Abstract
The cytosol of a cell is a concentrated milieu of a variety of different molecules, including small molecules (salts and metabolites) and macromolecules such as nucleic acids, polysaccharides, proteins and large macromolecular complexes. Macromolecular crowding in the cytosolic environment is proposed to influence various properties of proteins, including substrate binding affinity and enzymatic activity. Here we chose to use the synthetic crowding agent Ficoll, which is commonly used to mimic cytosolic crowding conditions to study the crowding effect on the catalytic properties of glycolytic enzymes, namely phosphoglycerate kinase, glyceraldehyde 3-phosphate dehydrogenase, and acylphosphatase. We determined the kinetic parameters of these enzymes in the absence and in the presence of the crowding agent. We found that the Michaelis constant, Km, and the catalytic turnover number, kcat, of these enzymes are not perturbed by the presence of the crowding agent Ficoll. Our results support earlier findings which suggested that the Michaelis constant of certain enzymes evolved in consonance with the substrate concentration in the cell to allow effective enzyme function in bidirectional pathways. This conclusion is further supported by the analysis of nine other enzymes for which the Km values in the presence and absence of crowding agents have been measured.
Introduction
The interior of cells, namely the cytosol, is not only filled with water and salts but also with a variety of different soluble metabolites and macromolecules (proteins, nucleic acids, oligosaccharides). The concentration of macromolecules can vary between 200–400 g/l depending on the organism (eukaryotes vs. prokaryotes) [1], [2]. Recently the intracellular metabolite pool of E. coli was assessed to have an approximate concentration of 300 mM [3].
All of these solute macromolecules have an influence on each other and might affect the mobility, stability, association property, and activity of proteins [4]. The effect of macromolecules on each other, also known as macromolecular crowding or the excluded volume effect, has been studied extensively over the last decade [5], [6]. Many different aspects of crowding have been discussed including the thermal stabilization of flavodoxin by synthetic crowders such as Ficoll [7], or the thermal stabilization of lens crystallin at high concentrations of protein crowders [8]. Interestingly, a recent study by Miklos et al. shows that the synthetic crowder PVP can stabilize the protein Cl2 in contrast to protein crowders such as bovine serum albumin or lysozyme which destabilize this protein [9]. In terms of protein association, different effects of crowding have been reported. Crowders were shown to enhance polymerization, self-association, and hetero-oligomerization [10], [11], [12], [13]. On the other hand, crowders have little effect on the association of heterodimers in other model systems [14]. The studies of the effects of crowding on enzyme activity have also produced opposing results, as most studies were focused on the effects of crowding agents on the specific activity [15], [16].
In this study we examined the effects of a crowding agent on the kinetic parameters of three different enzymes (yeast phosphoglycerate kinase - PGK, rabbit muscle glyceraldehyde 3-phosphate dehydrogenase – GAPDH, and human acylphosphatase 1 - ACP) in the terms of changes in the Michaelis constant, Km. This was inspired by Bennett et al. [3] who investigated the influence of intracellular metabolite concentrations on the active-site occupancy of enzymes. In their work, the authors compared the Km values of enzymes (as compiled in BRENDA information system [17]) with the measured intracellular substrate concentrations. They find that the Km values are directly correlated (with a slope of one) to the corresponding substrate concentrations for several major classes of enzymes, including enzymes involved in carbon metabolism [3]. It is noted, however, that the reported Km values in BRENDA are based on studies performed in dilute aqueous solution, i.e. in the absence of crowding agents. Nevertheless, this study suggests that the Michaelis constant is directly related to the available substrate concentration in the metabolite pool and that thermodynamic constraints dictate the effective cellular enzymatic activity. The question is whether the crowded cellular environment significantly affects the Km values of enzymes, and thus the correlation between the Km and cellular substrate concentrations, as observed by Bennett et al. [3], is circumstantial.
We find that the addition of 200 g/l of Ficoll, a neutral polymer that is often used to mimic crowded cellular environment, has a very small effect on Km of the three studied enzymes, PGK, GAPDH, and ACP. Analysis of the published data for several other enzymes further supports this finding. Overall, our results support the hypotheses put forward by Bennett et al. [3] that to ensure a rapid response to the changes in the metabolic flux, enzymes have Michaelis constants close to the cellular substrate concentrations.
Materials and Methods
Reagents
All reagents were obtained in the highest purity from Sigma-Aldrich Co. LLC. (USA). Ficoll PM 70 was obtained from GE Healthcare Bio-Sciences Corp. (USA).
Protein Preparations
Yeast PGK wt with an N-terminal 6×His-Tag followed by a TEV restriction site was expressed from a pGia [18] vector in Escherichia Coli strain BL21 (DE3) pLys. The cells were grown in TB-media at 37°C until the OD600 reached 0.8. The temperature was then decreased to 25°C and protein expression was induced by the addition of 250 µM Isopropyl β-D-1-thiogalactopyranoside (IPTG) overnight. The cells were harvested and disrupted using a French Laboratory Press (Thermo Fisher). Cell debris was pelleted at 8,900 rpm and 4°C. The supernatant was applied to a column packed with Ni-NTA resin (Novagen). The column was washed with buffer containing 50 mM potassium phosphate (KPi), 300 mM sodium chloride (NaCl), 10 mM Imidazole pH 7.4 until the OD280 reached a minimum baseline. The protein was then eluted from the column using a buffer containing 300 mM Imidazole. The protein solution was immediately dialyzed against a buffer containing 50 mM KPi, 300 mM NaCl, 0.5 mM ethylenediaminetetraacetic acid (EDTA) and 1 mM dithiothreitol (DTT) at 4°C for 2 hours. To cleave off the N-terminal 6×His-Tag, TEV protease [19] was added directly to the dialysis bag. The reaction mixture was left on dialysis overnight. The uncleaved protein and the TEV protease were removed by reapplying the reaction mixture to the Ni-NTA column. The flow through containing PGK was concentrated using an Amicon Ultra-15 Centrifugal Filter Unit (EMD Millipore, MA). Protein purity was verified by SDS-PAGE. The concentration was determined by the protein absorbance at 280 nm (ε280 (yPGK) = 21,360 M−1 cm−1) in buffer containing 20 mM KPi, 6 M guanidinium hydrochloride, pH 6.5 according to the method described by Gill and von Hippel [20]. Expression and purification of acylphosphatase was performed as described earlier [21]. Rabbit muscle glyceraldehyde 3-phosphate dehydrogenase (GAPDH, EC: 1.2.1.12) was purchased from Sigma-Aldrich Co. LLC. (USA).
Phosphoglycerate Kinase Activity
The enzymatic activity of yeast PGK was monitored using a coupled reaction with GAPDH [22]. The reaction rates for different concentrations of the substrates (3-phosphoglycerate (3-PGA) or adenosine diphosphate (ADP)) were determined from the changes in the concentration of reduced nicotinamide adenine dinucleotide (NADH). The concentration of NADH was monitored spectrophotometrically at 340 nm (ε340 (NADH) = 6,220 M−1 cm−1). The reaction rates were obtained by analyzing the initial slope of changes in absorbance during the first 5 seconds. The forward reaction (Figure 1A) was carried out in 50 mM Tris, 50 mM KPi, 3 mM magnesium chloride (MgCl2), 1 mM EDTA, 1.5 mM DTT at pH 7.4 with and without 200 g/l Ficoll PM70. The concentrations of glyceraldehyde-3-phosphate (GAP) and nicotinamide adenine dinucleotide (NAD+) were 830 µM and 415 µM, respectively and the ADP concentration was varied between 12.5–3,000 µM. For the reverse reaction (Figure 1A), 50 mM Tris, 3 mM MgCl2, 1 mM EDTA, and 1.5 mM DTT at pH 7.4 with or without 200 g/l Ficoll PM70 was used as a buffer. The concentrations of ATP and NADH were 2,500 µM and 100 µM, respectively, while the concentration of 3-PGA was varied between 250–30,000 µM. In all reactions, the concentration of PGK was 5 nM and the concentration of GAPDH was 1 µM. The observed rates were normalized to the concentration of PGK.
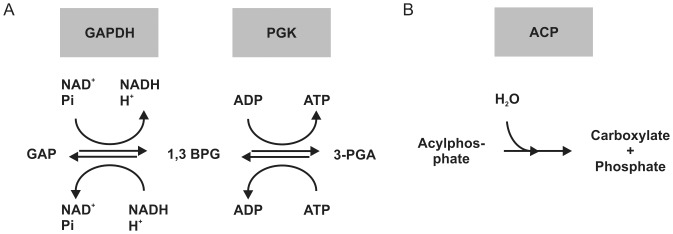
Figure 1. Reaction schemes for the tested enzymes.
(A) Reactions in the glucose metabolism catalyzed by GAPDH and PGK. In the forward reaction for GAPDH, conversion of substrate GAP is monitored directly by changes in NADH concentration. In the forward reaction for PGK, conversion of substrate ADP is monitored in a linked assay with forward reaction of GAPDH as a source for 1,3 BPG. In the reverse reaction for PGK, conversion of 3-PGA is monitored by the changes in NADH concentration in a linked assay with 1,3 BPG as a substrate for GAPDH [22]. (B) Reaction catalyzed by ACP. Hydrolysis of acylphosphate leads to the formation of a carboxylate and an inorganic phosphate.
Glyceraldehyde 3-phosphate Dehydrogenase Activity
The enzymatic activity of GAPDH was monitored by the increase in NADH concentration (ε340 (NADH) = 6,220 M−1 cm−1). The initial slope of the reaction covering the first 5 seconds was used for analysis. The forward reaction was carried out in 50 mM Tris, 50 mM KPi, 3 mM magnesium chloride (MgCl2), 1 mM EDTA, 1.5 mM DTT at pH 7.4 without or with the addition of 200 g/l Ficoll PM70. The concentration of NAD+ was 500 µM, while the concentration of glyceraldehyde-3-phosphate (GAP) was varied between 125–10,000 µM. In all reactions, the concentration of GAPDH was 20 nM. The observed rates were normalized to the concentration of GAPDH.
Acylphosphatase 1 Activity
The enzymatic activity of ACP was monitored by a decrease in absorbance upon hydrolysis of benzoyl phosphate (BP) using an extinction coefficient of ε283 (BP) = 960 M−1 cm−1 [23]. The initial slope of the reaction covering the first 5 seconds was used for analysis. The reaction was carried out in 100 mM sodium acetate pH 5.5 with or without the addition of 200 g/l Ficoll PM70. The concentration of the substrate benzoyl phosphate was varied between 25–1,000 µM. In all reactions, the ACP concentration was 10 nM. The observed rates were normalized to the concentration of ACP.
Michaelis-Menten Kinetics
All activity measurements were performed in triplicate using an SX.18MV-R stopped-flow apparatus (Applied Photophysics Ltd, UK) at 25°C. The initial reaction rates at different substrate concentrations were analyzed by nonlinear regression fit to Michaelis-Menten equation (Eq. 1) using Origin:
| (1) |
Where ν is the reaction rate, Vmax is the maximum reaction rate, Km is the Michaelis constant and [S] is the substrate concentration.
Results and Discussion
Phosphoglycerate Kinase Activity for ADP as a Substrate
The rate of the yeast PGK activity in the forward reaction (See Figure 1A) versus the ADP substrate concentration is shown in Figure S1A. Nonlinear regression analysis according to the Michaelis-Menten equation gives the following kinetic parameters of the reaction: Km = 340±40 µM and kcat = 860±40 s−1. These results are in agreement with earlier studies on yeast PGK which reported similar kinetic parameters under comparable conditions with a Km value of 180 and a kcat of 963 s−1 [24] or a Km = 500 µM and kcat of 613 s−1 [25]. The kinetic parameters for yeast PGK are also comparable to the kinetic parameters of PGK from different organisms (see supplementary Table S1 for comparison).
We also performed the activity measurements in the presence of 200 g/l Ficoll to elucidate the effects of the crowding agent on the kinetic parameters. Figure S1B shows the Michaelis-Menten plot for the forward reaction in the presence of Ficoll. The Km for ADP as the substrate was 430±40 s−1 µM, while the kcat was 920±30 s−1. These kinetic parameters obtained in the presence of the crowding agent Ficoll are similar to the parameters obtained in the absence of Ficoll, suggesting that the crowding agent does not have a significant effect on the activity of PGK (also see Table 1).
Table 1. Kinetic parameters of PGK, GAPDH and ACP in the absence or presence of the crowding agent Ficoll.
| Substrate | Without Crowder | With Crowder |
| PGK | ||
| ADP | ||
| Km (µM) | 340±40 | 430±40 |
| kcat (s−1) | 860±40 | 920±30 |
| 3-PGA | ||
| Km (µM) | 3300±500 | 2000±300 |
| kcat (s−1) | 490±30 | 490±20 |
| GAPDH | ||
| GAP | ||
| Km (µM) | 1000±160 | 1100±160 |
| kcat (s−1) | 50±3 | 37±2 |
| ACP | ||
| Benzoyl phosphate | ||
| Km (µM) | 100±18 | 80±17 |
| kcat (s−1) | 1100±60 | 300±17 |
Phosphoglycerate Kinase Activity for 3-PGA as a Substrate
In Figure S2, the rate of PGK in the reverse reaction (See Figure 1A) is plotted versus the 3-PGA substrate concentration. The substrate concentration was varied between 250–30,000 µM. The kinetic parameters from Michaelis- Menten analysis (Equation 1) were: Km = 3,300±500 µM and kcat = 490±30 s−1. In the presence of the crowding agent Ficoll we do not observe a change in kcat, 490±20 s−1 in comparison to the absence of the crowding agent (490±30 s−1). Similarly, there is an insignificant decrease in the Km value (2,000±300 µM) between the crowded and uncrowded conditions. Thus, the activity of PGK for 3-PGA as a substrate is not influenced by the addition of the crowding agent Ficoll. This is consistent with the effect of the crowding on the activity of PGK for the other substrate, ADP (see Table 1). If we assume that Ficoll is a suitable mimic of the crowded environment in the cell, our results suggest that the activity of PGK in the cell should be similar to the activity observed in vitro.
Glyceraldehyde 3-phosphate Dehydrogenase Activity
Figure S3 shows the activity measurements of GAPDH in the absence and presence of Ficoll to assess the influence of a crowded environment. The activity measurements were performed in the forward reaction (Figure S1A) by varying the concentration of the substrate GAP between 125–10,000 µM. The Km value obtained from the Michaelis-Menten plot in the absence of Ficoll is 1000±160 µM and the kcat value is 50±3 s−1. In the presence of Ficoll, the values of the kinetic parameters are Km = 1,100±150 µM and kcat = 37±2 s−1. The small decrease in kcat in the presence of Ficoll is related to the changes in the monomer-tetramer equilibrium of GAPDH in the presence of the crowding agent. It has been shown that crowding increases the association constant of tetramer formation in GAPDH, which will have an indirect effect on the activity, as the tetramer has a 30 times lower activity than the monomer [26]. Importantly, the Km value did not change in the presence of the crowding agent. This is in agreement with the observations we made for PGK, where the Michaelis constant also does not change in the presence of Ficoll.
Acylphosphatase 1 Activity
The activity of ACP was directly assayed using the model substrate benzoyl phosphate [23]. The reaction scheme is depicted in Figure S1B. The substrate concentration was varied between 25–1,000 µM (Figure S4). The Km and kcat values were determined from a fit to the Michaelis-Menten equation (Equation 1). The Km value is 100±18 µM, while the kcat value is 1100±60 s−1 at 25°C, in agreement with previous measurements [18]. We also measured the activity of ACP in the presence of 200 g/l Ficoll PM70. The kcat value in the presence of Ficoll is only three times lower (300±17 s−1) than in the absence of Ficoll. The Km value in the presence of Ficoll is 80±17 µM, which is within experimental error of the Km obtained in the absence of crowding agent. Since the kinetic parameters for benzoyl phosphate are similar in the absence and presence of crowding agent, we can assume that they will also be similar for the natural substrate acetyl phosphate. It is known that the Km for acetyl phosphate is 30 times higher than for benzoyl phosphate, which puts an estimate for Km for this substrate at 2,900 µM [27].
Relevance to the in vivo Activity
The activities for three different enzymes were analyzed in the presence of the crowding agent Ficoll to mimic a cell like environment with a high concentration of macromolecules. This was done to evaluate potential differences in their enzymatic activity in vitro (in dilute aqueous solution) and in vivo (in the crowded environment). Our goal was to investigate if the kinetic parameters were altered in the presence of the crowding agent. We have shown that the presence of a crowding agent does not influence the kinetic parameters and in particular the Km for PGK, GAPDH and ACP. The Km, in a first approximation, is the dissociation constant of the Michaelis complex and, thus, defines the fraction of the enzyme-substrate complex at a given substrate concentration. When the substrate concentration is equal to Km, only half of the enzyme is in the enzyme-substrate complex. For the enzymes involved in central carbon metabolism, it has been suggested that they have a Km value which is not significantly different (within 10-fold) from their substrate concentration to ensure rapid enzyme response to changes in substrate concentration in either direction [3], [28], [29]. However, these observations were made using Km values measured in vitro. Our measurements show that the activities of PGK, GAPDH and ACP in the absence or presence of the crowding agent Ficoll (which is used to mimic the crowded cell interior) are similar, thus, supporting this idea.
Indeed, the concentration of ADP, a substrate for PGK, is 560 µM in exponentially growing E. coli [3]. Our measured Km values are less than 2-fold lower than the cellular concentrations of ADP (Table 1). The reported concentration of 3-PGA, another PGK substrate, in E. Coli is 1,500 µM [3]. The Km value measured for PGK in the presence of Ficoll is similar to the cellular concentration of 3-PGA (Table 1). The concentration of GAP in E. Coli is 1,200 µM [30], and our results suggests that GAPDH also has a Km value which is similar to the corresponding substrate concentration (Table 1). Finally, the concentration of the natural substrate for ACP, acetyl phosphate, in E. Coli is 1,100 µM [3]. The Km of ACP for acetyl phosphate is 2,900 µM [27] and, as we discussed above, this value should not be affected by the crowded cellular environment. For all three studied enzymes, the cellular concentrations of the substrates and the Km values are very similar, which allows the enzymes to provide an immediate response to the changes in the substrate concentration. PGK and GAPDH are of special interest because both enzymes are part of central carbon metabolism and, therefore, perform their activity in two directions as needed. Our observations underline the hypothesis by Bennett et al. which proposes that the substrate binding sites of the enzymes involved in bidirectional carbon metabolism cannot be fully saturated with their corresponding substrates in order to allow efficient catalysis in both directions [3]. Such a conclusion is not limited to the three enzymes studied here. Table 2 shows a compilation of the known effects of various crowding agents on the Km values of nine other enzymes. Some of these enzymes are also involved in both catabolic and anabolic metabolism (e.g. lactate dehydrogenase and hexokinase) but others are only involved in catabolic processes (e.g. trypsin, hyaluronate lyase). Nevertheless, the Km values for all of these enzymes are not significantly affected by the presence of crowding agents.
Table 2. Crowding agent effects on the kinetic parameter, Km, of different enzymes.
| Enzyme | Crowding Agent | Conc. (g/l) | Substrate | Km change | Reference |
| ACP | Ficoll | 200 | Benzoyl phosphate | 1.3 | This work |
| GAPDH | Ficoll | 200 | GAP | 0.9 | This work |
| PGK | Ficoll | 200 | ADP | 0.9 | This work |
| Ficoll | 200 | 3-PGA | 1.7 | This work | |
| AspP | PEG 6000 | 50 | ADP-glucose | 3.3 | [31] |
| EntB | Ficoll | 300 | Isochorismate | 1.6 | [32] |
| EntC | Ficoll | 300 | Chorismate | 2.2 | [32] |
| HK | BSA | 200 | Glucose | 1.3 | [33] |
| Hyal | PEG 4000 | 50 | Hyaluronic acid | 1.4 | [34] |
| LDH | Dextran 40,000 | 200 | Lactate | 1.8 | [34] |
| 200 | NAD+ | 1.6 | [34] | ||
| 200 | Pyruvate | 1.9 | [34] | ||
| LDH | Ficoll | 300 | Pyruvate | 3.1 | [32] |
| MCO | Ficoll/Dextran | 200 | o-Dianisidine HCL | 0.1 | [35] |
| MenF | Ficoll | 300 | Chorismate | 2.5 | [32] |
| Trypsin | Dextran 40,000 | 257 | Benzoyl-arginine-p-nitroanilide | 1.4 | [34] |
The Km change is represented as the ratio of the Km in the absence of the crowding agent divided by the Km in the presence of the crowding agent. AspP – ADP-sugar pyrophosphatase; EntB – isochorismatase; EntC – isochorismate synthase; HK – hexokinase; Hyal – hyaluronate lyase; LDH – lactate dehydrogenase, MCO – multi-copper oxidase; MenF – monomeric isochorismate synthase.
To summarize, we conclude that 1. crowding agents that mimic the cellular environment insignificantly affect the Km of enzymes, and 2. cellular concentrations of many substrates are very similar to the Km values of the corresponding enzymes in the presence of crowding agents. These two findings support the previous idea that many enzymes in the cell are always under conditions where they can efficiently respond to the changes in the concentration of the substrate and, thus, provide an efficient and simple initial regulation of the flux of metabolites.
Supporting Information
Michaelis-Menten plots for PGK in the forward reaction. (A) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of ADP in the absence of Ficoll. (B) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of ADP in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Michaelis-Menten plots for PGK in the reverse reaction. (A) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of 3-PGA in the absence of Ficoll. (B) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of 3-PGA in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Michaelis-Menten plots for GAPDH in the forward reaction. (A) Michaelis-Menten plot for GAPDH (20 nM) with varying concentrations of GAP in the absence of Ficoll. (B) Michaelis-Menten plot for GAPDH (20 nM) with varying concentrations of GAP in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Michaelis-Menten plots for ACP with varying concentrations of benzoyl phosphate. (A) Michaelis-Menten plot for ACP (10 nM) with varying concentrations of benzoyl phosphate in the absence of Ficoll. (B) Michaelis-Menten plot for ACP (10 nM) with varying concentrations of benzoyl phosphate in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Summary of Km values for PGK from different species.
(PDF)
Acknowledgments
We are thankful to Dr. Joel E. Morgan from RPI/CBIS Analytical Biochemistry Core for the help with setting up stopped-flow experiments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant from the National Science Foundation (MCB-0818419) (www.nsf.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman SB, Trach SO. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991;222:599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]
- 3.Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuffee SR, Elcock AH. Diffusion, crowding & protein stability in a dynamic molecular model of the bacterial cytoplasm. PLoS Comput Biol. 2010;6:e1000694. doi: 10.1371/journal.pcbi.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman SB, Minton AP. Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu Rev Biophys Biomol Struct. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 7.Stagg L, Zhang SQ, Cheung MS, Wittung-Stafshede P. Molecular crowding enhances native structure and stability of alpha/beta protein flavodoxin. Proc Natl Acad Sci U S A. 2007;104:18976–18981. doi: 10.1073/pnas.0705127104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steadman BL, Trautman PA, Lawson EQ, Raymond MJ, Mood DA, et al. A differential scanning calorimetric study of the bovine lens crystallins. Biochemistry. 1989;28:9653–9658. doi: 10.1021/bi00451a017. [DOI] [PubMed] [Google Scholar]
- 9.Miklos AC, Sarkar M, Wang Y, Pielak GJ. Protein crowding tunes protein stability. J Am Chem Soc. 2011;133:7116–7120. doi: 10.1021/ja200067p. [DOI] [PubMed] [Google Scholar]
- 10.Bookchin RM, Balazs T, Wang Z, Josephs R, Lew VL. Polymer structure and solubility of deoxyhemoglobin S in the presence of high concentrations of volume-excluding 70-kDa dextran. Effects of non-s hemoglobins and inhibitors. J Biol Chem. 1999;274:6689–6697. doi: 10.1074/jbc.274.10.6689. [DOI] [PubMed] [Google Scholar]
- 11.Rivas G, Fernandez JA, Minton AP. Direct observation of the enhancement of noncooperative protein self-assembly by macromolecular crowding: indefinite linear self-association of bacterial cell division protein FtsZ. Proc Natl Acad Sci U S A. 2001;98:3150–3155. doi: 10.1073/pnas.051634398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilf J, Minton AP. Evidence for protein self-association induced by excluded volume. Myoglobin in the presence of globular proteins. Biochim Biophys Acta. 1981;670:316–322. doi: 10.1016/0005-2795(81)90103-3. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman SB, Trach SO. Effects of macromolecular crowding on the association of E. coli ribosomal particles. Nucleic Acids Res. 1988;16:6309–6326. doi: 10.1093/nar/16.14.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillip Y, Sherman E, Haran G, Schreiber G. Common crowding agents have only a small effect on protein-protein interactions. Biophys J. 2009;97:875–885. doi: 10.1016/j.bpj.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhar A, Samiotakis A, Ebbinghaus S, Nienhaus L, Homouz D, et al. Structure, function, and folding of phosphoglycerate kinase are strongly perturbed by macromolecular crowding. Proc Natl Acad Sci U S A. 2010;107:17586–17591. doi: 10.1073/pnas.1006760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris MG, Malys N. What is the true enzyme kinetics in the biological system? An investigation of macromolecular crowding effect upon enzyme kinetics of glucose-6-phosphate dehydrogenase. Biochem Biophys Res Commun. 2011;405:388–392. doi: 10.1016/j.bbrc.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Scheer M, Grote A, Chang A, Schomburg I, Munaretto C, et al. BRENDA, the enzyme information system in 2011. Nucleic Acids Res. 2011;39:D670–676. doi: 10.1093/nar/gkq1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribenko AV, Makhatadze GI. Role of the charge-charge interactions in defining stability and halophilicity of the CspB proteins. J Mol Biol. 2007;366:842–856. doi: 10.1016/j.jmb.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 19.Kapust RB, Tozser J, Copeland TD, Waugh DS. The P1' specificity of tobacco etch virus protease. Biochem Biophys Res Commun. 2002;294:949–955. doi: 10.1016/S0006-291X(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 20.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 21.Strickler SS, Gribenko AV, Gribenko AV, Keiffer TR, Tomlinson J, et al. Protein stability and surface electrostatics: a charged relationship. Biochemistry. 2006;45:2761–2766. doi: 10.1021/bi0600143. [DOI] [PubMed] [Google Scholar]
- 22.Bücher T. Phosphoglycerate kinase from Brewer's yeast. Methods Enzymol: Academic Press. 1955. pp. 415–422.
- 23.Camici G, Manao G, Cappugi G, Ramponi G. A new synthesis of benzoyl phosphate: a substrate for acyl phosphatase assay. Experientia. 1976;32:535–536. doi: 10.1007/BF01920843. [DOI] [PubMed] [Google Scholar]
- 24.Hurth C, Tassius C, Talbot JC, Maali A, Moskalenko C, et al. Enzymatic activity of immobilized yeast phosphoglycerate kinase. Biosens Bioelectron. 2007;22:2449–2455. doi: 10.1016/j.bios.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Mas MT, Bailey JM, Resplandor ZE. Site-directed mutagenesis of histidine-388 in the hinge region of yeast 3-phosphoglycerate kinase: effects on catalytic activity and activation by sulfate. Biochemistry. 1988;27:1168–1172. doi: 10.1021/bi00404a015. [DOI] [PubMed] [Google Scholar]
- 26.Minton AP, Wilf J. Effect of macromolecular crowding upon the structure and function of an enzyme: glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1981;20:4821–4826. doi: 10.1021/bi00520a003. [DOI] [PubMed] [Google Scholar]
- 27.Liguri G, Camici G, Manao G, Cappugi G, Nassi P, et al. A new acylphosphatase isoenzyme from human erythrocytes: purification, characterization, and primary structure. Biochemistry. 1986;25:8089–8094. doi: 10.1021/bi00372a044. [DOI] [PubMed] [Google Scholar]
- 28.Cornish-Bowden A. The effect of natural selection on enzymic catalysis. J Mol Biol. 1976;101:1–9. doi: 10.1016/0022-2836(76)90062-0. [DOI] [PubMed] [Google Scholar]
- 29.Fendt SM, Buescher JM, Rudroff F, Picotti P, Zamboni N, et al. Tradeoff between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Mol Syst Biol. 2010;6:356. doi: 10.1038/msb.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber J, Kayser A, Rinas U. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Microbiology. 2005;151:707–716. doi: 10.1099/mic.0.27482-0. [DOI] [PubMed] [Google Scholar]
- 31.Moran-Zorzano MT, Viale AM, Munoz FJ, Alonso-Casajus N, Eydallin GG, et al. Escherichia coli AspP activity is enhanced by macromolecular crowding and by both glucose-1,6-bisphosphate and nucleotide-sugars. FEBS Lett. 2007;581:1035–1040. doi: 10.1016/j.febslet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Jiang M, Guo Z. Effects of macromolecular crowding on the intrinsic catalytic efficiency and structure of enterobactin-specific isochorismate synthase. J Am Chem Soc. 2007;129:730–731. doi: 10.1021/ja065064+. [DOI] [PubMed] [Google Scholar]
- 33.Olsen SrN. Applications of isothermal titration calorimetry to measure enzyme kinetics and activity in complex solutions. Thermochimica Acta. 2006;448:12–18. [Google Scholar]
- 34.Laurent TC. Enzyme reactions in polymer media. Eur J Biochem. 1971;21:498–506. doi: 10.1111/j.1432-1033.1971.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 35.Pozdnyakova I, Wittung-Stafshede P. Non-linear effects of macromolecular crowding on enzymatic activity of multi-copper oxidase. Biochim Biophys Acta. 2010;1804:740–744. doi: 10.1016/j.bbapap.2009.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Michaelis-Menten plots for PGK in the forward reaction. (A) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of ADP in the absence of Ficoll. (B) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of ADP in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Michaelis-Menten plots for PGK in the reverse reaction. (A) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of 3-PGA in the absence of Ficoll. (B) Michaelis-Menten plot for PGK (5 nM) with varying concentrations of 3-PGA in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Michaelis-Menten plots for GAPDH in the forward reaction. (A) Michaelis-Menten plot for GAPDH (20 nM) with varying concentrations of GAP in the absence of Ficoll. (B) Michaelis-Menten plot for GAPDH (20 nM) with varying concentrations of GAP in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Michaelis-Menten plots for ACP with varying concentrations of benzoyl phosphate. (A) Michaelis-Menten plot for ACP (10 nM) with varying concentrations of benzoyl phosphate in the absence of Ficoll. (B) Michaelis-Menten plot for ACP (10 nM) with varying concentrations of benzoyl phosphate in the presence of Ficoll. The black lines represent the fit to the Michaelis-Menten equation. The fit results are collated in Table 1.
(PDF)
Summary of Km values for PGK from different species.
(PDF)