure of body weight. Mice in the exercise group had significantly reduced body weight at sacrifice (24.8±0.7 versus 26.9±0.4g) (P=0.024; F=5.781).
3.2 Tumor Burden
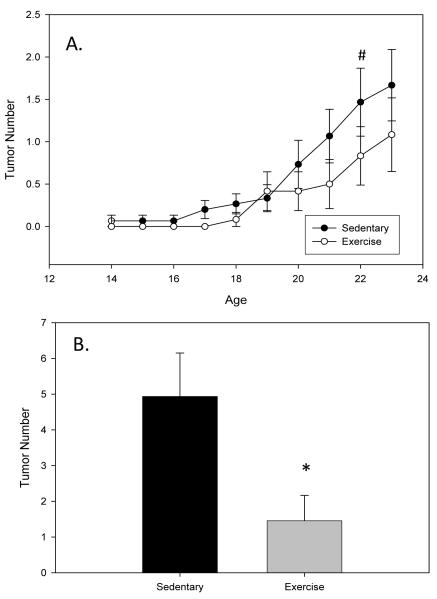
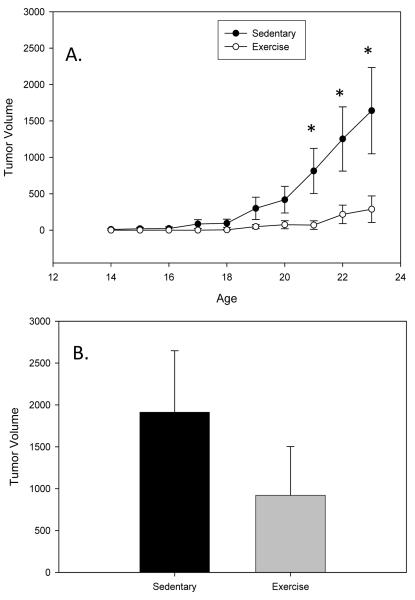
Tumor number and size (volume) were measured via external palpation weekly throughout the treatment period as well as at the time of sacrifice to get a more direct measurement following dissection. While most of the mammary tumors in C3(1)SV40Tag mice are visible from the exterior some of the tumors can not be detected until sacrifice and therefore the tumor burden at sacrifice is a more accurate measure of tumor progression. All mice survived until the sacrifice time-point. Data indicates a trend toward a significant effect of exercise at reducing tumor number at 17 wks (P=0.098; F=2.778) (Figure 1A). At sacrifice (24 wks) tumor number was reduced by approximately 70% (1.5±0.7 versus 4.9±1.2; P=0.036; F=4.959) (Figure 1B). Similarly, exercise training reduced tumor volume at 21 (P=0.045; F=4.467) and 22 wks (P=0.046; F=4.430) and there was a trend for a decrease at 23 wks (P=0.062; F=3.843) (Figure 2A). Tumor volume at sacrifice was also reduced in the exercise group; however, this did not reach statistical significance (Figure 2B).
Figure 1.
Exercise reduces tumor number in C3(1)SV40Tag mice. Mice were run on a treadmill (20m/min and 5% grade for 60 minutes) from 4 wks to 24 wks of age (n=12-14/group). Tumor number was examined weekly (A; palpable tumors) and at sacrifice (B; total tumors). Values are means ± SEM. * significantly different from Sedentary P<0.05. # trend toward a significant effect of exercise P<0.1.
Figure 2.
Exercise reduces tumor volume in C3(1)SV40Tag mice. Mice were run on a treadmill (20m/min and 5% grade for 60 minutes) from 4 wks to 24 wks of age (n=12-14/group). Tumor volume was determined weekly (A) and at sacrifice (B). Values are means ± SEM. * significantly different from Sedentary P<0.05.
3.3 Spleen Weight
Spleen weight was examined as an indirect measure of inflammation; the spleen is an immunological tissue that increases in size during various inflammatory challenges including infection and cancer, initiating an immune response directed towards specific tissues that need repair [31]. We have previously reported that a reduction in spleen weight was associated with reduced IL-6 in male ApcMin/+ mice following exercise [23]. Our data shows that there was a significant difference in spleen weight across groups (P=0.036; F=3.652). Spleen weight was significantly increased in
C3(1)SV40Tag mice compared to wildype mice (P=0.029) and exercise offset this effect; there was no difference in spleen weight in the exercise group versus the wildtype control group. Average spleen weight was 229.7±35 mg in the sedentary group versus 174.4±25 mg in the exercise group. Spleen weight in the wildtype mice was 118±3 mg. Because mice were sacrificed 24 h following the last exercise bout it is unlikely that these effects are due to changes in splenocyte mobilization that have been documented to occur following acute exercise bouts.
3.4 Plasma Concentration of Inflammatory Markers
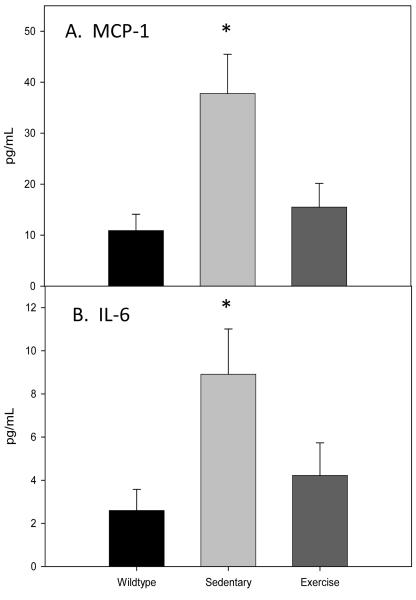
MCP-1 and IL-6 were measured in the plasma of C3(1)SV40Tag mice following exercise or sedentary treatment. A wildtype control group also was included as a comparison. All samples were run in duplicate; coefficient of variation between duplicates was < 4%. Our data indicate a difference in MCP-1 (P=0.008; F= 5.644) (Figure 3A) and IL-6 (P=0.035; F=3.698) (Figure 3B) across groups. MCP-1 and IL-6 were increased in C3(1)SV40Tag mice (P=0.011 and P=0.038 , respectively) and exercise offset this effect (P=0.018 and P=0.059), respectively). MCP-1 concentration was 37.8±7.7, 15.5±4.7 and 10.9±3.2 and IL-6 concentration was 8.9±2.1, 4.2±1.5 and 2.6±1.0 pg/mL for sedentary, exercise and wildtype, respectively. There was a positive correlation between tumor number (R = 0.828; P<0.001) and volume (R = 0.809; P<0.001) for MCP-1. IL-6 also was positively associated with tumor number (R = 0.642; P<0.001) and volume (R = 0.442; P=0.024) although the effects were not as strong as those observed with MCP-1.
Figure 3.
Exercise blocks the increase in plasma MCP-1 and IL-6 in C3(1)SV40Tag mice. Mice were run on a treadmill (20m/min and 5% grade for 60 minutes) from 4 wks to 24 wks of age. Plasma concentration of MCP-1 (A) and IL-6 (B) were determined at 24wks of age. Wildtype mice were used as a control. Values are means ± SEM. * Significantly different from Wildtype P<0.05.
4. Discussion
While the mechanisms involved in exercise-induced prevention of breast cancer have yet to be completely elucidated, recent epidemiological studies have proposed that the anti-inflammatory effects of exercise may play a role [12, 13]. This study represents the first time that a well established genetically engineered mouse model of triple negative breast cancer (C3(1)SV40Tag) has been used to examine the relationship between exercise and systemic inflammation in relation to breast cancer progression. Our findings indicate that exercise can reduce tumor progression (number and volume) in this mouse model and that these effects may, at least in part, be mediated by a reduction in inflammation (MCP-1 and IL-6). This is also the first experimental study to report an exercise-induced reduction in inflammation (MCP-1 and IL-6) in association with a reduction in breast cancer.
Animal models provide a tool to examine the effects of exercise on tumorigenesis in an experimental environment in which the type and intensity of exercise can be controlled. They allow for detailed study of stage-specific responses to exercise, and help to identify the optimal mode, intensity and duration of exercise. To date there are few animal models that have been used to study the relationship between exercise and mammary tumorigenesis, therefore controlled experimental studies in rodent models are limited and those that are available often report inconsistent findings [4]. The largest number of experimental studies on the exercise-induced prevention of breast cancer has been in rodent models of chemically induced mammary carcinogenesis [5-8]. However, this does not mimic the human disease, and inconsistencies in mammary tumor outcomes have been reported using this approach which has been linked to methodological issues with the dose, timing, route and type of carcinogen. Genetically engineered mouse models of breast cancer have been generated to more closely mimic the spontaneous development of breast cancer in humans. However, to our knowledge there has been only one study that has examined the effects of exercise on breast cancer using a genetically engineered mouse model [9]. Colbert et al., reported a detrimental effect of both treadmill and wheel-running activity on mammary tumorigenesis in a p53-deficient (p53+/−): MMTV-Wnt-1 mouse model of breast cancer [9]. Treadmill running increased the rate of tumor development and the proportion of mice with multiple carcinomas and decreased survival time, while wheel-running resulted in an increased incidence and multiplicity of mammary carcinomas [9]. The differences in findings between our study and that of Colbert et al., are likely due, at least in part, to the model used; it has been proposed that the negative effects of exercise in the (p53+/−): MMTV-Wnt-1 model may result from an exercise-induced increase in expression of the Wnt-1 transgene or an interaction between p53 dosage and exercise [9].
While the lack of appropriate animal models is likely to play a role in the inconsistencies among studies, differences in the type, intensity, duration, and timing of exercise are likely to also contribute. Thompson et al. reported that exercise intensity is the most important determinant of whether a protective effect is observed [32]. In general, exercise intensity that is >70% of maximal aerobic capacity appears to consistently inhibit mammary tumorigenesis whereas studies involving lower intensity exercise (<35% of maximal aerobic capacity) show both decreases and increases in tumorigenesis [32]. The treadmill protocol used in our investigation is estimated to elicit approximately 70% VO2max for one hour which is consistent with those findings that have reported a beneficial effect of exercise on mammary tumorigenesis. The timing of exercise in relation to development of tumorigenesis is also likely to play a role in whether a protective effect of exercise exists. While the intensity and duration of exercise between that of Colbert et al. and the present study were similar, our exercise training began at 4 wks of age whereas that of Colbert et al., began after 10 wks of age [9] which provides another possible explanation for the differences in findings, one with particularly important implications for human health (e.g., when should girls be encouraged to get serious about physical activity?).
The preventive effects of exercise on mammary tumorigenesis are likely to involve several inter-related mechanisms including energy balance, anthropomorphic characteristics, hormonal status and immune function [33]. However, there have been limited animal data to substantiate these claims. We show that exercise can offset the increase in markers of inflammation, including plasma MCP-1 and IL-6 concentration, as well as spleen weight in C3(1)SV40Tag mice. MCP-1 has been identified as a major chemokine for macrophage recruitment in several human tumors [17] and has been correlated with increased grade of the tumor [34] and poor prognosis in breast cancer as well as other cancers [17, 35]. Serum MCP-1 levels have been correlated with advanced tumor stage and lymph node involvement [36]. Similarly, the pro-inflammatory cytokine IL-6 has been associated with protumorigenic activities within the breast tumor microenvironment and elevated expression of this cytokine is directly correlated with advanced disease states [16, 20, 21] and circulating levels of this cytokine have been reported to be a predictor of survival in breast cancer patients [20]. While it is clear from our findings that the benefits of exercise on tumorigenesis are associated with a reduction in inflammation, this study does not address whether or not this is a result of a direct effect of exercise training or an indirect effect of the benefits of exercise on tumorigenesis.
Although it was not the purpose of this study it is worth noting that our findings show that exercise reduces body weight in the C3(1)SV40Tag mice even though food consumption is increased which is consistent with most training studies in healthy and diseased animals. There is evidence to support a relationship between changes in energy balance, body composition and breast cancer progression [37]; however, the complexity of this relationship makes it difficult to draw firm conclusions about the relative importance of each [6-8, 38]. Changes in both energy expenditure and energy intake can have distinct effects on various factors that can affect cancer progression including, hormones [4], immune function [39] and mammary gland gene expression [40] and are therefore likely to modulate their effects through different biologically mechanisms that further highlights the intricacy of this relationship. While this study was not designed to distinguish the relative importance of these exercise-related factors on breast cancer progression it does contribute to the available literature that supports a role for reduced body weight as a possible contributing factor.
In summary, these data are the first to show a benefit of long-term rigorous exercise training on mammary tumorigenesis (number and volume) in a genetically engineered mouse model of triple negative breast cancer that progresses through stages closely resembling human DCIS. Further, this is the first report of an exercise-induced reduction in systemic inflammation as a possible mechanism for the benefits of exercise in any mouse model of mammary tumorigenesis. Our findings provide strong support for further development of the C3(1)SV40Tag mouse model for use in understanding the mechanisms of an apparent benefit of exercise training on breast cancer progression. The results of this animal model of mammary tumorigenesis may have special relevance to African-American women who are more like to present with triple negative tumors [41] and to have generally poor prognoses [42].
ACKNOWLEDGEMENTS
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute to JR Hébert (K05 CA136975).
REFERENCES
- 1.Adams SA, et al. Association of physical activity with hormone receptor status: the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1170–8. doi: 10.1158/1055-9965.EPI-05-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tehard B, et al. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(1):57–64. doi: 10.1158/1055-9965.EPI-05-0603. [DOI] [PubMed] [Google Scholar]
- 3.Dal Maso L, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123(9):2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman-Goetz L. Physical activity and cancer prevention: animal-tumor models. Med Sci Sports Exerc. 2003;35(11):1828–33. doi: 10.1249/01.MSS.0000093621.09328.70. [DOI] [PubMed] [Google Scholar]
- 5.Whittal KS, Parkhouse WS. Exercise during adolescence and its effects on mammary gland development, proliferation, and nitrosomethylurea (NMU) induced tumorigenesis in rats. Breast Cancer Res Treat. 1996;37(1):21–7. doi: 10.1007/BF01806628. [DOI] [PubMed] [Google Scholar]
- 6.Lane HW, et al. Reduced energy intake and moderate exercise reduce mammary tumor incidence in virgin female BALB/c mice treated with 7,12-dimethylbenz(a)anthracene. J Nutr. 1991;121(11):1883–8. doi: 10.1093/jn/121.11.1883. [DOI] [PubMed] [Google Scholar]
- 7.Thompson HJ, et al. Exercise intensity dependent inhibition of 1-methyl-1-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis. 1995;16(8):1783–6. doi: 10.1093/carcin/16.8.1783. [DOI] [PubMed] [Google Scholar]
- 8.Westerlind KC, et al. Moderate exercise training slows mammary tumour growth in adolescent rats. Eur J Cancer Prev. 2003;12(4):281–7. doi: 10.1097/00008469-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Colbert LH, et al. Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc. 2009;41(8):1597–605. doi: 10.1249/MSS.0b013e31819f1f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson HJ, et al. Effect of type and amount of dietary fat on the enhancement of rat mammary tumorigenesis by exercise. Cancer Res. 1989;49(8):1904–8. [PubMed] [Google Scholar]
- 11.Thompson HJ, et al. Effect of exercise on the induction of mammary carcinogenesis. Cancer Res. 1988;48(10):2720–3. [PubMed] [Google Scholar]
- 12.Neilson HK, et al. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18(1):11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 13.Pierce BL, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114(1):155–67. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 16.Rao VS, et al. Potential prognostic and therapeutic roles for cytokines in breast cancer (Review) Oncol Rep. 2006;15(1):179–85. doi: 10.3892/or.15.1.179. [DOI] [PubMed] [Google Scholar]
- 17.Ueno T, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–9. [PubMed] [Google Scholar]
- 18.Vieira VJ, et al. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab. 2009;296(5):E1164–71. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieira VJ, et al. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 2009;46(3):339–45. doi: 10.1016/j.cyto.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salgado R, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103(5):642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 21.Snoussi K, et al. Genetic variation in pro-inflammatory cytokines (interleukin-1beta, interleukin-1alpha and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur Cytokine Netw. 2005;16(4):253–60. [PubMed] [Google Scholar]
- 22.Oberbach A, et al. Long-term exercise training decreases interleukin-6 (IL-6) serum levels in subjects with impaired glucose tolerance: effect of the −174G/C variant in IL-6 gene. Eur J Endocrinol. 2008;159(2):129–36. doi: 10.1530/EJE-08-0220. [DOI] [PubMed] [Google Scholar]
- 23.Mehl KA, et al. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98(6):2219–25. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 24.Bennett CN, Green JE. Genomic analyses as a guide to target identification and preclinical testing of mouse models of breast cancer. Toxicol Pathol. 38(1):88–95. doi: 10.1177/0192623309357074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tennant RW, et al. Chemical effects in transgenic mice bearing oncogenes expressed in mammary tissue. Carcinogenesis. 1993;14(1):29–35. doi: 10.1093/carcin/14.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Maroulakou IG, et al. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A. 1994;91(23):11236–40. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green JE, et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene. 2000;19(8):1020–7. doi: 10.1038/sj.onc.1203280. [DOI] [PubMed] [Google Scholar]
- 28.Shibata MA, et al. The C3(1)/SV40 T antigen transgenic mouse model of prostate and mammary cancer. Toxicol Pathol. 1998;26(1):177–82. doi: 10.1177/019262339802600121. [DOI] [PubMed] [Google Scholar]
- 29.Kavanaugh C, Green JE. The use of genetically altered mice for breast cancer prevention studies. J Nutr. 2003;133(7 Suppl):2404S–2409S. doi: 10.1093/jn/133.7.2404S. [DOI] [PubMed] [Google Scholar]
- 30.Gupta V, et al. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci U S A. 2005;102(9):3219–24. doi: 10.1073/pnas.0409709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowdler AJ. The Complete Spleen: Structure, Function, and Clinical Disorders. Humana; Totowa, NJ: 2002. [Google Scholar]
- 32.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat. 1997;46(2-3):135–41. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman-Goetz L, et al. Possible mechanisms mediating an association between physical activity and breast cancer. Cancer. 1998;83(3 Suppl):621–8. doi: 10.1002/(sici)1097-0142(19980801)83:3+<621::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Amann B, et al. Urinary levels of monocyte chemo-attractant protein-1 correlate with tumour stage and grade in patients with bladder cancer. Br J Urol. 1998;82(1):118–21. doi: 10.1046/j.1464-410x.1998.00675.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka K, et al. The expression of monocyte chemotactic protein-1 in papillary thyroid carcinoma is correlated with lymph node metastasis and tumor recurrence. Thyroid. 2009;19(1):21–5. doi: 10.1089/thy.2008.0237. [DOI] [PubMed] [Google Scholar]
- 36.Lebrecht A, et al. Monocyte chemoattractant protein-1 serum levels in patients with breast cancer. Tumour Biol. 2004;25(1-2):14–7. doi: 10.1159/000077718. [DOI] [PubMed] [Google Scholar]
- 37.Thompson HJ, Zhu Z, Jiang W. Weight control and breast cancer prevention: are the effects of reduced energy intake equivalent to those of increased energy expenditure? J Nutr. 2004;134(12 Suppl):3407S–3411S. doi: 10.1093/jn/134.12.3407S. [DOI] [PubMed] [Google Scholar]
- 38.Cohen LA, et al. Modulation of N-nitrosomethylurea-induced mammary tumor promotion by dietary fiber and fat. J Natl Cancer Inst. 1991;83(7):496–501. doi: 10.1093/jnci/83.7.496. [DOI] [PubMed] [Google Scholar]
- 39.Rogers CJ, et al. Energy restriction and exercise differentially enhance components of systemic and mucosal immunity in mice. J Nutr. 2008;138(1):115–22. doi: 10.1093/jn/138.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padovani M, et al. Distinct effects of calorie restriction and exercise on mammary gland gene expression in C57BL/6 mice. Cancer Prev Res (Phila) 2009;2(12):1076–87. doi: 10.1158/1940-6207.CAPR-09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52(1):108–18. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 42.Hebert JR, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115(11):2539–52. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]