Abstract
SNARE complexes mediate membrane fusion in the endomembrane system. They consist of coiled-coil bundles of four helices designated as Qa, Qb, Qc and R. A critical intermediate in the fusion pathway is the trans-SNARE complex generated by the assembly of SNAREs residing in opposing membranes. Mechanistic details of trans-SNARE complex formation and topology in a physiological system remain largely unresolved. Our studies on native yeast vacuoles revealed that SNAREs alone are insufficient to form trans-SNARE complexes and that additional factors, potentially tethering complexes and Rab GTPases, are required for the process. Here we report a novel finding that a HOPS tethering complex dimer catalyzes Rab GTPase-dependent formation of a topologically preferred QbQcR-Qa trans-SNARE complex.
Keywords: HOPS tethering complex dimer, QbQcR-Qa trans-SNARE complex, Rab GTPase
Introduction
Membrane fusion is a fundamental process marking the culmination of every vesicular trafficking route in the endomembrane system. Soluble N-ethylmaleimide sensitive factor Attachment protein receptors, or SNAREs, are central players mediating the fusion of apposed membrane bilayers through the assembly of distinct trans-SNARE complexes. A trans-SNARE complex bridging two apposed membranes is composed of a parallel four-helix bundle with the SNARE domain helices designated as Qa, Qb, Qc and R.1,2 As demonstrated in our previous work,3 the compatibility for fusion is determined by a particular combination of SNAREs contributed by each membrane and that such a preferred trans-SNARE topology is guided by additional factors such as tethering complexes and Rab GTPases. Vesicular transport can be sectioned into a progression of interdependent subreactions that comprise vesicle budding, movement, priming, tethering, docking, lipid mixing and content mixing.4 However, the precise protein interaction scheme underlying the evolution of priming through docking is still unresolved.
Essential components of the yeast vacuolar fusion machinery include SNAREs—Vam3 (Qa), Vti1 (Qb), Vam7 (Qc) and Nyv1 (R); the HOPS (HOmotypic fusion and vacuole Protein Sorting) tethering complex and the Rab GTPase Ypt7.5-7 HOPS is a highly conserved tethering complex operating within the endolysosomal transport circuit. The HOPS complex is composed of six different subunits namely Vps11, Vps16, Vps18, Vps33, Vps39 and Vps41. Vps39 and Vps41 are vacuole-specific subunits while the remaining four form a conserved core. Individual subunits of the HOPS complex exhibit domain architecture designed to mediate protein-protein interactions, and in particular, signify oligomerization potential. Typical domains include C-terminal zinc binding RING finger in Vps11 and Vps18 and clathrin heavy chain repeat in Vps18, Vps39 and Vps41.8 HOPS subunits show remarkable structural similarity to other membrane-shaping multisubunit building blocks such as COPI, COPII, clathrin and the nuclear pore complex.8 Therefore it is likely that one or more subunits might assemble into high-order structures in a cis and/or trans configuration. Additionally, by virtue of its modular structural architecture and multiple affinities toward small G proteins, SNAREs and lipids, the heterohexameric HOPS tethering complex is poised to link the recognition of membranes (via lipids/adaptors/small G proteins in general) with subsequent fusion (via SNAREs) through highly dynamic interactions with multiple sets of proteins.9-11 In the simplest sense, dimeric HOPS would create a highly stable platform capable of (1) binding activated Rab proteins, (2) recruiting primed SNARE subcomplexes dependent on the action of Sec18/NSF(N-ethylmaleimide Sensitive Factor) and (3) catalyzing the formation of a topologically restricted trans-SNARE complex, thus imparting additional specificity and fidelity to the process of membrane fusion. It is easy to visualize how a scaffold in the form of an oligomerized tethering complex would, by means of its size, reach over the distance between apposed membranes and, through multiple affinities, enhance the local concentration of fusion factors ultimately facilitating SNARE pairing in trans.
We have shown earlier that a QbQcR-Qa trans-SNARE distribution is biochemically distinguishable and functionally relevant for membrane fusion. To further investigate our previous finding that the formation of a QbQcR cis-SNARE subcomplex depends upon the HOPS complex and the Rab Ypt7, we now sought more evidence that might indicate a direct participation of these components in trans-SNARE complex assembly as well. We employed various approaches to investigate (1) whether the HOPS complex possesses any oligomerization tendencies and (2) whether it is able to create an asymmetry in SNARE function. First we resorted to gel filtration chromatography to detect if any higher-order oligomers of the entire HOPS complex processed from detergent extracts of purified yeast vacuoles exist and if so, determine their stability. Second, taking advantage of the fact that HOPS subunits contain complete or partial RING domains or Cysteine/Histidine-rich regions in general, we used a disulfide bridge dependent crosslinking strategy to ascertain if any multimerization of individual subunits occurs on native membranes. Most importantly, we used specific co-immunoprecipitation schemes involving differentially tagged versions of HOPS subunits and SNAREs to be able to categorically pinpoint HOPS-SNARE associations leading to trans-SNARE complex establishment. We propose a novel concept that specificity in eukaryotic intercompartmental communications and regulation over trans-SNARE pairing is imparted by tethering complex dimerization more robustly than just the specific occurrence of SNAREs.
Results
HOPS is a dimeric complex on the surface of yeast vacuoles
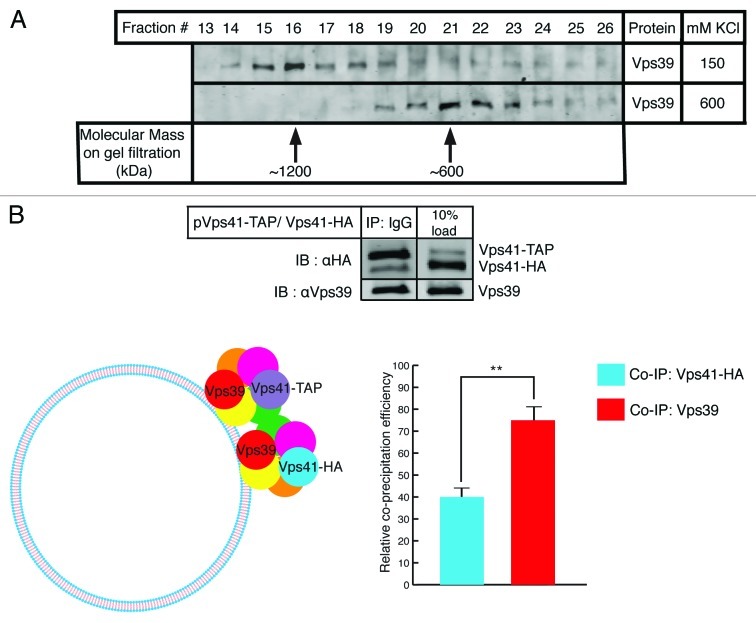
A straightforward way to evaluate complex formation tendency of a protein or multiprotein complex is to perform gel filtration analysis. Vacuoles purified from wild type yeast cells were detergent extracted and the solubilizate was run through a Superose 6 column able to resolve proteins within a molecular size range of 10–2,000 kDa. Fractions from gel filtration were analyzed by SDS-PAGE and immunoblotted by probing with antibodies raised against Vps39. The predicted molecular mass of the HOPS complex obtained by summing the masses of individual subunits is around 600 kDa. Remarkably however, we observed that HOPS predominantly migrates at exactly double the expected molecular mass which is around 1,200 kDa as depicted in Figure 1A (top lane). Upon modifying the solubilization conditions by increasing the salt concentration beyond physiological levels HOPS was found to run at its predicted monomeric mass of 600 kDa (Fig. 1A bottom lane). There is almost complete transposition of a dimeric population to a monomeric population of HOPS under high salt concentration. To further confirm whether the observed mobility shift corresponds to a true HOPS dimer or involves other binding partners, we co-expressed a particular HOPS subunit, namely Vps41, as two differently tagged versions in the same strain—Vps41-HA through its native genomic locus and Vps41-TAP through an exogenous plasmid source. It is to be noted that any interaction between identical subunits carrying different epitope tags cannot originate from intra-complex binding but rather signifies interaction between two different HOPS complexes. As shown in Figure 1B (top panel), we assayed for HOPS complex dimerization by IgG pull down of Vps41-TAP and probing for any co-precipitating Vps41-HA and ascertained holocomplex precipitation by probing for Vps39 since these are known to be the HOPS-specific subunits. Relative co-precipitation efficiencies of Vps41-HA and Vps39 plotted in Figure 1B (bottom panel) indicate that HOPS quantitatively forms a dimer in this assay.
Figure 1. HOPS is a dimeric complex on the surface of yeast vacuoles. (A) Gel filtration analysis of purified yeast vacuoles. Fractions 13–26 from Superose 6 gel filtration of solubilized vacuoles were inspected using SDS-PAGE followed by immunoblotting against Vps39. When vacuoles were processed at 150 mM KCl in the solubilization buffer, HOPS predominantly runs in fraction 16 (top lane). At 600 mM KCl, HOPS predominantly runs in fraction 21 (bottom lane). Arrows indicate molecular weights. (B) Cis-HOPS complexes assayed by differently tagged Vps41. Vacuoles from the Vps41-HA strain harboring a Vps41-TAP plasmid were processed as described in Materials and Methods. After IgG pull down of Vps41-TAP, co-precipitating proteins were analyzed by SDS-PAGE and western blotting. In the top panel, the left lane shows precipitated Vps41-TAP, co-precipitating Vps41-HA and Vps39 and the right lane displays corresponding protein inputs. The bottom panel depicts relative co-precipitation efficiencies of Vps41-HA and Vps39 quantified by Odyssey densitometry and normalized from three independent experiments. Vps39 is consistently found to co-precipitate at approximately twice the efficiency of that of Vps41-HA (p < 0.01).
HOPS dimerizes in cis via Vps11
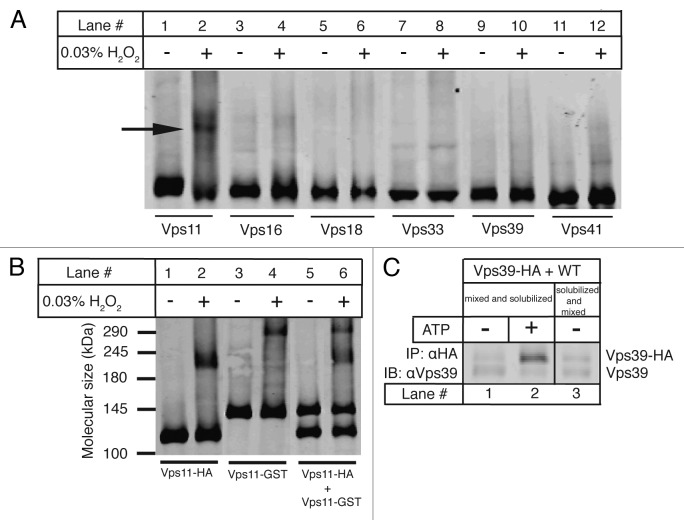
The HOPS subunits Vps11,9 Vps3912 and Vps4113 have previously been reported to interact with themselves in different yeast two-hybrid analyses. Also Vps11 and Vps18 have been proposed to potentially multimerize via their C-terminal RING domains, which are susceptible to oxidation. We therefore devised a chemical crosslinking strategy in which disulfide bridges are formed between Cysteine residues when treated with hydrogen peroxide. Vacuoles harboring HA tagged versions of individual HOPS subunits were incubated with and without 0.03% H2O2 under standard fusion conditions and then subjected to non-reducing SDS-PAGE followed by immunoblotting with antiHA antibody. As indicated in Figure 2A, a higher band corresponding to a disulfide crosslinked product appears exclusively in the case of Vps11 (Fig. 2A, lane 2) among all of the HOPS subunits suggesting a role of the Cysteine-rich RING domain of Vps11 in mediating its homo-oligomerization. To deduce the order of oligomerization of Vps11 we employed the same protocol on a GST-tagged version of Vps11 (Vps11-GST~145 kDa) along with the HA-tagged version of Vps11 (Vps11-HA~120 kDa). It is expected that addition of another tag differing in size will alter the apparent mobility of Vps11 on SDS-PAGE. We compared the molecular mass shifts between crosslinked and non-crosslinked proteins from vacuoles harboring Vps11-HA and Vps11-GST individually and observed that the crosslinked product in each case runs at exactly double the molecular mass of the non-crosslinked one confirming that Vps11 forms a homodimer (~240 kDa for Vps11HA/Vps11-HA and ~290 for Vps11-GST/Vps11-GST) as shown in Figure 2B (lanes 1–4). Interestingly, upon crosslinking Vps11-HA and Vps11-GST together in a mixture, we do not observe any intermediate crosslinked product corresponding to Vps11-HA/Vps11-GST interaction (~265 kDa) (Fig. 2B, lanes 5 and 6). This implies that there is only a preferred cis-interaction occurring between Vps11 molecules on apposed native membranes since no intermixing between the differently tagged Vps11 populations is seen. Trans-interactions, if any, cannot be detected at this level of sensitivity. To further verify whether the HOPS complex in general dimerizes in a trans configuration, we looked at inter-vacuolar HOPS interactions by employing Vps39-HA and non-tagged Vps39 versions. Vacuoles from the Vps39-HA strain and those from the wild type strain were mixed and incubated with or without ATP, solubilized and immunoprecipitated against the HA epitope (Fig. 2C, lanes 1 and 2). Additionally, vacuoles from these two strains were separately solubilized and the solubilizates were then mixed to control for interactions forming in the immunoprecipitation buffer (Fig. 2C, lane 3). The precipitates were separated on SDS-PAGE and immunoblotted using antiVps39 antibody. As depicted in Figure 2C, there is no significant increase in Vps39 co-precipitation efficiency over background levels validating the absence of any trans-HOPS dimers.
Figure 2. HOPS dimerizes via the core subunit Vps11. (A) Crosslinking of HOPS subunits. Vacuoles harboring HA-tagged versions of all HOPS subunits were incubated under standard fusion conditions with or without 0.03% H2O2, centrifuged and analyzed on non-reducing SDS-PAGE followed by western blotting with antiHA antibody. Lanes 1–12 show the effect of peroxide treatment on each HOPS subunit labeled below. Only Vps11 shows a crosslinked product marked by the arrow (lane 2). (B) Crosslinking of Vps11-HA and Vps11-GST. Crosslinking of vacuoles harboring C-terminal 6HA or GST/3HA tags on Vps11 was done as described in 2A. Molecular weights in kDa are as marked. Vps11-HA (~120 kDa, lane 1) forms a higher molecular weight crosslinked product (~240 kDa, lane 2). Similarly Vps11-GST (~145 kDa, lane 3) forms a crosslinked product (~290 kDa, lane 4). A mixture of Vps11-HA and Vps11-GST (lane 5) shows two crosslinked products (~240 and 290 kDa, lane 6) identical to those observed in lanes 2 and 4, but no intermediate band. (C) Trans-HOPS complexes assayed by Vps39 variants. Vacuoles from Vps39-HA and wild type strains were processed as described in Materials and Methods. Vps39-HA was precipitated using antiHA antibody and co-precipitating Vps39 was analyzed using SDS-PAGE and western blotting. Interactions between Vps39-HA and non-tagged Vps39 from the two fusion partners include those observed in the absence of ATP (lane 1), in the presence of ATP (lane 2) and in the solubilization buffer (lane 3).
HOPS catalyzes a Rab GTPase-dependent trans Qa-QbQbR SNARE complex establishment
We have established previously that the ATPase Sec18/NSF creates an activated QbQcR cis-SNARE pool that serves as an acceptor subcomplex for the single Qa SNARE originating from the opposite membrane. Additionally we demonstrated that the HOPS complex and the Rab GTPase Ypt7 are necessary to maintain a stable QbQcR cis-SNARE subcomplex. It has been previously shown that functional Ypt7 is necessary on each partner for successful homotypic vacuolar fusion.14 This led us to inquire whether Ypt7 in trans is involved in SNARE pairing. We used two approaches to alter Ypt7 and hence study its role on trans-SNARE complex establishment—(1) the Ypt7-T22N point mutant strain which delivers a GDP-locked inactive form of Ypt7 and (2) recombinant GDI (GDP dissociation inhibitor) which extracts Ypt7 from its membrane-bound form and thereby inactivates it.
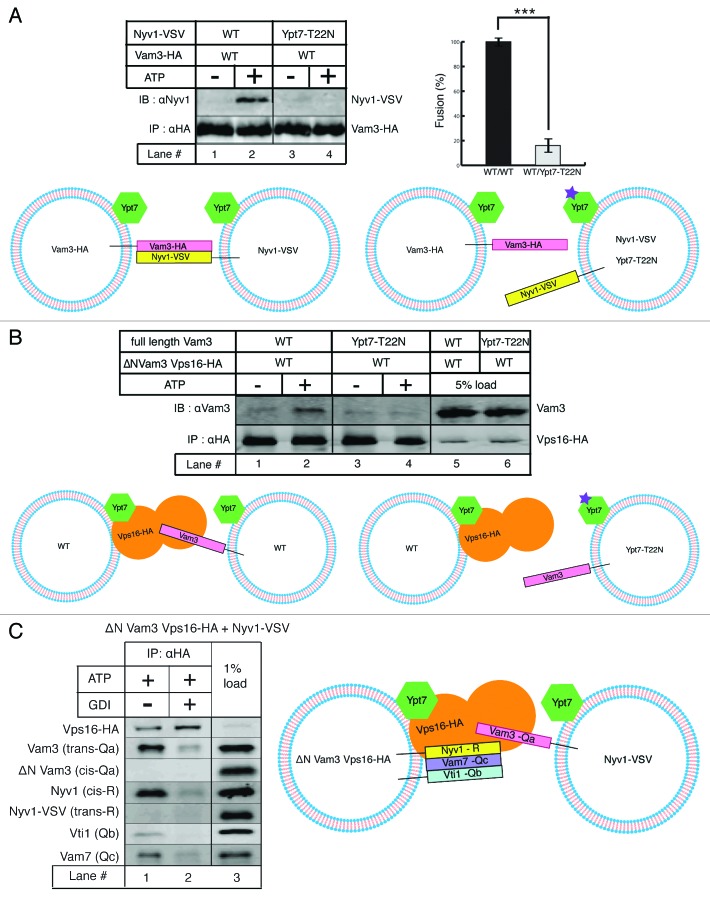
Trans-SNARE complexes are generated upon addition of ATP to purified vacuoles. Differential tagging of peptides allows us to distinguish interactions occurring on the same (cis) membrane from those on opposing (trans) membranes. We adjusted the experimental conditions to be able to capture only trans-SNARE complexes and eliminate the possibility of obtaining any post-fusion cis-SNARE complexes.3 First we assayed for trans-SNARE complex formation between Vam3 (Qa) and Nyv1 (R) using differently tagged versions of these SNAREs. As displayed in Figure 3A, Vam3-HA vacuoles from the wild type strain were mixed with Nyv1-VSV vacuoles from either the wild type or the Ypt7-T22N point mutant strain and incubated with or without ATP. The vacuole mixtures were solubilized and immunoprecipitated against the HA epitope. The precipitate was resolved on SDS-PAGE and western blotting using the indicated antibodies was performed to analyze the extent of trans-interactions. A clear trans-SNARE signal is observed in the presence of ATP where Nyv1 (R) from the wild type strain significantly co-precipitates with Vam3 (Qa) compared with that in absence of ATP (lanes 1 and 2). In contrast, Nyv1 (R) from the Ypt7-T22N strain is unable to form a trans-SNARE complex with Vam3 (Qa) (lanes 3 and 4). Concomitantly, no significant fusion was observed between wild type and Ypt7-T22N vacuoles compared with that between both wild type partners, as plotted in Figure 3A.
Figure 3.
A HOPS dimer catalyzes Ypt7-dependent trans QbQcR-Qa SNARE complex formation. (A) trans-SNARE complexes assayed by tagged SNAREs. Vam3-HA vacuoles from the wild type strain were mixed with Nyv1-VSV vacuoles from either the wild type strain (lanes 1 and 2) or the Ypt7-T22N strain (lanes 3 and 4) and incubated with or without ATP under standard fusion conditions. Vam3-HA was precipitated using Protein-G/antiHA antibody and co-precipitating Nyv1-VSV was detected by SDS-PAGE and western blotting with antiNyv1 antibody. A trans-SNARE complex is observed only in the presence of ATP when both partners are wild type (lane 2) but not in a Rab mutant background for one of the partners (lane 4). The panel on the right side compares fusion efficiency between both wild type partners with that between the wild type and Ypt7-T22N mutant quantified and normalized from three independent experiments (p < 0.001). (B) trans-HOPS/Qa SNARE interactions. Vacuoles from the ΔN-terminal Vam3 strain harboring Vps16-HA were mixed with vacuoles from either the wild type strain (lanes 1 and 2) or the Ypt7-T22N strain (lanes 3 and 4) and incubated with or without ATP under standard fusion conditions. Vps16-HA was precipitated using Protein-G/antiHA antibody and co-precipitating Vam3 was detected by SDS-PAGE and western blotting with antiVam3 antibody. Vam3 (trans-Qa SNARE) co-precipitates with Vps16-HA only in presence of ATP when both partners are wild type (lane 2) but not in a Rab mutant background for one of the partners (lane 4). Lanes 5–8 show corresponding protein inputs. (C) Vacuoles from the ΔN-terminal Vam3 strain harboring Vps16-HA were mixed with vacuoles from the Nyv1-VSV strain and incubated with ATP alone (lane 1) or both ATP and GDI (lane 2) under standard fusion conditions. Vps16-HA was precipitated using Protein-G/antiHA antibody and co-precipitating SNAREs were analyzed by SDS-PAGE and western blotting against the indicated proteins. Full length Vam3 (trans Qa SNARE) and Nyv1 (cis R SNARE) co-precipitate with Vps16-HA in an ATP-dependent manner as. Lane 3 shows corresponding protein inputs.
To further address the issue of requirement of Rab function on each fusion partner, we resorted to the following co-immunoprecipitation strategies involving HOPS, SNARE and Rab variants. As represented in Figure 3B, vacuoles were purified from the Vps16-HA strain deleted for the N-terminal region of Vam3, and mixed with vacuoles from either the wild type or the Ypt7-T22N strain. This combination allows us to make a distinction between the Qa SNAREs arriving from apposed fusion partners since full-length Vam3 runs higher on SDS-PAGE than its truncated form. The vacuole mixture was solubilized and immunoprecipitated against the HA epitope. The precipitate was separated on SDS-PAGE and blotted against the indicated antibodies. We observed that Vam3 (Qa) originating from the wild type background co-precipitates significantly with Vps16-HA in an ATP-dependent manner recapitulating a classical trans-SNARE signal (lanes 1 and 2). Remarkably however, no Vam3 (Qa) from the Ypt7-T22N background is able to co-precipitate with Vps16-HA (lanes 3 and 4). Taken together, these results suggest that small G proteins are necessary for trans-SNARE formation on both fusion partners—one delivering a QbQcR SNARE subcomplex and the other delivering the Qa SNARE.
To be able to assess HOPS-SNARE topology more completely, we used vacuoles from the Vps16-HA strain deleted for the N-terminal region of Vam3 in combination with vacuoles from the Nyv1-VSV strain. This permits us to distinguish Qa and R SNAREs originating from each fusion partner. Also, using GDI as a Ypt7 inactivating agent it is simultaneously possible to assay for Rab protein requirement for the process. As illustrated in Figure 3C, vacuoles from the two strains mentioned above were mixed and incubated in the presence of ATP only or both ATP and GDI, solubilized and immunoprecipitated against the HA epitope. The precipitate was resolved on SDS-PAGE and analyzed by western blotting using the indicated antibodies. We observe that in the presence of ATP, Vps16-HA preferentially interacts with Nyv1 in cis and with full length Vam3 in trans (lane 1). The co-precipitation efficiencies of both these SNAREs diminish significantly in the presence of GDI (lane 2). It is worthwhile to note that the reciprocal Qa (truncated Vam3) and R (Nyv1-VSV) SNAREs are not detectible in the HOPS precipitation indicating selective recruitment of SNAREs to the HOPS complex in preparation for trans-SNARE complex formation. These results, in conjunction with our previous studies describing the existence of a stable QbQcR cis-SNARE subcomplex, lead us to present a model of a HOPS dimer-mediated assembly of a QbQcR-Qa trans-SNARE complex.
Conclusions
It has been reported that there is promiscuity and diversity in SNARE-SNARE associations.15-18 Unrestricted pairing of SNAREs by random chance in a cell where continuous membrane flux is required between dynamically evolving compartments would create tremendous disturbances in overall homeostasis, including inappropriate cargo delivery and premature protein turnover to cite a few. This makes the pathway leading to trans-SNARE complex formation a critical target for regulation.
SNARE-driven membrane fusion serves different functions at different locations in a cell, ranging from neurotransmitter release to phagosome maturation. The kinetics of each fusion event must therefore be adjusted by local factors and trans-SNARE distribution influences their fusion efficiency as we have demonstrated in our previous functional studies. Also, Figure 3A implies that mere existence of SNAREs on opposing membranes is not sufficient for the formation of trans-SNARE complexes. Hence, orientation of the relevant SNARE topology at the appropriate location in a cell becomes an important aspect to be governed by additional factors. The requirement for SNAREs to function as the sole determinants of fusion specificity is not absolutely critical since a number of factors, by virtue of their size, abundance or localization, can see each other upstream of trans-SNARE pairing. Faithful membrane fusion would require one or more agents to exercise stringent control over and dictate specific channelization of SNARE molecules entering into trans-complexes. Based on our previous and current studies on vacuolar fusion, the dimeric HOPS tethering complex and the activated Rab GTPase Ypt7 appear as prime candidates having an intrinsic or acquired capability to perform this job.
Various lines of evidence seem to support our general idea that control over trans-SNARE complex formation occurs through collaborative action of tethers and Rabs, and that interactions among different tethering factors and the corresponding Rab proteins sets up the stage for SNARE-driven fusion. In the context of physiological membranes the vesicle tethering protein p115 was biochemically shown to selectively catalyze the assembly of Gos28 (v-SNARE) and syntaxin5 (t-SNARE) during NSF-driven Golgi reassembly. The syntaxin binding SM(Sec1/Munc18) family member Sly1 also coprecipitated in this complex.19 Another study demonstrated a Rab1-mediated recruitment of p115 to COPII vesicles on which the cis-complex of cognate v-SNAREs was stabilized.20 In these reports, however, trans-SNARE complex formation and topology were not explicitly assayed. Studies on heterotypic fusion involving ER-derived COPII vesicles and the Golgi hint toward a QbQcR-Qa trans-SNARE distribution with the Qa SNARE acting from the Golgi membrane although each membrane contains the full complement of SNAREs.21 The asymmetry in SNARE function was suggested to be caused by an asymmetric requirement for functional Rab GTPase Ypt1 and the SM family protein Sly1 specifically on the Golgi compartment suggesting a possible role of these factors in contributing to fusion specificity.
Purified HOPS, when added to SNARE-reconstituted proteoliposomes, was recently shown to accelerate their fusion22 and HOPS was proposed to be the direct agent of tethering, but evidence for a pathway of HOPS-mediated trans-SNARE complex formation is lacking. It was shown using a liposome-based system that an endosomal Rab GTPase can dimerize in trans with itself to tether membranes.23 However, in contrast to typical multisubunit or elongated coiled-coil tethering factors, the scope for small G proteins to function as tethering agents solely by themselves appears rather limited by their overall size and surface area available for orchestrating multifarious sequential interactions. Studies involving purified SNAREs, HOPS and Rabs reconstituted into synthetic liposomes do provide critical clues about probable functions of these fusion elements along with mechanistic insights.24-26 However, to be able to firmly establish and extrapolate fundamental mechanisms underlying membrane fusion, evidence from model systems incorporating physiological membranes is essential.
Studies on rat liver Golgi membranes have shown that the coiled-coil tether Golgin-84 on COPI vesicles interacts with the cis-Golgi localized heterooctameric COG (conserved oligomeric Golgi) complex through its Cog7 subunit and it was suggested that this tether-tether interaction in trans may somehow aid SNARE complex formation.27 EEA1(early endosomal antigen 1), the rod-like coiled coil tether functioning on endosomes was shown to form a parallel coiled coil dimer. Importantly, EEA1 (both recombinant and that derived from rat brain) can be crosslinked to yield a product with double the molecular mass of the monomer. It contains an N-terminal Zinc finger, a C-terminal PI(3)P binding FYVE domain and two binding sites for a GTP-bound form of Rab5—one at the N-terminal and other at the C-terminal. It was postulated in this study that the EEA1 dimer was likely to link Rab5-enriched compartments to each other.28 TRAPPII is a tethering complex composed of ten different subunits localized at the Golgi network. Native TRAPPII purified from yeast was found to exist predominantly in dimeric form as judged by the 2-fold symmetry apparent in negatively stained electron micropraphs.29 Also gel filtration studies have shown that TRAPPII elutes at approximately twice the value of molecular masses of its individual components.30 However, it remains to be determined whether this TRAPPII dimer is able to link native membranes via interactions with Rab GTPases or SNAREs.
Experiments on HOPS subunits implicate Vps39 and Vps41 in Rab recognition and emphasize that Vps11 functions as a platform for HOPS complex assembly with its C-terminal RING domain being functionally critical for traffic to the vacuole.31 A recent advance illustrated the cryo-EM structure of the HOPS complex stating that HOPS is a flexible, seahorse-like structure, 28–35 nm long, and was not found to exist as a dimer.32 To account for previously reported interactions and modes of action of HOPS, it was suggested that HOPS could oscillate between different conformations that bring its head (Vps41, Vps33) and tail (Vps39) subunits closer or farther to accommodate binding partners, especially Ypt7 and SNAREs. We conceptualize that a HOPS dimer assimilating this behavior can indeed sequentially stabilize a QbQcR cis-SNARE complex (likely on one HOPS complex in the dimer); execute, in concert with Ypt7 from trans, long-range membrane recognition and tethering over tens of nanometers (likely through Vps39/Vps41 on the partner HOPS complex in the dimer); followed by topologically regulated QbQcR-Qa trans-SNARE complex assembly (likely through Vps33 as suggested in Bröcker et al.32).
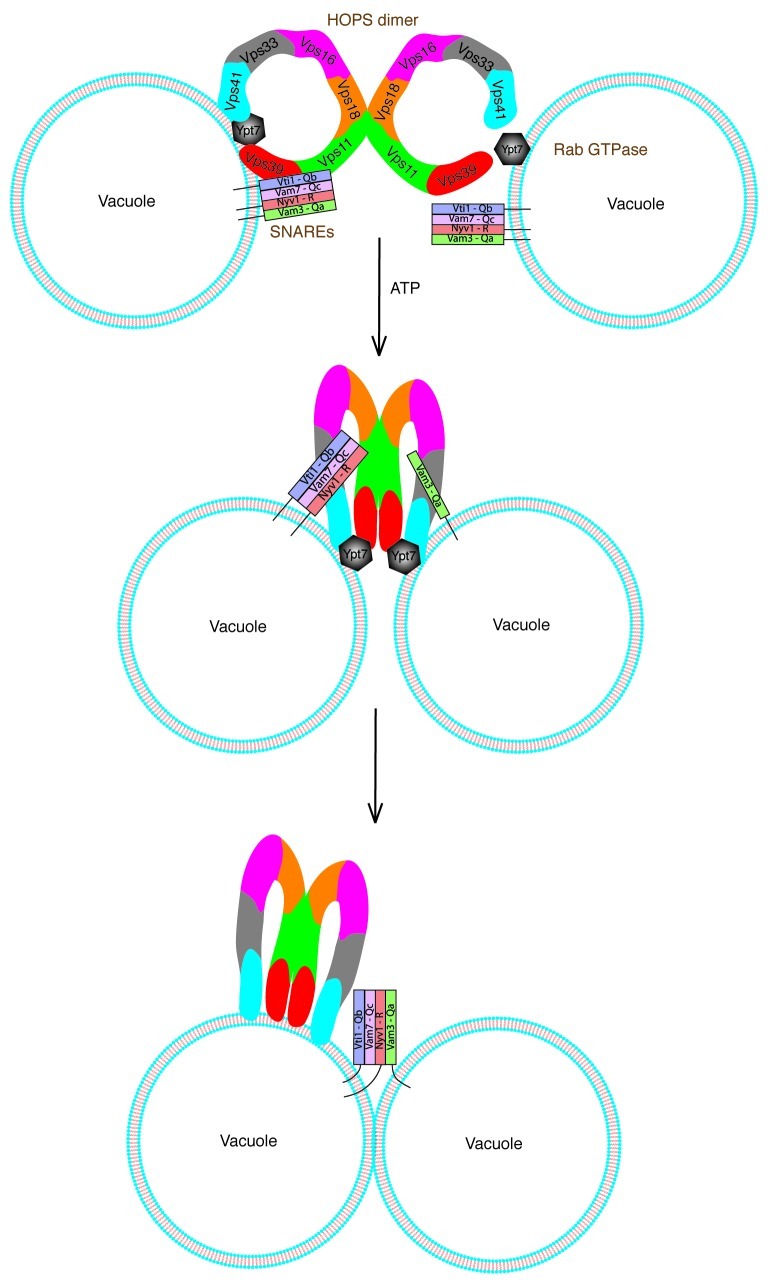
According to our homotypic model depicted in Figure 4, a fusion reaction starts out with identical fusion partners each possessing a full complement of the tetrameric cis-SNARE complex. Upon Sec18/NSF-induced activation of SNAREs in the presence of ATP, a HOPS dimer, accompanied by activated Ypt7 in cis, coordinates a stable QbQcR cis-SNARE complex having displaced the Qa SNARE. The HOPS dimer recognizes activated Ypt7 in trans and incorporates the single Qa SNARE from the apposed fusion partner ultimately catalyzing Rab-dependent assembly of a QbQcR-Qa trans-SNARE complex.
Figure 4. Working model for trans-SNARE complex establishment in homotypic fusion. Each fusion partner (vacuole) is exactly identical and possesses similar fusion machinery including four vacuolar SNAREs, a HOPS dimer and Ypt7. In the presence of ATP, the HOPS dimer along with Ypt7 in cis coordinates a QbQcR acceptor subcomplex having displaced the Qa SNARE.3 A HOPS dimer on one fusion partner (left) recognizes activated Ypt7 on the opposing fusion partner (right) and incorporates the single Qa SNARE from the opposing partner. Ultimately a HOPS dimer-dependent QbQcR-Qa trans-SNARE complex is assembled. HOPS subunits are color coded throughout.
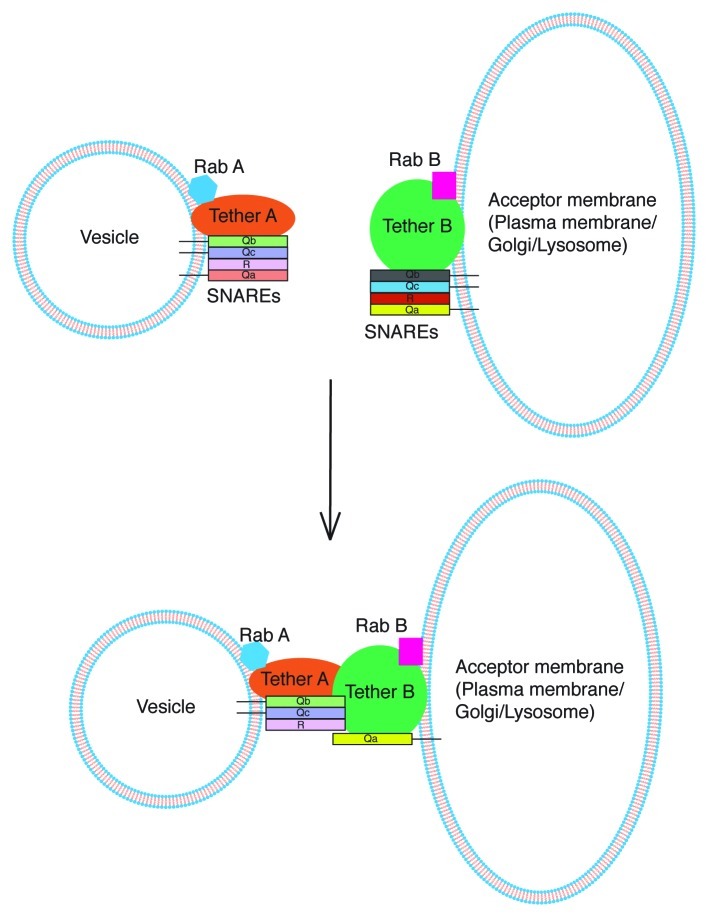
Similarly a model explaining trans-SNARE complex formation in a more general heterotypic scenario can be proposed as shown in Figure 5. Each fusion partner possesses its own distinct fusion machinery consisting of SNAREs, tethering factors and Rab proteins. Here, initial recognition of apposed membranes is likely to occur through interaction between their cognate tethering complexes in trans. Functional Rab proteins are required on both membranes for the heterogeneous tether dimer to be able to coordinate trans-SNARE complex assembly. Since the tethers and Rabs are already dissimilar on each fusion partner as opposed to homotypic fusion, asymmetry in SNARE pairing becomes intrinsic. Eventually the tether dimer organizes a QbQcR-Qa trans-SNARE complex leading to fusion.
Figure 5. Proposed model for trans-SNARE complex establishment in heterotypic fusion. Each fusion partner possesses its own distinct set of fusion factors. Initial recognition between the non-identical fusion partners (vesicle and plasma membrane for example) is likely through occur through interaction between their cognate tethering complexes. This heterogeneous tether dimer catalyzes the formation of a QbQcR-Qa trans-SNARE complex in the presence of functional Rab GTPases on each partner.
Altogether our results suggest that both recognition of the appropriate fusion partner as well as coordination of a preferred trans-SNARE topology by a common Rab GTPase-dependent tether-tether scaffold may be a general mechanism that leads to homotypic and heterotypic fusion events.
Materials and Methods
Vacuole purification
BJ3505 strains were grown in YPD at 30°C at 150 rpm to OD600 = 2 and harvested (3 min, 5,000 g). Vacuoles were isolated as described33 and cell walls were hydrolyzed by lyticase,34 recombinantly expressed in E. coli RSB805 (provided by Dr Randy Schekman, Berkeley), and prepared from a periplasmic supernatant. Harvested cells were resuspended in reduction buffer (30 mM Tris/HCl pH 8.9, 10 mM DTT) and incubated for 5 min at 30°C. After harvesting as described above cells were resuspended in 15 ml digestion buffer (600 mM sorbitol, 50 mM K-phosphate pH 7.5 in YP medium with 0.2% glucose and 0.1 mg/ml lyticase preparation). After 20 min at 30°C, cells were centrifuged (1 min, 5,800 rpm in JLA25.5 rotor). The spheroplasts were resuspended in 2.5 ml 15% Ficoll-400 in PS buffer (10 mM PIPES/KOH pH 6.8, 200 mM sorbitol) and 200 µl DEAE-Dextran (0.4 mg/ml in 15% Ficoll-400 in PS). After 90 sec of incubation at 30°C, the cells were transferred to SW41 tubes and overlaid with steps of 8%, 4% and 0% Ficoll-400 in PS. Cells were centrifuged for 60–75 min at 2°C and 30,000 rpm in an SW41 rotor.
Gel filtration
Four-hundred migrocrams of purified yeast vacuoles were solubilized in buffer containing 1% Triton X-100, 3 mM EDTA, 2 mM DTT and 150 mM or 600 mM KCl. After centrifugation for 4 min at 20,000 g the supernatant was applied on a Superose 6 column and the collected fractions were inspected using SDS-PAGE and western blotting.
Cis-HOPS assay
The yeast strain Vps41-HA containing a Vps41-TAP plasmid was treated with 1mM PMSF for the last one hour of its growth period prior to harvesting the cells. One millimolar PMSF was included in the digestion buffer and in 15% Ficoll-400 in PS buffer during vacuole preparation. Five-hundred micrograms of vacuoles purified from this strain were incubated in buffer containing 120 mM KCl, 500 μM MnCl2 for 15 min at 27°C. The vacuoles were then centrifuged for 2 min at 20,000 g and subsequently solubilized in buffer containing 1% Triton X-100 and 120 mM KCl. Vps41-TAP was precipitated using IgG/Sepharose beads (GE Healthcare) and co-precipitating proteins were analyzed employing SDS-PAGE and western blotting.
Crosslinking assay
Five hundred micrograms of yeast vacuoles were purified from Vps11-HA, Vps16-HA, Vps18-TAP, Vps33-HA, Vps39-HA and Vps41-HA strains. Vacuoles from each strain were incubated in buffer containing 120 mM KCl and 500 μM MnCl2 for 5 min at 27°C. H2O2 was then added to a final concentration of 0.03% and the vacuoles were further incubated for 15 min at 27°C. After centrifugation for 2 min at 20000 g at 4°C, the supernatant was discarded and the pellet was analyzed on non-reducing SDS-PAGE followed by western blotting.
Trans-SNARE assay
Five-hundred micrograms of yeast vacuoles purified from Vam3-HA and Nyv1-VSV strains were mixed and incubated in PS buffer with 120 mM KCl, 500 μM MnCl2 and 1 mM DTT for 5 min at 27°C in the absence of ATP. The fusion reaction was started by adding ATP-regenerating system (0.25 mg/ml creatine kinase, 20 mM creatine phosphate, 500 mM ATP, 500 mM MgCl2). After 5 min at 27°C, the vacuoles were cooled down to 7°C and incubated further for 30 min at this temperature. Thereafter, 3 mM EDTA was added and vacuoles were centrifuged for 2 min at 4°C at 20,000 g and subsequently solubilized in buffer containing 0.5% Triton, 50 mM KCl, 3 mM EDTA, 3 mM DTT in PS. Vam3-HA was precipitated using Protein G/anti-HA antibodies (Covance, mouse monoclonal) and co-precipitating proteins were analyzed employing SDS-PAGE and western blotting.
HOPS precipitations
Five-hundred micrograms of yeast vacuoles purified from a ΔN-terminal Vam3 strain harboring Vps16-HA were mixed with 500 μg of vacuoles harboring Nyv1-VSV or Ypt7-T22N or with wild type and incubated in PS buffer containing 120 mM KCl, 500 μM MnCl2 and ATP-regenerating system. After 15 min at 27°C, vacuoles were centrifuged for 2 min at 20,000 g and subsequently solubilized in buffer containing 1% Triton X-100, 75 mM KCl, 3 mM EDTA and 2 mM DTT. Vps16-HA was precipitated using Protein G/anti-HA antibodies (Covance, mouse monoclonal) and co-precipitating proteins were analyzed employing SDS-PAGE and western blotting.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grant GM087333.
Glossary
Abbreviations:
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
- HOPS
homotypic fusion and vacuole protein sorting
- NSF
N-ethylmaleimide sensitive factor
- GDI
GDP dissociation inhibitor
- SM
Sec1/Munc18
- COPII
coat protein complex II
- COG
conserved oligomeric Golgi
- EEA1
early endosomal antigen 1
- TRAPPII
transport protein particle II
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/20359
References
- 1.Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005;38:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 3.Alpadi K, Kulkarni A, Comte V, Reinhardt M, Schmidt A, Namjoshi S, et al. Sequential analysis of trans-SNARE formation in intracellular membrane fusion. PLoS Biol. 2012;10:e1001243. doi: 10.1371/journal.pbio.1001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–82. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Ungermann C, von Mollard GF, Jensen ON, Margolis N, Stevens TH, Wickner W. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol. 1999;145:1435–42. doi: 10.1083/jcb.145.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seals DF, Eitzen G, Margolis N, Wickner WT, Price AA. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci U S A. 2000;97:9402–7. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–36. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 8.Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol. 2009;21:543–51. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostrowicz CW, Bröcker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, et al. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11:1334–46. doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 10.Krämer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol Biol Cell. 2011;22:2601–11. doi: 10.1091/mbc.E11-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–89. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan S, Hartnell LM, Aguilar RC, Naslavsky N, Bonifacino JS. Human Vam6p promotes lysosome clustering and fusion in vivo. J Cell Biol. 2001;154:109–22. doi: 10.1083/jcb.200102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darsow T, Katzmann DJ, Cowles CR, Emr SD. Vps41p function in the alkaline phosphatase pathway requires homo-oligomerization and interaction with AP-3 through two distinct domains. Mol Biol Cell. 2001;12:37–51. doi: 10.1091/mbc.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–70. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J Biol Chem. 1999;274:15440–6. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- 16.Wendler F, Tooze S. Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic. 2001;2:606–11. doi: 10.1034/j.1600-0854.2001.20903.x. [DOI] [PubMed] [Google Scholar]
- 17.Bethani I, Lang T, Geumann U, Sieber JJ, Jahn R, Rizzoli SO. The specificity of SNARE pairing in biological membranes is mediated by both proof-reading and spatial segregation. EMBO J. 2007;26:3981–92. doi: 10.1038/sj.emboj.7601820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weninger K, Bowen ME, Chu S, Brunger AT. Single-molecule studies of SNARE complex assembly reveal parallel and antiparallel configurations. Proc Natl Acad Sci U S A. 2003;100:14800–5. doi: 10.1073/pnas.2036428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–8. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 21.Cao X, Barlowe C. Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J Cell Biol. 2000;149:55–66. doi: 10.1083/jcb.149.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21:2297–305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo SY, Brett CL, Plemel RL, Vignali M, Fields S, Gonen T, et al. Intrinsic tethering activity of endosomal Rab proteins. Nat Struct Mol Biol. 2012;19:40–7. doi: 10.1038/nsmb.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–72. doi: 10.1016/S0092-8674(00)81404-X. [DOI] [PubMed] [Google Scholar]
- 25.Parlati F, McNew JA, Fukuda R, Miller R, Söllner TH, Rothman JE. Topological restriction of SNARE-dependent membrane fusion. Nature. 2000;407:194–8. doi: 10.1038/35025076. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Jun Y, Thompson J, Yates J, Wickner W. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29:1948–60. doi: 10.1038/emboj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, et al. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic. 2010;11:1552–66. doi: 10.1111/j.1600-0854.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 28.Callaghan J, Simonsen A, Gaullier JM, Toh BH, Stenmark H. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J. 1999;338:539–43. doi: 10.1042/0264-6021:3380539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip CK, Berscheminski J, Walz T. Molecular architecture of the TRAPPII complex and implications for vesicle tethering. Nat Struct Mol Biol. 2010;17:1298–304. doi: 10.1038/nsmb.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, et al. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell 2001; 7:433-442; PMID:11239471; 0.1016/S1097-2765(01)00190-3. [DOI] [PubMed]
- 31.Plemel RL, Lobingier BT, Brett CL, Angers CG, Nickerson DP, Paulsel A, et al. Subunit organization and Rab interactions of Vps-C protein complexes that control endolysosomal membrane traffic. Mol Biol Cell. 2011;22:1353–63. doi: 10.1091/mbc.E10-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bröcker C, Kuhlee A, Gatsogiannis C, Balderhaar HJ, Hönscher C, Engelbrecht-Vandré S, et al. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci U S A. 2012;109:1991–6. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieren M, Schmidt A, Mayer A. The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening. Nat Struct Mol Biol. 2010;17:710–7. doi: 10.1038/nsmb.1809. [DOI] [PubMed] [Google Scholar]
- 34.Scott JH, Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980;142:414–23. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]