Abstract
OBJECTIVE
Colonization pressure is an important infection control metric. The aim of this study was to describe the definition and measurement of and adjustment for colonization pressure in nosocomial-acquisition risk factor studies of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile.
METHODS
We performed a computerized search of studies of nosocomial MRSA, VRE, and C. difficile acquisition published before July 1, 2009, through MEDLINE. Studies were included if a study outcome was MRSA, VRE, or C. difficile acquisition; the authors identified risk factors associated with MRSA, VRE, or C. difficile acquisition; and the study measured colonization pressure.
RESULTS
The initial MEDLINE search yielded 505 articles. Sixty-six of these were identified as studies of nosocomial MRSA, VRE, or C. difficile acquisition; of these, 18 (27%) measured colonization pressure and were included in the final review. The definition of colonization pressure varied considerably between studies: the proportion of MRSA- or VRE-positive patients (5 studies), the proportion of MRSA- or VRE-positive patient-days (6 studies), or the total or mean number of MRSA-, VRE-, or C. difficile–positive patients or patient-days (7 studies) in the unit over periods of varying length. In 10 of 13 studies, colonization pressure was independently associated with MRSA, VRE, or C. difficile acquisition.
CONCLUSION
There is a need for a simple and consistent method to quantify colonization pressure in both research and routine clinical care to accurately assess the effect of colonization pressure on cross-transmission of antibiotic-resistant bacteria.
Colonization pressure is an important infection control metric that was first described by Bonten et al.1 in 1994. It is defined as the proportion of patients colonized with a particular organism in a defined geographic area within a hospital during a specified time period. Therefore, colonization pressure can be used to quantify the burden of antibiotic-resistant bacteria in a hospital unit and can also represent an estimate of the probability of cross-transmission of antibiotic-resistant bacteria within the unit. For example, the risk of transmission is likely higher when 80% of the patients are already colonized than when only 10% of the patients are colonized.2 Thus, colonization pressure may potentially provide a method for adjusting for the burden of antibiotic-resistant bacteria while assessing the independent associations of other hypothesized causal factors with acquisition of antibiotic-resistant bacteria in epidemiology studies.
Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile are prevalent antibiotic-resistant bacteria in healthcare settings, and there is strong evidence that these organisms can be transmitted from patient to patient.3,4 Thus, colonization pressure is likely an important risk factor for acquisition of these antibiotic-resistant bacteria, and these species are the subject of this review. The aim of this study was to describe the definition and measurement of and adjustment for colonization pressure in nosocomial-acquisition risk factor studies of MRSA, VRE, and C. difficile.
METHODS
Identification of Relevant Articles
We performed a computerized search of studies of nosocomial MRSA, VRE, and C. difficile acquisition published before July 1, 2009. The search terms “MRSA acquisition,” “VRE acquisition,” “Clostridium difficile acquisition,” “colonization pressure,” “MRSA acquisition AND colonization pressure,” “VRE acquisition AND colonization pressure,” and “Clostridium difficile acquisition AND colonization pressure” were used for the search in MEDLINE. MRSA, VRE, and C. difficile acquisition was defined as isolation of MRSA or VRE or a positive toxin assay for C. difficile from a surveillance culture sample, a clinical culture sample, or both after the first 24 hours of admission if a patient had a negative culture result for MRSA, VRE, or C. difficile or no stool culture positive for C. difficile on admission to the hospital unit.
Additional studies were identified by a review of cited references from all retrieved articles. The complete set of titles and abstracts from the search was independently reviewed by one investigator (A.O.A.) to identify those that met the inclusion and exclusion criteria. The studies selected for the final review were further reviewed by another author (J.P.F.) to ensure that they met the inclusion criteria. Because this study did not involve patient or research subject data, it did not require approval by the Institutional Review Board.
Inclusion and Exclusion Criteria
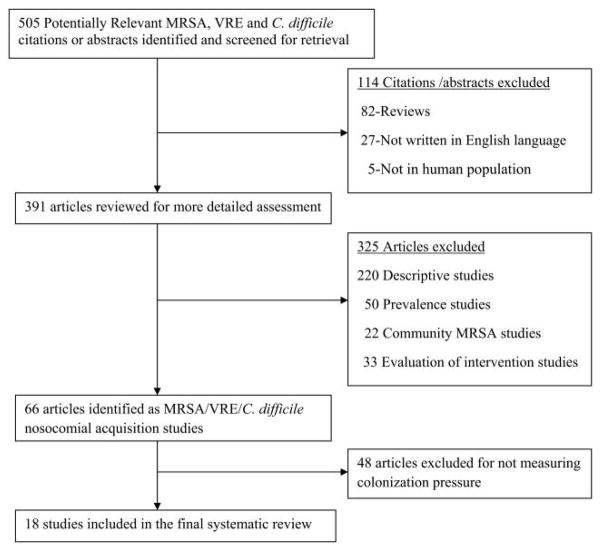
Studies were included if all three of the following criteria were met: (1) a study outcome was MRSA, VRE, or C. difficile acquisition; (2) the authors aimed to identify risk factors associated with MRSA, VRE, or C. difficile acquisition, that is, the study used epidemiologic and statistical methods to identify variables (eg, antibiotic exposures, medical devices, patient comorbidities) associated with MRSA, VRE, or C. difficile acquisition; and (3) the study measured colonization pressure. Studies were excluded if the study described only the demographic and clinical characteristics of patients colonized or infected with MRSA, VRE, or C. difficile; described only the risk factors for patients colonized or infected with MRSA, VRE, or C. difficile on hospital admission; described only the molecular subtypes of the colonizing or infecting MRSA, VRE, or C. difficile isolates; reported only the prevalence of MRSA, VRE, or C. difficile; or reported only the effect of an intervention on the incidence of MRSA, VRE, or C. difficile but did not explore factors associated with MRSA, VRE, or C. difficile acquisition. In addition, studies were excluded if they did not have humans as subjects, were not written in English, or were review articles (Figure 1).
FIGURE 1.
Article selection tree for systematic review.
Data Extraction
The following information was extracted for each study that met the inclusion criteria: author(s) and year of publication; country of study origin; study design; hospital setting (eg, intensive care unit [ICU]); body sites of culture samples and organisms isolated; definition of colonization pressure; culture type (eg, surveillance or clinical) used to determine MRSA, VRE, or C. difficile acquisition; methods of statistical analysis; and study results.
RESULTS
The initial search yielded 505 articles: 378 on MRSA, 72 on VRE, and 55 on C. difficile. Following initial review and application of the inclusion criteria, 66 studies (31 of MRSA, 18 of VRE, and 17 of C. difficile) were identified as nosocomial-acquisition studies. Of these 66 studies, 18 (27%) measured colonization pressure and were included in the final review (Figure 1).
General Description of the Studies Included in the Systematic Review
Of the 18 studies, 10 were conducted in the United States, 6 in Europe, 1 in Canada, and 1 in Hong Kong. Thirteen studies were prospective cohort studies, 3 were retrospective cohort studies, and 2 were case-control studies (Table 1). Fourteen studies included ICU patients only, 1 included only general medical patients, and 3 included both ICU and general medical patients. Seven studies assessed only MRSA acquisition, 7 assessed only VRE acquisition, 1 assessed both MRSA and VRE acquisition, and 3 assessed only C. difficile acquisition.
TABLE 1.
Characteristics of Studies Included in the Final Review
| First author, year | Country | Study design | Hospital setting |
Organism isolated |
Body sites of culture sample |
Definition of colonization pressure |
Culture type (frequency) |
Reported adjusted measurea |
Other factorsb |
|---|---|---|---|---|---|---|---|---|---|
| Bloemendaal 20095 | Netherlands, France, Portugal, Spain, Italy | Prospective cohort | ICUs (77 beds) | MRSA | Nares, perineum, and clinical samples | No. of MRSA- positive patients in the unit during the 3 days preceding acquisition or during the study period for patients without acquisition | Surveillance (admission, twice weekly, discharge), clinical culture | Multivariable analysis not performed | |
| Williams 20096 | Canada | Prospective cohort | General medicine (36 beds) | MRSA | Nares, perineum, and clinical samples (wound) | Proportion of MRSA-positive patient-days among total patient-days in a month | Surveillance (admission, point prevalence every 4 months), clinical culture | Multivariable analysis not performed | |
| Drees 20087 | United States | Prospective cohort | ICU (20 beds) | VRE | Rectum, stool specimen | Average daily pro- portion of VRE-positive patients in the ICU until VRE acquisition or discharge | Surveillance (admission, twice weekly, discharge) | HR = 1.4 (95% CI, 1.12–1.84) | Environmental contamination, mean no. of antibiotics per day, leukemia |
| Dubberke 20078 | United States | Retrospective cohort | General hospital patients | Clostridium difficile | Stool specimen | Average daily no. of CDAD-positive patients present in the ward during patient’s stay at risk in the ward | Clinical culture | OR = 2.9 (95% CI, 2.1–4.2) for mean CDAD pressure of 0.3–1.4; OR = 4.0 (95% CI, 2.9–5.6) for mean CDAD pressure of >1.4 | Mechanical ventilation, low albumin level, leukemia/lymphoma, antimobility agent, H2 blockers, PPI, >7-day course of FQ, vancomycin, or 1G or 3G cephalosporin, >0-day course of metronidazole or 4G cephalosporin |
| Dubberke 20079 | United States | Nested case-control | General hospital patients | C. difficile | Stool specimen | Total daily no. of CDAD-positive patients present in the ward during patient’s stay in the ward; average daily no. of CDAD-positive patients present in the ward during patient’s stay at risk in the ward | Clinical culture | OR = 2.9 (95% CI, 2.0–4.3) for sum CDAD pressure of 2–8; OR = 4.0 (95% CI, 2.7–6.0) for sum CDAD pressure of >8; OR = 3.9 (95% CI, 2.6–5.8) for mean CDAD pressure of 0.3–1.4; OR = 5.4 (95% CI, 3.4–8) for mean CDAD pressure of >1.4 | Age ≥45 years; admission to the facility in the previous 60 days; CMI of 1–2; modified APS of 3–5; receipt of gastric acid suppressor, narcotic, or antidiarrheal agent; low albumin level; receipt of FQ or 3G or 4G cephalosporin |
| Lawrence 200710 | United States | Retrospective cohort | ICU (19 beds) | C. difficile | Stool specimen | Total daily no. of CDAD-positive patients in the ICU unit during patient’s time at risk in the ICU | Clinical culture | OR = 3.77 (95% CI, 1.14–12.49) for CCP of >30 case-days of exposure | VRE colonization or infection |
| Dancer 200611 | Scotland | Retrospective cohort | ICU (8 beds) | MRSA | Blood | Proportion of MRSA-positive patient-days per total patient-days in the unit for the week | Surveillance (admission, alternate days), clinical culture | Multivariable analysis not performed | |
| Cepeda 200512 | United Kingdom | Prospective cohort | ICU (28 beds) | MRSA | Nares, groin, and clinical samples (sputum, wound, blood) | Total daily no. of MRSA-positive patients present in the ICU during patient’s ICU stay | Surveillance (admission, weekly, discharge), clinical culture | HR = 1.19 (95% CI, 0.86–1.65) | Anti-MSSA antibiotic use |
| Lucet 200513 | France | Prospective cohort | ICU (47 beds) | MRSA | Nares | Proportion of MRSA-positive patient-days per total patient- days in the unit during the week preceding acquisition or discharge | Surveillance (admission, weekly), clinical culture | OR = 1.019; P < .0001 | Age, severity of illness (SAPS II) ICU LOS |
| Winston 200414 | United States | Prospective cohort | ICU (30 beds) | VRE | Rectum | Average daily pro- portion of VRE-positive patients in the ICU during each patient’s ICU stay | Surveillance (twice weekly) | CP not significant in multivariable analysis (HR not reported) | End-stage renal disease, ICU LOS, ceftria-zone and piper-acillin-tazobactam use |
| Martínez 200315 | United States | Case-control | ICU (10 beds) | VRE | Rectum, clinical sample | Average daily pro- portion of VRE-positive patients in the ICU during each patient’s ICU stay | Surveillance (admission, weekly), clinical culture | CP not significant in multivariable analysis | Pre-ICU LOS, pre-ICU vancomycin and quinolone use, location in high-risk MICU room |
| Ho 200316 | Hong Kong | Prospective cohort | ICU (92–170 beds) | MRSA, VRE | Nares, throat, rectum | Proportion of MRSA-positive patients at ICU entry | Surveillance (admission, discharge) | CP not included in multivariable analysis | |
| Muller 200317 | France | Prospective cohort | ICU, general medical, surgical unit | MRSA | Nares, clinical sample | Proportion of MRSA-positive patient-days per total patient-days in the unit during the study period | Surveillance (admission), clinical culture | OR = 1.02 (95% CI, 1.02–1.03) | Admission to the ICU; antibiotic exposure to β-lactams, FQ, and macrolides during ICU stay |
| Srinavasan 200218 | United States | Prospective cohort | ICU (16 beds) | VRE | Perineum, rectum | Proportion of VRE-positive patient-days per total patient-days in the unit during the study period | Surveillance (admission, weekly) | CP not included in multivariable analysis | |
| Puzniak 200219 | United States | Quasi-experimental prospective cohort | ICU (19 beds) | VRE | Rectum, stool sample | Total no. of VRE-positive patients present during each patient’s ICU stay | Surveillance (admission, weekly, discharge) | CP significant in multivariable analysis but OR not reported | Anaerobic therapy duration |
| Puzniak 200120 | United States | Quasi- experimental prospective cohort | ICU (19 beds) | VRE | Rectum, stool sample, blood | Total no. of VRE-positive patients present during each patient’s ICU stay | Surveillance (admission, weekly, discharge), clinical culture | OR = 1.06 (95% CI, 1.00–1.12) | Enteral feeding, anaerobic therapy duration |
| Merrer 200021 | France | Prospective cohort | ICU (12 beds) | MRSA | Nares, axilla, perineum, and clinical samples | Proportion of MRSA-positive patient-days per total patient-days in the unit for the week | Surveillance (admission, weekly) clinical culture | OR not reported; CP significant in multivariable analysis (P = .002) | |
| Bonten 19982 | United States | Prospective cohort | ICU (16 beds) | VRE | Rectum | Average daily pro-portion of VRE-positive patients in the ICU until VRE acquisition or discharge | Surveillance (admission and daily thereafter) | HR = 1.03 (95% CI, 1.01–1.05) | Enteral feeding, proportion of patient-days with cephalosporin use |
NOTE. 1G, first-generation; 3G, third-generation; 4G, fourth-generation; CCP, C. difficile colonization pressure; CDAD, C. difficile–associated disease; CMI, Charlson comorbidity index; CP, colonization pressure; FQ, fluoroquinolone; H2, histamine-2; HR, hazard ratio; ICU, intensive care unit; LOS, length of stay; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; OR, odds ratio; PPI, proton pump inhibitor; SAPS II, simplified Acute Physiology Score; VRE, vancomycin-resistant enterococci.
Measure of effect for colonization pressure. An odds ratio of 1.02 for colonization pressure indicates that for each 1% increase in colonization pressure, the odds of acquiring the antibiotic-resistant bacteria increased by 2%. A hazard ratio of 1.4 for colonization pressure indicates that for each 1% increase in colonization pressure, the risk of acquiring the antibiotic-resistant bacteria increased by 40%.
Other factors associated with MRSA, VRE, and/or C. difficile acquisition after adjustment for colonization pressure.
Of the 8 MRSA studies, 7 defined MRSA acquisition using surveillance and clinical cultures, while 1 did so using only surveillance cultures. Seven of the 8 MRSA studies indicated that the nares were the primary body site for culture samples to determine MRSA colonization. Five studies used culture samples from other body sites, such as the perineum,5,6,21 throat,16 groin,12 and axilla,21 in addition to the nares, to determine MRSA colonization. Clinical cultures used to determine MRSA acquisition included blood, sputum, and wound cultures.6,11,12
Of the 8 VRE studies, 3 defined VRE acquisition using both surveillance and clinical cultures,15,19,20 while 5 defined VRE acquisition using only surveillance cultures.2,7,14,16,20 All 8 VRE studies indicated that the rectum was the primary body site for culture samples to determine VRE colonization. Three studies used stool samples in addition to rectal swab samples to determine VRE colonization.7,19,20
All 3 C. difficile studies defined C. difficile acquisition using only clinical cultures.8–10 None of the 3 studies used stool cultures in asymptomatic patients to identify those who were colonized with C. difficile. Unformed clinical stool samples were used for C. difficile toxin assay to determine C. difficile acquisition in all 3 studies.
Definitions of Colonization Pressure
The definition of colonization pressure varied considerably between studies (Table 1). The three broad definitions of colonization pressure used were the proportion of MRSA- or VRE-positive patients (5 studies), the proportion of MRSA-or VRE-positive patient-days (6 studies), and the total or mean number of MRSA-, VRE-, or C. difficile–positive patients or patient-days (7 studies) in the unit over study periods of varying length.
Of the 5 studies that defined colonization pressure as the proportion of MRSA- or VRE-positive patients in the unit, 1 defined colonization pressure as the proportion of MRSA-or VRE-colonized patients on entry to the ICU.16 Two studies defined colonization pressure as the daily proportion of patients in the unit colonized with VRE prior to VRE acquisition or discharge.2,7 Two studies defined colonization pressure as the daily proportion of patients in the unit colonized with VRE throughout a patient’s ICU stay.14,15
Of the 6 studies that defined colonization pressure as the proportion of MRSA- or VRE-positive patient-days in the unit, 1 defined colonization pressure as the proportion of MRSA-positive patient-days in the unit during the week preceding acquisition or discharge.13 Two studies defined colonization pressure as the weekly proportion of MRSA-positive patient-days in the unit.11,21 One study defined colonization pressure as the monthly proportion of MRSA-positive patient-days in the unit.6 Two studies defined colonization pressure as the proportion of MRSA-positive patient-days in the unit during the entire study period.17,18
Of the 7 studies that defined colonization pressure as the total or mean number of MRSA-, VRE-, or C. difficile–positive patients or patient-days in the unit, 1 defined colonization pressure as the mean number of MRSA-positive patients on the unit in the 3 days preceding MRSA acquisition or discharge.5 One study defined colonization pressure as the total number of MRSA-positive patients in the unit during a patient’s ICU stay.12 Two studies defined colonization pressure as the total number of VRE-positive patients or patient-days in the unit during a patient’s ICU stay.19,20 One study defined colonization pressure as the total number of C. difficile–positive patients present during a patient’s susceptible days in the unit.16 Two studies defined colonization pressure as the total number of C. difficile–positive patients present during a patient’s stay in the unit or the mean number of C. difficile–positive patients present during a patient’s susceptible days in the unit.8,9
MRSA Acquisition Study Results
Of the 8 MRSA studies, 3 did not include multivariable analysis.5,6,11 Of the remaining 5 studies, 4 included colonization pressure in their multivariable analysis.12,13,17,21 Three of the 4 studies found colonization pressure to be significantly associated with MRSA acquisition.13,17,21
The definitions of colonization pressure that yielded a significant association between colonization pressure and MRSA acquisition were the proportion of MRSA-positive patient-days in the unit during the week preceding MRSA acquisition or discharge, the weekly proportion of MRSA-positive patient-days in the unit, and the proportion of MRSA-positive patient-days in the unit during the entire study period. Risk factors identified as associated with MRSA acquisition when colonization pressure was controlled for in a multivariable analysis were age, admission to the ICU, severity of illness (Simplified Acute Physiology Score II), and ICU length of stay.13,17
VRE Acquisition Study Results
All 8 VRE studies measured colonization pressure, but only 6 included colonization pressure in their multivariable analysis. Four of the 6 studies found colonization pressure to be significantly associated with VRE acquisition.2,7,19,20 One study found that colonization pressure modified the association between gown use and VRE acquisition, that is, that gown use was protective against VRE acquisition for patients exposed to a high level of VRE (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.27–0.68) but that gown use was not protective against VRE acquisition for patients exposed to a low level of VRE (OR, 1.50; 95% CI, 0.57–3.98).19
The definitions of colonization pressure that yielded a significant association between colonization pressure and VRE acquisition were the daily proportion of patients in the unit colonized with VRE prior to VRE acquisition or discharge and the total number of VRE-positive patients or patient-days in the unit during a patient’s ICU stay. Risk factors identified as associated with VRE acquisition when colonization pressure was controlled for in a multivariable analysis were environmental contamination, enteral feeding, leukemia, end-stage renal disease, pre-ICU and ICU length of stay, and pre-ICU and ICU antibiotic use (anaerobic therapy, cephalosporin, ceftriazone, piperacillin-tazobactam, vancomycin, and quinolones).2,14,15,19,20
C. difficile Acquisition Study Results
All 3 C. difficile studies included colonization pressure in their multivariable analysis and found colonization pressure to be significantly associated with C. difficile acquisition.8–10 The definitions of colonization pressure that yielded significant association between colonization pressure and C. difficile acquisition were the total number of C. difficile–positive patients present during a patient’s susceptible days or during a patient’s total stay in the unit and the mean number of C. difficile–positive patients present during a patient’s susceptible days in the unit. Risk factors identified as associated with C. difficile acquisition when colonization pressure was controlled for in a multivariable analysis were age of at least 45 years; admission to the facility in the previous 60 days; mechanical ventilation; receipt of gastric acid suppressor, narcotic, or antidiarrheal agent; low albumin level; VRE colonization or infection; leukemia or lymphoma; Charlson comorbidity index of 1–2; Modified Acute Physiology Score of 3–5; and receipt of fluoroquinolone, vancomycin, metronidazole, or first-, third-, or fourth-generation cephalosporin.8–10
DISCUSSION
Colonization pressure is an important infection control metric that quantifies the burden of antibiotic-resistant bacteria in a hospital unit over a period of time. Colonization pressure has been shown to be an important risk factor for nosocomial acquisition of MRSA,13,17,21 VRE,2,7,19,20 and C. difficile.8–10 Previous studies have also assessed risk factors for acquisition of other antibiotic-resistant bacteria, such as extended-spectrum β-lactamase–producing Klebsiella and Escherichia coli, and have concluded that patient-to-patient transmission is likely an important contributor.22,23 However, these studies were not included in this review because of the limited data on colonization pressure and in an attempt to focus the manuscript. The aim of this study was to describe the definition and measurement of and adjustment for colonization pressure in nosocomial-acquisition risk factor studies of MRSA, VRE, and C. difficile.
We systematically reviewed the colonization pressure literature for MRSA, VRE, and C. difficile acquisition studies, and we found significant heterogeneity in the definition of and adjustment for colonization pressure. To summarize, colonization pressure was broadly defined as the proportion of antibiotic-resistant-bacteria-positive patients, the proportion of antibiotic-resistant bacteria–positive patient-days, or the total number of antibiotic-resistant bacteria–positive patients or patient-days in the unit or the mean number of antibiotic-resistant bacteria–positive patients in the unit daily, weekly, monthly, or for the duration of the study period. Positivity was determined using surveillance cultures, clinical cultures, or both. This review did not provide sufficient data to determine the most accurate definition of colonization pressure, but it is clear that there is a need for a simple and consistent but optimal definition of colonization pressure for use in both research and routine clinical care.24
The majority of the studies included in this review were performed in ICUs, where patients are screened more often to identify asymptomatic carriers of antibiotic-resistant bacteria. However, there are other healthcare settings, such as non-ICU hospital wards and long-term care facilities, where MRSA, VRE, and C difficile are endemic but surveillance for these bacteria is not routinely performed. In these settings, clinical cultures from symptomatic patients may be the only data available to quantify colonization pressure, and a definition of colonization pressure that quantifies only asymptomatic carriers could be problematic. The use of a definition that incorporates clinical-culture positivity or possibly prior history of colonization or infection may be more applicable and may still provide some useful data in these settings.
However, it is important to note that using clinical cultures from symptomatic patients to quantify colonization pressure may be prone to ascertainment bias. Clinical cultures are requested by the treating physician as clinically indicated; therefore, sicker patients and patient populations are more likely to have clinical cultures collected. This may lead to a subgroup of the patient population who are less sick and thus are less likely to have clinical cultures collected. This would likely result in an underestimation of colonization pressure.
For a definition of colonization pressure to be useful, it should be applicable in both research and routine clinical care. For example, a useful definition of colonization pressure should be applicable in a healthcare unit to routinely monitor colonization pressure, and when colonization pressure is found to be especially high (ie, above an indicated level), for example, during an outbreak, more intensive infection prevention efforts can be implemented.
In summary, the optimal definition of colonization pressure would quantify asymptomatic carriers present in the unit daily. Colonization pressure would thus be defined as the average daily proportion of patients colonized with the bacterial species under study during the period prior to acquisition or discharge from the unit. However, because of limited resources in routine clinical care, daily surveillance cultures are not often feasible, and this definition of colonization pressure may not be applicable in every healthcare setting. In the absence of daily surveillance culture data, the best available data, such as weekly surveillance data, clinical cultures, or possibly prior history of colonization or infection, may prove useful for quantifying colonization pressure. Computer simulation models may also be useful in estimating colonization pressure. Readers interested in the use of computer simulation models to study transmission of antibiotic-resistant bacteria may benefit from several available resources.25–27
In conclusion, further study is needed to determine a simple and consistent method to quantify colonization pressure in research and routine clinical care to accurately assess the effect of colonization pressure on cross-transmission of antibiotic-resistant bacteria.
Acknowledgments
We thank Angela C. Comer, MPH, for her careful review and assistance with formatting the manuscript.
Financial support. A.D.H. was supported by National Institutes of Health Midcareer Investigator Award 1K24AI079040. J.P.F. was supported by National Institutes of Health Career Development Award 1K01AI071015-03. J.C.M. was supported by National Institutes of Health Career Development Award KL2RR024141.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
Presented in part: 5th Decennial International Conference on Healthcare-Associated Infections; Atlanta, Georgia; March 2010
References
- 1.Bonten MJ, Gaillard CA, Johanson WG, Jr, et al. Colonization in patients receiving and not receiving topical antimicrobial pro-phylaxis. Am J Respir Crit Care Med. 1994;150(5):1332–1340. doi: 10.1164/ajrccm.150.5.7952561. [DOI] [PubMed] [Google Scholar]
- 2.Bonten MJM, Slaughter S, Ambergen AW, et al. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998;158(10):1127–1132. doi: 10.1001/archinte.158.10.1127. [DOI] [PubMed] [Google Scholar]
- 3.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 5.Bloemendaal AL, Fluit AC, Jansen WMT, et al. Acquisition and cross-transmission of Staphylococcus aureus in European intensive care units. Infect Control Hosp Epidemiol. 2009;30(2):117–124. doi: 10.1086/593126. [DOI] [PubMed] [Google Scholar]
- 6.Williams VR, Callery S, Vearncombe M, Simor AE. The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2009;37(2):106–110. doi: 10.1016/j.ajic.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Drees M, Snydman DR, Schmid CH, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46(5):678–685. doi: 10.1086/527394. [DOI] [PubMed] [Google Scholar]
- 8.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile–associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45(12):1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 9.Dubberke ER, Reske KA, Olsen MA, et al. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C. difficile–associated disease. Arch Intern Med. 2007;167(10):1092–1097. doi: 10.1001/archinte.167.10.1092. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28(2):123–130. doi: 10.1086/511793. [DOI] [PubMed] [Google Scholar]
- 11.Dancer SJ, Coyne M, Speekenbrink A, Samavedam S, Kennedy J, Wallace PG. MRSA acquisition in an intensive care unit. Am J Infect Control. 2006;34(1):10–17. doi: 10.1016/j.ajic.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet. 2005;365:295–304. doi: 10.1016/S0140-6736(05)17783-6. [DOI] [PubMed] [Google Scholar]
- 13.Lucet J-C, Paoletti X, Lolom I, et al. Successful long-term program for controlling methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2005;31(8):1051–1057. doi: 10.1007/s00134-005-2679-0. [DOI] [PubMed] [Google Scholar]
- 14.Winston LG, Charlebois ED, Pang S, Bangsberg DR, Perdreau-Remington F, Chambers HF., III Impact of a formulary switch from ticarcillin-clavulanate to piperacillin-tazobactam on colonization with vancomycin-resistant enterococci. Am J Infect Control. 2004;32(8):462–469. doi: 10.1016/j.ajic.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Martínez JA, Ruthazer R, Hansjosten K, Barefoot L, Snydman DR. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch Intern Med. 2003;163(16):1905–1912. doi: 10.1001/archinte.163.16.1905. [DOI] [PubMed] [Google Scholar]
- 16.Ho P-L Hong Kong intensive care unit antimicrobial resistance study (HK-ICARE) Group. Carriage of methicillin-resistant Staphylococcus aureus, ceftazidime-resistant Gram-negative bacilli, and vancomycin-resistant enterococci before and after intensive care unit admission. Crit Care Med. 2003;31(4):1175–1182. doi: 10.1097/01.CCM.0000059437.01924.97. [DOI] [PubMed] [Google Scholar]
- 17.Muller AA, Mauny F, Bertin M, et al. Relationship between spread of methicillin-resistant Staphylococcus aureus and antimicrobial use in a French university hospital. Clin Infect Dis. 2003;36(8):971–978. doi: 10.1086/374221. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan A, Song X, Ross T, Merz W, Brower R, Perl TM. A prospective study to determine whether cover gowns in addition to gloves decrease nosocomial transmission of vancomycin-resistant enterococci in an intensive care unit. Infect Control Hosp Epidemiol. 2002;23(8):424–428. doi: 10.1086/502079. [DOI] [PubMed] [Google Scholar]
- 19.Puzniak LA, Leet T, Mayfield J, Kollef M, Mundy LM. To gown or not to gown: the effect on acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2002;35(1):18–25. doi: 10.1086/340739. [DOI] [PubMed] [Google Scholar]
- 20.Puzniak LA, Mayfield J, Leet T, Kollef M, Mundy LM. Acquisition of vancomycin-resistant enterococci during scheduled antimicrobial rotation in an intensive care unit. Clin Infect Dis. 2001;33(2):151–157. doi: 10.1086/321807. [DOI] [PubMed] [Google Scholar]
- 21.Merrer J, Santoli F, Appéré-De Vecchi C, Tran B, De Jonghe B, Outin H. “Colonization pressure” and risk of acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol. 2000;21(11):718–723. doi: 10.1086/501721. [DOI] [PubMed] [Google Scholar]
- 22.Harris AD, Kotetishvili M, Shurland S, et al. How important is patient-to-patient transmission in extended-spectrum β-lactamase Escherichia coli acquisition. Am J Infect Control. 2007;35(2):97–101. doi: 10.1016/j.ajic.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Harris AD, Perencevich EN, Johnson JK, et al. Patient-to-patient transmission is important in extended-spectrum β-lactamase–producing Klebsiella pneumoniae acquisition. Clin Infect Dis. 2007;45(10):1347–1350. doi: 10.1086/522657. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AL, Calfee D, Fridkin SK, et al. Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC position paper. Infect Control Hosp Epidemiol. 2008;29(10):901–913. doi: 10.1086/591741. [DOI] [PubMed] [Google Scholar]
- 25.McBryde ES, Pettitt AN, McElwain DLS. A stochastic mathematical model of methicillin resistant Staphylococcus aureus transmission in an intensive care unit: predicting the impact of interventions. J Theor Biol. 2007;245(3):470–481. doi: 10.1016/j.jtbi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Raboud J, Saskin R, Simor A, et al. Modeling transmission of methicillin-resistant Staphylococcus aureus among patients admitted to a hospital. Infect Control Hosp Epidemiol. 2005;26(7):607–615. doi: 10.1086/502589. [DOI] [PubMed] [Google Scholar]
- 27.Forrester M, Pettitt AN. Use of stochastic epidemic modeling to quantify transmission rates of colonization with methicillin-resistant Staphylococcus aureus in an intensive care unit. Infect Control Hosp Epidemiol. 2005;26(7):598–606. doi: 10.1086/502588. [DOI] [PubMed] [Google Scholar]