Abstract
Manipulation of the actin cytoskeleton is a commonly used process by which bacterial pathogens and viruses are able to neutralize host defense mechanisms and subvert them in order to replicate in a hostile environment. Diverse bacteria display a wide array of mechanisms of regulation of microfilaments to enter, move within or exit the host cell. A less studied subject is how pathogens may co-opt the actin cytoskeleton to disturb vesicle trafficking pathways, namely phagolysosomal fusion, and avoid degradation. In fact, although actin plays a role in endosomal trafficking and phagosome maturation, the knowledge on the exact mechanisms and additional players is still scarce. Recently, we found that the Legionella pneumophila virulence factor VipA is an actin nucleator, associates with actin filaments and early endosomes during infection, and interferes in yeast organelle trafficking pathways, suggesting it may be linking actin dynamics to endosome biogenesis. Further studies on this protein, together with work on other bacterial effectors, may help shed light in the role of actin in endosomal maturation.
Keywords: Legionella pneumophila, Type IV Secretion System, VipA, actin, effector, multivesicular body, organelle trafficking
Introduction
Over long processes of coevolution, microbial pathogens have developed a multitude of mechanisms to survive and replicate in the host organism. They have not only neutralized host cell pathways that would ultimately lead to their killing, but have also hijacked eukaryotic networks for their own benefit. Depending on the pathogen displaying or not an obligatory intracellular phase during the life cycle, these mechanisms may yield responses as diverse and contrasting as promotion or prevention of fundamental eukaryotic processes, such as phagocytosis, apoptosis, autophagy or vesicle trafficking. In order to modulate these pathways, bacterial pathogens inject into the host cell virulence factors, or effector proteins, through secretion systems. These effectors act by targeting specific eukaryotic components, regulating their subcellular localization and/or activity.
Legionella pneumophila Effectors: The Legion of Doom
Legionella pneumophila is a facultative intracellular bacterium that is able to invade and replicate inside phagocytic cells. In its natural habitat, aquatic environments, it parasitizes a wide and diversified group of amoebae. It is believed that the large repertoire of virulence mechanisms acquired during coevolution with protozoa has made L. pneumophila competent for infection of human lung macrophages, leading to a frequently fatal type of pneumonia known as Legionnaires’ disease. Legionellae thrive in a remodeled compartment inside the host cell, the Legionella-containing vacuole (LCV). Immediately after uptake, the LCV avoids the endocytic pathway and sequentially recruits vesicles trafficking between the ER and the Golgi, mitochondria and ribosomes, becoming a rough ER-like compartment. In this modified niche the bacteria divide until they finally exit the host cell, either by lytic or non-lytic egress mechanisms. Fundamental to these processes is a Type IV-B secretion system, called Icm/Dot, which translocates over 300 effectors into the host cell.1 A particular feature of the Legionella effector repertoire is its functional redundancy, which derives from the absence of infection-related phenotypes when one or more of the effector encoding genes are deleted, and therefore complicates the assessment of their role during infection. Nevertheless, during recent years functions have been assigned to a number of them in targeting host cell processes such as apoptosis, the NFkB pathway or the secretory pathway (for more detailed reviews on Legionella effector function please consult1,2,3).
Pathogen Invasion and Actin as a (Cyto)skeleton Key
As actin is one of the most abundant eukaryotic proteins and used in a great variety of structural and regulatory functions in the cell, it is not surprising that the actin cytoskeleton is a major target of pathogenic bacteria and viruses. Co-opting actin dynamics is fundamental in diverse steps of the infection cycle, both for extracellular and intracellular pathogens. Phagocytosis by immune system cells is the first defense mechanism triggered by the host to fight a pathogen. Some extracellular bacteria such as Yersinia use effectors to cripple the actin machinery, thus avoiding phagocytosis and consequent degradation, while E. coli pathogenic strains hijack the actin cytoskeleton to form an actin pedestal in the site of adhesion, fundamental to the infection cycle, and Vibrio promotes microfilament rearrangements disrupting cellular tight junctions in order to disturb the epithelial barrier. In contrast, some intracellular pathogens, namely Salmonella, Shigella and Listeria, stimulate actin rearrangements to promote their own engulfment into otherwise non-phagocytic cells. Once inside the cell, pathogens like Listeria or Rickettsia evade the phagosome and are able to move within the host cell cytosol and spread to neighboring cells, by triggering the formation of actin comet tails in one pole of the bacterial cell. Vacuolar pathogens, on the other hand, have to counteract the deleterious effects of phagolysosomal fusion and develop a niche viable for replication. However, although actin has been shown to play a role in processes such as the biogenesis of the Chlamydia inclusion or in enhancing the translocation of type III secretion substrates, relatively less is known about its importance in the establishment of a replicative niche that allows for microbe replication (for references and pathogen subversion of other cytoskeleton components please consult recent comprehensive reviews on this topic by4-6).
Actin cytoskeleton dynamics also play a fundamental role in Legionella infection. Actin remodeling is essential for phagocytic uptake and several actin-binding proteins have been implicated in the entry process, such as coronin, villidin, α-actinin and filamin.7 Analysis of the composition of the LCV during infection of D. discoideum has identified, in addition to actin, many other actin related proteins at later times post infection such as coronin, cofilin, myosin II and profilin, Arp2/3 components, actin bundling and capping proteins.8,9 However, the function of all these actin related proteins on the LCV membrane and the cross-talk between L. pneumophila effectors and the host actin cytoskeleton components has remained for the most part elusive.
Bridging Actin and the Endosomal Pathway
In our recent paper,10 we found that the L. pneumophila effector VipA binds and nucleates actin polymerization in vitro. During infection of macrophages, translocated VipA co-localizes with actin filaments and early endosomes, an association also observed when VipA-GFP was ectopically expressed in S. cerevisiae and in mammalian CHO cells. In previous work,11 it had been shown that this effector impaired vacuolar protein trafficking in S. cerevisiae, interfering with the Multivesicular Body (MVB) pathway, which is supported by its colocalization with some MVB components. In addition, we isolated a VipA mutant carrying a mutation in the N-terminal region of the protein (VipA-1), which is affected in its binding and polymerization of actin, in its ability to interfere with the MVB pathway and subcellular localization. Taken together, these results suggest a role for VipA as a link between the host actin cytoskeleton and endosomal trafficking, a novel function among prokaryotic virulence factors.
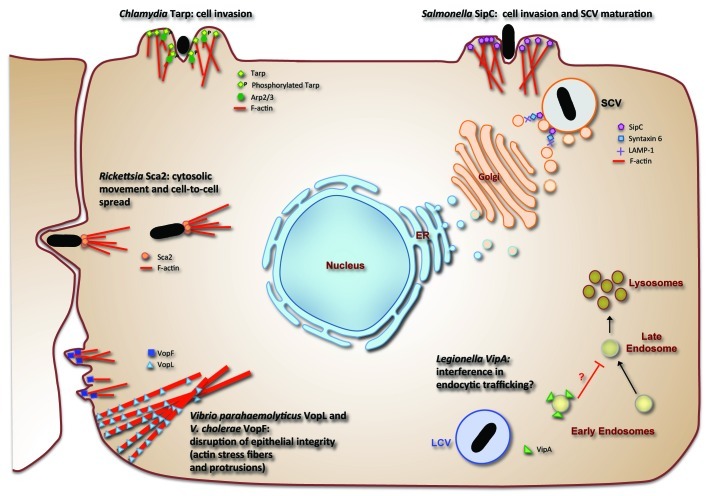
To date, only a small number of bacterial actin nucleators have been identified and characterized (Fig. 1). Chlamydia Tarp and Salmonella SipC are involved in actin-mediated uptake of the pathogen, while Rickettsia Sca2 is a formin-like nucleator that mediates intracellular movement of the bacterium via polymerization of actin comet tails.12-14 In addition, the extracellular bacterium Vibrio parahaemolyticus injects into host epithelial cells VopL and VopF, which lead to a rearrangement of the actin network and cause disruption of cell-cell contacts, facilitating enteric infection.15,16 None of these bacterial actin nucleators have however been implicated in vesicle trafficking, and to our knowledge only two effectors have given clues as to how the pathogen may link actin dynamics to endosomal trafficking for their own benefit. The Salmonella enterica phosphoinositide phosphatase SopB plays multiple roles during infection. In addition to mediating actin-dependent internalization of the bacteria, it also prevents the transition of PI(3)P to PI(3,5)P in the Salmonella containing vacuole (SCV) membrane and therefore may contribute to arresting SCV maturation along the endosomal pathway and consequently lysosomal fusion.17-19 Enteropathogenic Escherichia coli (EPEC) EspF nucleates a multiprotein complex containing the Arp2/3 activator N-WASP and the endocytic regulator sorting nexin 9 (SNX9), which mediates clathrin-dependent endocytosis. Although the mechanism or pathogenic significance of this has not been yet elucidated, EspF has been proposed to alter SNX9 regulation of endocytosis during EPEC infection, leading to the formation of aberrant tubular vesicles.20-22
Figure 1. Bacterial actin nucleators and their role in infection. Schematic diagram of cell rearrangements led by bacterial effector-driven actin polymerization. In Chlamydia, a cooperative mechanism leading to the formation of cell protrusions and invasion has been proposed, in which Tarp nucleates short actin filaments and when phosphorylated also leads to the recruitment of Arp2/3 via Rac signaling.12,30 Salmonella SipC promotes entry by direct microfilament assembly and, in addition, is involved in the recruitment of Golgi-derived vesicles with the SCV, by associating with host Syntaxin-6, which in turn binds lysosomal LAMP-113,31. The formin-like Sca2 nucleator from Rickettsia leads to the formation of actin comet tails, which enable the movement of the pathogen within the host cell cytosol, as well as invasion of neighboring cells.14Vibrio cholerae VopF and Vibrio parahaemolyticus VopL (and ortholog VopN from V. cholerae) lead to membrane protrusions and formation of stress fibers, respectively, implicated in disruption of tight junctions and consequent epithelial integrity.15,16,32 In addition to nucleating actin filaments, L. pneumophila VipA binds early endosomes and may play a role in the endocytic pathway.10
Clues From Eukaryotes
Even among eukaryotic proteins only a few have been reported to be simultaneously involved in actin dynamics and endosomal trafficking. Alix (also known as Aip1) is an adaptor protein with multiple functions and binding partners. It associates with actin, tubulins, focal adhesion kinase (FAK), Src and proline-rich tyrosine kinase 2 (PYK-2). It is involved in the actin-dependent positioning of endosomes by connecting these organelles to the cortical actin cytoskeleton.23,24 In addition, it interacts with ESCRT components, being involved in the MVB Pathway and thus playing a role in related processes such as EGFR downregulation, HIV budding and also cytokinesis.25,26
Annexins have also been implicated in a dual function as modulators of endosomal trafficking and actin dynamics. Annexin A6 is a Ca2+-dependent protein that binds lipid rafts in the plasma membrane and endosomes. It also binds F-actin, presumably acting as a scaffold link between membrane microdomains and the cytoskeleton, and regulating transient membrane-actin interactions during endocytic and exocytic transport.27,28 The related protein annexin A2 plays a role in early to late endosome transport. Together with Spire1 and Arp2/3, it is involved in MVB formation, by nucleating actin patches in early endosomes that are responsible for the actin-dependent endosome biogenesis, presumably by driving the membrane remodeling process.29
Recent insights into actin regulation of endosome biogenesis came from studies on the Arp2/3 activators WASH and N-WASP. Both play a role in endocytic trafficking, although their functions seem to be temporally and spatially distinct. WASH is endosome-specific (locating mainly in early and recycling endosomes) and it was implicated in actin-regulated endosome shape and scission via Arp2/3 activation.26 In addition, analysis of the trafficking of different receptors (of transferrin, EGF and the acid hydrolase CI-MPR) suggest that membrane remodeling during endosome biogenesis is controlled by WASH-mediated actin assembly. In contrast, N-WASP appears to function in previous and subsequent endocytic events, by promoting the internalization of plasma membrane patches and later actin-mediated vesicle propulsion through the cytoplasm (reviewed in ref. 4).
Future Perspectives
The localization of VipA to endosomes and its effect on MVB trafficking suggest a role in endosome biogenesis. In the context of a Legionella infection, a possible goal would be a modulation of the interaction of the LCV with the endocytic machinery, thus aiding in preventing its delivery and degradation in the lysosome. Alternative functions related to the MVB pathway could be a disturbance in the adaptive immune response, by interfering with antigen presentation, or in the regulation of membrane lipid and protein composition. In the past, many eukaryotic cell pathways and protein functions have been elucidated by studies on microbe-host interactions. The role played by actin in the endocytic pathway, particularly in the formation of the MVB, is still scarce and insights may come from further studies on this bacterial effector.
Acknowledgments
This work was supported by funding from: Fundação para a Ciência e Tecnologia through grant # PEst-OE/EQB/LA0004/2011; the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° PCOFUND-GA-2009-246542; and NIH grant AI23549.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/20422
References
- 1.Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One. 2011;6:e17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isberg RR, O’Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco IS, Shuman HA, Charpentier X. The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol. 2009;11:1435–43. doi: 10.1111/j.1462-5822.2009.01351.x. [DOI] [PubMed] [Google Scholar]
- 4.Haglund CM, Welch MD. Pathogens and polymers: microbe-host interactions illuminate the cytoskeleton. J Cell Biol. 2011;195:7–17. doi: 10.1083/jcb.201103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carabeo R. Bacterial subversion of host actin dynamics at the plasma membrane. Cell Microbiol. 2011;13:1460–9. doi: 10.1111/j.1462-5822.2011.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stolp B, Fackler OT. How HIV takes advantage of the cytoskeleton in entry and replication. Viruses. 2011;3:293–311. doi: 10.3390/v3040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fajardo M, Schleicher M, Noegel A, Bozzaro S, Killinger S, Heuner K, et al. Calnexin, calreticulin and cytoskeleton-associated proteins modulate uptake and growth of Legionella pneumophila in Dictyostelium discoideum. Microbiology. 2004;150:2825–35. doi: 10.1099/mic.0.27111-0. [DOI] [PubMed] [Google Scholar]
- 8.Shevchuk O, Batzilla C, Hägele S, Kusch H, Engelmann S, Hecker M, et al. Proteomic analysis of Legionella-containing phagosomes isolated from Dictyostelium. Int J Med Microbiol. 2009;299:489–508. doi: 10.1016/j.ijmm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Urwyler S, Nyfeler Y, Ragaz C, Lee H, Mueller LN, Aebersold R, et al. Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic. 2009;10:76–87. doi: 10.1111/j.1600-0854.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 10.Franco IS, Shohdy N, Shuman HA. The Legionella pneumophila effector VipA is an actin nucleator that alters host cell organelle trafficking. PLoS Pathog. 2012;8:e1002546. doi: 10.1371/journal.ppat.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shohdy N, Efe JA, Emr SD, Shuman HA. Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc Natl Acad Sci U S A. 2005;102:4866–71. doi: 10.1073/pnas.0501315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, et al. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A. 2004;101:10166–71. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayward RD, Koronakis V. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 1999;18:4926–34. doi: 10.1093/emboj/18.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat Cell Biol. 2010;12:1057–63. doi: 10.1038/ncb2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ. A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe. 2007;1:95–107. doi: 10.1016/j.chom.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Liverman AD, Cheng HC, Trosky JE, Leung DW, Yarbrough ML, Burdette DL, et al. Arp2/3-independent assembly of actin by Vibrio type III effector VopL. Proc Natl Acad Sci U S A. 2007;104:17117–22. doi: 10.1073/pnas.0703196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou D, Chen LM, Hernandez L, Shears SB, Galán JE. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol. 2001;39:248–59. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou D, Mooseker MS, Galán JE. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science. 1999;283:2092–5. doi: 10.1126/science.283.5410.2092. [DOI] [PubMed] [Google Scholar]
- 19.Dukes JD, Lee H, Hagen R, Reaves BJ, Layton AN, Galyov EE, et al. The secreted Salmonella dublin phosphoinositide phosphatase, SopB, localizes to PtdIns(3)P-containing endosomes and perturbs normal endosome to lysosome trafficking. Biochem J. 2006;395:239–47. doi: 10.1042/BJ20051451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alto NM, Weflen AW, Rardin MJ, Yarar D, Lazar CS, Tonikian R, et al. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178:1265–78. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weflen AW, Alto NM, Hecht GA. Tight junctions and enteropathogenic E. coli. Ann N Y Acad Sci. 2009;1165:169–74. doi: 10.1111/j.1749-6632.2009.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weflen AW, Alto NM, Viswanathan VK, Hecht GE. E. coli secreted protein F promotes EPEC invasion of intestinal epithelial cells via an SNX9-dependent mechanism. Cell Microbiol. 2010;12:919–29. doi: 10.1111/j.1462-5822.2010.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabezas A, Bache KG, Brech A, Stenmark H. Alix regulates cortical actin and the spatial distribution of endosomes. J Cell Sci. 2005;118:2625–35. doi: 10.1242/jcs.02382. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MH, Dikic I, Bögler O. Src phosphorylation of Alix/AIP1 modulates its interaction with binding partners and antagonizes its activities. J Biol Chem. 2005;280:3414–25. doi: 10.1074/jbc.M409839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt MH, Hoeller D, Yu J, Furnari FB, Cavenee WK, Dikic I, et al. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–93. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–27. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornely R, Rentero C, Enrich C, Grewal T, Gaus K. Annexin A6 is an organizer of membrane microdomains to regulate receptor localization and signalling. IUBMB Life. 2011;63:1009–17. doi: 10.1002/iub.540. [DOI] [PubMed] [Google Scholar]
- 28.Enrich C, Rentero C, de Muga SV, Reverter M, Mulay V, Wood P, et al. Annexin A6-Linking Ca(2+) signaling with cholesterol transport. Biochimica et biophysica acta 2011; 1813:935-47. [DOI] [PubMed]
- 29.Morel E, Parton RG, Gruenberg J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell. 2009;16:445–57. doi: 10.1016/j.devcel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog. 2008;4:e1000014. doi: 10.1371/journal.ppat.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan R, Rastogi R, Parashuraman S, Mukhopadhyay A. Salmonella acquires lysosome-associated membrane protein 1 (LAMP1) on phagosomes from Golgi via SipC protein-mediated recruitment of host Syntaxin6. J Biol Chem. 2012;287:5574–87. doi: 10.1074/jbc.M111.286120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam VC, Suzuki M, Coughlin M, Saslowsky D, Biswas K, Lencer WI, et al. Functional analysis of VopF activity required for colonization in Vibrio cholerae. MBio 2010; 1. [DOI] [PMC free article] [PubMed]