Abstract
Endometriosis is associated with infertility; however the etiology of this association is unclear, thus complicating management. Several mechanisms of pathogenesis have been proposed, however no one theory has been implicated. Medical therapy can be helpful in managing symptoms, but does not improve pregnancy rates. The role of surgical treatment remains controversial. There is little data regarding ovulation induction treatments for endometriosis only, while superovulation with intrauterine insemination has shown modest improvement in pregnancy rates in women who may have endometriosis. The most effective treatment for endometriosis-associated infertility is in-vitro fertilization. Recent focus on proteomics and genetics of the disease may aid in optimizing treatment options.
Keywords: endometriosis, infertility, endometrioma, pregnancy, surgery, treatment
INTRODUCTION
Endometriosis affects 5–10% of reproductive-age women and at least one third of women with infertility.1 This complex disease is defined by the presence of endometrial glands and stroma outside the uterine cavity, and can manifest as peritoneal or visceral lesions, adhesions, fibrosis, endometriomas or any combination of the above. Clinical manifestations range from dysmenorrhea, chronic pelvic pain, dyspareunia, dyschezia and infertility to completely asymptomatic disease. Patient history, examination findings of cul de sac nodularity, limited mobility, or adnexal mass may be suggestive of endometriosis, but the diagnosis is made by surgical visualization or histologic assessment of suspected lesions. Endometriosis is notorious for the lack of correlation between symptoms and disease severity, as defined by surgical diagnosis, which further complicates patient management.
Endometriosis has been associated with infertility; however, the exact cause of infertility is not definitively known. Some women with endometriosis will conceive without difficulty, however others may have a substantially longer time to conception. Several controlled trials have suggested reduced fecundity in women with endometriosis ranging from 2–10%2. A retrospective cohort study demonstrated a significantly lower likelihood of pregnancy amongst those with endometriosis-associated infertility compared to unexplained infertility over a three year period (36% versus 55% with p<0.05)3. Anti-mullerian hormone levels have been noted to be decreased in patients with mild endometriosis compared to tubal factor infertility alone (1.26 +/− 0.7ng/mL versus 2.02 +/− 0.72 ng/ml, p= 0.004), however how that AMH level correlates to pregnancy rates has not been established in this cohort.4 Currently, there is no method to distinguish which women with endometriosis have normal or reduced fecundity.
THEORIES OF PATHOGENESIS
Several mechanisms have been proposed for the association of endometriosis and infertility. Retrograde menstruation is among the most commonly accepted theories, but it incompletely explains the disease process, as not all women with retrograde menstruation have endometriosis.5 Others have proposed the metaplastic changes in the coelomic epithelium, in response to an undetermined stimulus as well as lymphovascular spread of endometriotic tissue to explain implants distant to the pelvis. Other contributing factors to reduced fertility may include altered peritoneal function resulting in impaired folliculogenesis and oocyte quality, pelvic anatomy distortion, immunologic dysfunction, and impaired implantation.6 The impact of endometriosis on oocyte quality has suggested by studies evaluating the donor oocytes of patients with and without endometriosis and implantation rates in recipients. Specifically, in a retrospective analysis women who received embryos from endometriotic ovaries had significantly reduced implantation rates.7
MEDICAL MANAGEMENT FOR ENDOMETRIOSIS-RELATED INFERTILITY
Medical management has been demonstrated to improve the quality of life for many patients with endometriosis. The benefit of oral contraceptives, progestins, androgens, and gonadotropin releasing hormone agonists in managing symptoms has been clearly demonstrated.8 Unfortunately, the medical therapies for endometriosis almost exclusively limit reproductive options due to their contraceptive effects. Some modalities, such a depot medroxyprogesterone acetate, while very effective for treatment of symptoms, may have lasting effects of ovulation suppression beyond the duration of treatment. So the natural question is, for patients with endometriosis, when should medical management be stopped if the patient is anticipating attempting conception, and is there a benefit to preceding ovulation suppression? A Cochrane review of 23 trials including over 3000 women addresses the latter question.9 It demonstrated no difference in pregnancy rates with preceding ovulation suppression with oral contraceptives, progestins, or danazol in subfertile women with endometriosis (OR 1.02, CI 0.70 to 1.52, p=0.82). Therefore, while oral contraceptive pills, progestins, and GNRH agonists can be very effective in treating symptoms of endometriosis before and after pregnancy, pretreatment with these agents does not appear to improve fecundity and therefore implementation of medical management will only delay attempts at conception.
SURGICAL MANAGEMENT OF ENDOMETRIOSIS FOR INFERTILITY
The surgical management of endometriosis has largely been guided by patient symptoms – specifically, complaints of dysmenorrheal, dyspareunia, dyschezia, and chronic pelvic pain. While the benefits of surgical management for improvement of endometriosis-related symptoms have been established there is much debate about the utility of surgery in management of endometriosis-related infertility.
Most of the available data is for patients with mild to moderate disease. There are no randomized controlled trials to determine the efficacy of surgical management of moderate to severe endometriosis to date. The Canadian Collaborative Group on Endometriosis studied 341 infertile women with minimal or mild endometriosis who were randomized to diagnostic laparoscopy alone or laparoscopic treatment of endometriosis by ablation or resection, and found a significantly higher 36-week cumulative probability of pregnancy continuing beyond 20 weeks in the treatment group (30.7% versus 17.7%, p=0.006) suggesting enhanced fecundity with surgical treatment of endometriosis.10 However, another randomized control trial of 101 women with minimal to mild endometriosis demonstrated no difference in live birth rates between women who underwent laparoscopic treatment of endometriosis by ablation or resection compared to diagnostic laparoscopy alone (19.6% versus 22.2% over one year, OR 0.75, 95% CI 0.30–1.85).11 A Cochrane review attempted to reconcile these conflicting studies with a meta-analysis.12 When the data from these studies were combined, there was a benefit of surgical treatment compared to diagnostic laparoscopy alone for clinical pregnancy, and ongoing pregnancy after 20 weeks (OR 1.66, 95% CI 1.09–2.51 and OR 1.64 95% CI 1.05–2.57, respectively).
While there is a suggestion that there may be a role for treatment of mild to moderate disease to improve fertility, overall pregnancy rate in these studies (both with and without treatment) remains very low. Thus, while there may be objective evidence that surgery is better than no treatment, surgery may not be the best treatment to improve fertility. The overall absolute difference is 8.6% in favor of therapy (95% CI 2.1–15) resulting in the number needed to treat of 12 (95% CI 7–49). Thus for every 12 patients having Stage I or Stage II endometriosis diagnosed at laparoscopy, there will be one additional successful pregnancy in up to one year of attempted conception if ablation or resection of visible endometriosis is performed compared to no treatment. It is important to note that the number needed to treat would apply only to those who have endometriosis. The chance of finding endometriosis at the time of laparoscopy in a subfertile woman who does not have signs or symptoms of endometriosis is low. Thus, the number needed to treat, taking into account the number of women who will have a negative laparoscopy, is much higher. Given the conservative assumption that approximately 30% of patients with otherwise unexplained infertility (and no signs of symptoms to suggest endometriosis) would be diagnosed with endometriosis, the number of laparoscopies needed to be performed to gain one additional pregnancy is actually 40. If the prevalence of endometriosis is even lower, the number needed to treat is even higher (Table 1). There is no evidence that the outcome is affected by the method of ablation, by electro-surgery, or laser delivery systems.10
Table 1.
Number Needed to Treat by Endometriosis Prevalence in a Population of Subfertile Women
| Endometriosis Prevalence in Subfertile Women | Number Needed to Treat |
|---|---|
| 10%* | 120 |
| 20% | 60 |
| 30% | 40 |
| 40% | 30 |
| 50% | 24 |
| 100%** | 12 |
Assumes 10% of patients undergoing laparoscopy have evidence of minimal to mild endometriosis
Assumes all patients undergoing laparoscopy have evidence of minimal to mild endometriosis
Another large area of debate surrounds the impact of surgical management of endometriomas on infertility. While there have been many studies exploring pregnancy rates after excision, ablation or drainage of endometriomas, most are small, uncontrolled, and there is an overall lack of data on live birth outcomes in this study population. A Cochrane review of two randomized controlled trials of laparoscopic management of endometriomas demonstrated an increased unassisted pregnancy rate in women with prior subfertility with laparoscopic cyst wall excision as opposed to cyst wall ablation (OR 5.21 95% CI 0.18–0.93) with pregnancy rates of 61.0% versus 23.4% within two years of laparoscopic treatment.13 There was minimal added benefit of laparoscopic cystectomy when combined with controlled ovarian stimulation (COH) and intrauterine insemination (IUI) (OR 1.4 95% CI 0.47–4.15).13 These studies were done on women with pain and endometriomas greater than 3cm, so are limited application to the asymptomatic woman with an endometrioma. There is added concern that surgical treatment of endometriomas may contribute to further decreasing ovarian reserve in an already subfertile population. Retrospective data suggest a significantly lower antral follicle count and ovarian volume after laparoscopic excision,14 however this has failed to translate to a difference in pregnancy rates.
Therefore, consideration of laparoscopy for treatment of endometriosis in infertility patients who are suspected to have the disease by symptoms may be of benefit. There is very little benefit to diagnostic laparoscopy to look for endometriosis in women without symptoms of the condition. Patients with symptomatic endometriomas, or those in whom removal of the endometrioma may improve access to ovarian follicles for assisted reproductive techniques may benefit from cystectomy; however routine removal is not recommended to improve fertility rates and may detrimentally impact ovarian reserve.
OVULATION INDUCTION FOR ENDOMETRIOSIS RELATED INFERTILITY
There are limited studies that specifically address the treatment benefit of ovulation induction specifically in women with endometriosis. Therefore, success of ovulation induction and superovulation for patients with endometriosis must be extrapolated from studies that included women with either unexplained infertility or male factor infertility. These studies, which include women with mild endometriosis, suggest modest benefit. One study suggested possible additional benefit with clomiphene citrate with IUI compared to controls (fecundity 0.095 versus 0.033).15 A small study of patients with surgically diagnosed endometriosis randomized to with human menopausal gonadrotrophin (HMG) with IUI and no treatment for four cycles demonstrated a cumulative live birth rate over 4 cycles of 11% versus 2% (p=0.002, OR 5.6 CI 1.8 to 17.4) suggesting that COH may improve pregnancy rates.16 Another multicenter trial included patients with unexplained infertility, endometriosis or mild male factor infertility that were randomized to intracervical insemination (ICI), IUI, FSH with ICI or FSH with IUI.17 The study demonstrated higher pregnancy rates in the FSH with IUI group over up to four cycles as defined by positive serum βHCG, when compared to the ICI group (33% v 10%, p <0.0001). In summary, the evidence suggests that in a woman with endometriosis and subfertility, it may be reasonable to start with ovulation induction in combination with intrauterine insemination.
IN-VITRO FERTILIZATION FOR ENDOMETRIOSIS RELATED INFERTILITY
There is little doubt that the most effective treatment for infertility associated with endometriosis is in-vitro fertilization (IVF). In 2009, over 5600 IVF cycles were performed for patients with endometriosis in the United States resulting in over 1400 live births.18 However, there is still much to be learned about the most effective stimulation protocols and embryo culture conditions for these patients. A meta-analysis of 22 studies including over 2000 in-vitro cycles of women with endometriosis and over 4000 cycles of women undergoing IVF for other indications demonstrated that pregnancy rates were significantly lower in patients with endometriosis, and in particular those with severe disease.19 Specifically, the oocyte yield, fertilization rates, implantation rates were all significantly decreased (adjusted OR 0.82, (95% CI 0.85–0.90), 0.86 (95% CI (0.85–0.88), and 0.81 (95% CI 0.79–0.83) respectively). Furthermore, the chance of achieving pregnancy as defined by a positive serum βHCG after embryo transfer, was significantly lower (OR 0.63, 95% CI 0.51–0.77).
The results of this meta-analysis seem to contradict the findings of a high pregnancy rate for women with endometriosis in the Society for Assisted Reproductive Technology Registry or other case series. This discrepancy is likely due to the inability of the meta-analysis to control for confounding factors such as young age, or other factors that may contribute to a more favorable prognosis for women with endometriosis compared to women undergoing IVF cycles for other indications. Moreover, data in the voluntary registry is known to be subject to misclassification and bias.20 Thus, while it is clear endometriosis adversely impacts IVF success rates, endometriosis is clearly successful in women with endometriosis. There is no evidence to support that treatment of endometriosis prior to IVF, improves success rates.
FUTURE DIRECTIONS OF RESEARCH
There is ongoing research regarding the genetics of endometriosis. A recent genome-wide association study in 3194 patients with surgically confirmed endometriosis and 7060 controls identified an association with rs12700667 on chromosome 7p15.2 (OR 1.22, 95% CI 1.13–1.32) and a slightly stronger association amongst patients with moderate to severe disease (OR 2.38 (95% CI 1.24–1.53).21 This single nucleotide polymorphism is located in a region upstream from the HOXA10 gene, which has been implicated in the pathogenesis of endometriosis related infertility as related to implantation.18 However urrent theories are exploring the possibility that endometriosis is related to epigenetic modification rather than Mendelian inheritance.22 Hypermethylation of genes for HOXA10, progesterone receptor- β, and E- cadherin as well as hypomethylation of genes for estrogen receptor- β and steroidogenic-factor 1 have all been implicated as potential contributors to endometriosis pathophysiology.22 There is also an evolving focus of research efforts to evaluate biomarkers that may serve as diagnostic aids or markers of treatment success.23 Currently no reliable markers to indicate which patients are more likely to have infertility. Targeting of altered molecular pathways within lesions themselves may provide insight and new therapeutic modalities. Studies involving use of aromatase inhibitors for management of pain have shown benefit; the use of these modalities in conjunction with current fertility treatments may be helpful, but clinical trials are necessary to further explore this further.24
APPROACH TO THE PATIENT WITH ENDOMETRIOSIS INTERESTED IN CONCEPTION
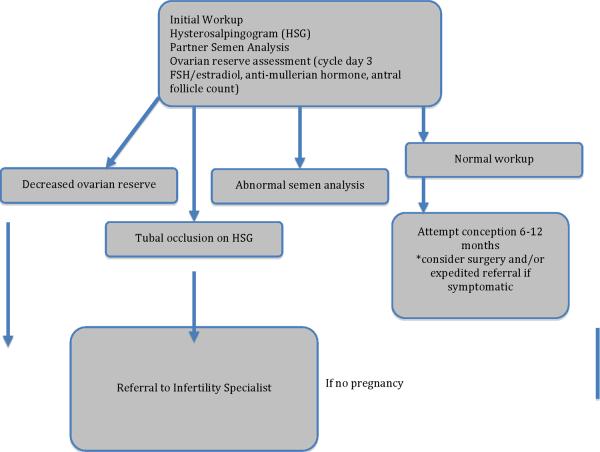
In conclusion, the management of couples facing infertility can be complicated by endometriosis. Management of these patients should focus on early recognition of a potential for subfertility, and prompt and appropriate referral for assisted reproductive techniques as needed. For those who have been attempting conception unsuccessfully, the initial infertility workup should be initiated and include a hysterosalpingogram to assess tubal patency and the endometrial cavity, the partner's semen analysis, and measures of ovarian reserve. Measuring cycle day 3 FSH with estradiol, and an antral follicular count can be helpful in assessing ovarian reserve. Anti-mullerian hormone assays are becoming increasingly available and have the added benefit of non-cycle dependent assessment. Abnormalities uncovered during the workup merit prompt referral to a specialist.
The decision regarding infertility treatment should consider the patient's baseline disease, as well as any factors that may justify more aggressive techniques (See Figure 1). Medical treatment of women who desire fertility is contraindicated as there is no proven benefit and it will delay conception. Surgical treatment should be reserved for symptomatic women or for women in whom reproductive anatomy is distorted but amenable to repair. It is not recommended to perform a laparoscopy in subfertile women to look for asymptomatic endometriosis. The ultimate choice of therapy for a subfertile woman with endometriosis depends on many factors including age, concomitant diagnoses, length of infertility, and desired aggressiveness. Depending on the circumstances, treatment may include expectant management, ovulation induction, and/or IVF. The complexity of endometriosis certainly warrants continued investigation into disease pathogenesis and options for treatment that may further enhance pregnancy rates.
Figure 1.
Approach to the patient with Endometriosis interested in conception
References
- 1.D'Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003 May;21(2):243–254. doi: 10.1055/s-2003-41330. [DOI] [PubMed] [Google Scholar]
- 2.Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil Steril. 1993 May;59(5):963–970. [PubMed] [Google Scholar]
- 3.Akande VA, Hunt LP, Cahill DJ, Jenkins JM. Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum Reprod. 2004 Jan;19(1):96–103. doi: 10.1093/humrep/deh045. [DOI] [PubMed] [Google Scholar]
- 4.Lemos NA, Arbo E, Scalco R, Weiler E, Rosa V, Cunha-Filho JS. Decreased anti-Mullerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil Steril. 2008 May;89(5):1064–1068. doi: 10.1016/j.fertnstert.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 5.Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927 Mar;3(2):93–110. 143. [PMC free article] [PubMed] [Google Scholar]
- 6.Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008 Apr;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon C, Gutierrez A, Vidal A, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994 Apr;9(4):725–729. doi: 10.1093/oxfordjournals.humrep.a138578. [DOI] [PubMed] [Google Scholar]
- 8.Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril. 1993 Jul;60(1):75–79. [PubMed] [Google Scholar]
- 9.Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev. 2007;(3):CD000155. doi: 10.1002/14651858.CD000155.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcoux S, Maheux R, Berube S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997 Jul 24;337(4):217–222. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- 11.Parazzini F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell'Endometriosi. Hum Reprod. 1999 May;14(5):1332–1334. doi: 10.1093/humrep/14.5.1332. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson TZ, Duffy JM, Barlow D, Farquhar C, Koninckx PR, Olive D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2010;(1):CD001398. doi: 10.1002/14651858.CD001398.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992. doi: 10.1002/14651858.CD004992.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Somigliana E, Ragni G, Benedetti F, Borroni R, Vegetti W, Crosignani PG. Does laparoscopic excision of endometriotic ovarian cysts significantly affect ovarian reserve? Insights from IVF cycles. Hum Reprod. 2003 Nov;18(11):2450–2453. doi: 10.1093/humrep/deg432. [DOI] [PubMed] [Google Scholar]
- 15.Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990 Dec;54(6):1083–1088. [PubMed] [Google Scholar]
- 16.Tummon IS, Asher LJ, Martin JS, Tulandi T. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril. 1997 Jul;68(1):8–12. doi: 10.1016/s0015-0282(97)81467-7. [DOI] [PubMed] [Google Scholar]
- 17.Guzick DS, Carson SA, Coutifaris C, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. National Cooperative Reproductive Medicine Network. N Engl J Med. 1999 Jan 21;340(3):177–183. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- 18.Society of Assisted Reproductive Technnology [Accessed June 30, 2011];2009 National Data Summary. 2009 Available at: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0.
- 19.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002 Jun;77(6):1148–1155. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 20.Molinaro TA, Shaunik A, Lin K, Sammel MD, Barnhart KT. A strict infertility diagnosis has poor agreement with the clinical diagnosis entered into the Society for Assisted Reproductive Technology registry. Fertil Steril. 2009 Dec;92(6):2088–2090. doi: 10.1016/j.fertnstert.2009.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painter JN, Anderson CA, Nyholt DR, et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011 Jan;43(1):51–54. doi: 10.1038/ng.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009 Oct;15(10):587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 23.Seeber B, Sammel MD, Fan X, et al. Proteomic analysis of serum yields six candidate proteins that are differentially regulated in a subset of women with endometriosis. Fertil Steril. 2010 May 1;93(7):2137–2144. doi: 10.1016/j.fertnstert.2008.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulun SE. Endometriosis. N Engl J Med. 2009 Jan 15;360(3):268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]