Abstract
Kinesin-5 mechanoenzymes drive mitotic spindle dynamics as slow, processive microtubule (MT)-plus-end directed motors. Surprisingly, the Saccharomyces cerevisiae kinesin-5 Cin8 was recently found to be bi-directional: it can move processively in both directions on MTs. Two hypotheses have been suggested for the mechanism of the directionality switch: (1) single molecules of Cin8 are intrinsically minus-end directed, but mechanical coupling between two or more motors triggers the switch; (2) a single motor can switch direction, and “cargo binding” i.e., binding between two MTs triggers the switch to plus-end motility. Single-molecule fluorescence data we published recently, and augment here, favor hypothesis (2). In low-ionic-strength conditions, single molecules of Cin8 move in both minus- and plus-end directions. Fluorescence photo bleaching data rule out aggregation of Cin8 while they move in the plus and in the minus direction. The evidence thus points toward cargo regulation of directionality, which is likely to be related to cargo regulation in other kinesins. The molecular mechanisms of this regulation, however, remain to be elucidated.
Keywords: Saccharomyces cerevisiae Cin8, kinesin directionality, kinesin-5, microtubules, mitosis
Members of the kinesin-5 family of motor proteins are conserved among eukaryotes, from yeast to humans. Among the cytoskeletal motors, kinesins, myosins and dyneins, kinesin-5 motors are the only ones that function as bipolar homotetramers, with two pairs of catalytic domains located at opposite ends of the active complex.1,2 This special architecture is thought to enable kinesin-5 motors to crosslink and slide apart antiparallel MTs emanating from the opposite poles of the mitotic spindle.3 By this mode of action, kinesin-5 motors are believed to fulfill their essential roles in spindle dynamics such as spindle assembly, maintenance of the bipolar spindle structure prior to the onset of anaphase,3-5 as well as anaphase B spindle elongation.6-11 Since MTs are organized with their plus ends overlapping in the midzone, kinesin-5 can only push spindle poles apart during spindle assembly and elongation via plus-end directed motility between antiparallel MTs. It has indeed been demonstrated in vitro, that the vertebrate kinesin-5 Eg5 moves simultaneously toward the plus ends of two antiparallel MTs that it crosslinks.12,13 This finding was consistent with the 20-year-old dogma that kinesin homologs which carry their catalytic domains at the N-terminus are plus-end directed.14
The majority of the members of the kinesin superfamily are plus-end directed. Minus-end motion was seen only for the structurally distinct kinesin-14 family members which carry the catalytic domain at their C-terminus.15-17 Being non-processive, these motors produce isolated power strokes and can only produce persistent motion in ensembles. Surprisingly, the S. cerevisiae kinesin-5 Cin8 was recently found to move processively in the minus-end direction of MTs in single-molecule fluorescence motility assays under close-to-physiological conditions.18,19 Cin8 was shown to switch directionality to plus-end directed motility in several experimental circumstances: in multi-motor MT gliding assays,18,20 under low-ionic-strength conditions, and when bound between two antiparallel MTs.18,19 Two possible mechanisms for this switch have been suggested: one is that single molecules of Cin8 can move only toward the minus end of MTs and that coupling between two or more motors triggers the plus-end directed switch;18 the second possibility is that the ability to switch directionality is contained within a single motor itself and that interaction between Cin8 and MTs can trigger the switch.19 Several lines of evidence support the second mechanism.
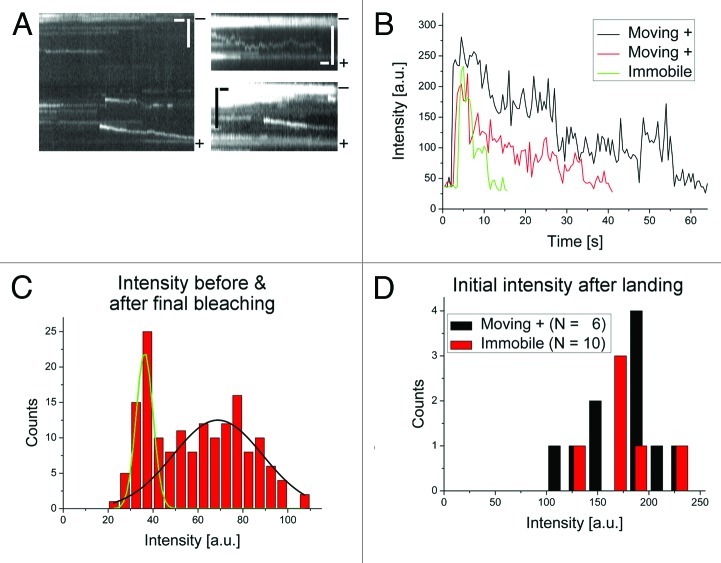
The central evidence for a motor-intrinsic switching mechanism is that individual Cin8 molecules can switch to plus-end directed motility under low-ionic-strength conditions.19 In our in vitro single-molecule fluorescence experiments,19 low total ionic strength below ~0.13 M (an unphysiological environment) induced plus-end-directed and high ionic strength promoted minus-end-directed motion of Cin8. In view of the controversy about the mechanism of the directionality switch, it is crucial to prove that, indeed, individual Cin8 molecules move toward the plus ends of MTs and that observed fluorescent traces do not originate from small clusters of motors acting collectively. To address this point, we followed the photo-bleaching of fluorescent Cin8-GFP tetramers, purified from S. cerevisiae cells, while they moved on polarity-marked MTs under low-ionic-strength conditions. Experiments are described in detail in Gerson-Gurwitz et al.19 and additional data is presented here (Fig. 1). Two buffer conditions were examined: motility buffer (MB) with 30 mM NaCl added (ionic strength 0.132 M) and MB with no added NaCl (ionic strength 0.102 M). We have previously shown that under these conditions, Cin8 moves toward the plus end of MTs for ~60% and ~70% of the time, respectively.19 To count the number of GFP fluorophores on each moving motor or (possibly) motor aggregate, we measured the intensity of several single spots in a given video recording, both for spots appearing during the recording (i.e., landing from the bulk on the MT) and then moving to the plus-end of the MT, and for spots appearing and remaining stationary on the MT during the recording. To obtain a scale for the intensity, i.e., to determine the intensity of a single GFP, intensities before and after the final bleaching step of immobile motors were analyzed (Fig. 1C). The measured intensity distribution was fitted with a sum of two Gaussians, resulting in a value for the background and a value for the intensity of a single GFP. We then measured the distribution of the initial fluorescence intensities right after landing for both moving and immobile motors. Intensities in a fixed area were averaged for the first three frames (1.5 sec) right after landing (Fig. 1D), during which time the motors moved much less than the size of the chosen area around the spots. Comparison of initial intensities to monomer intensities confirms that both, the motors moving to the plus end of MTs and the stationary motors were tetramers. Furthermore, some of the bleaching traces of Cin8 molecules that moved toward the plus ends of MTs showed four consecutive bleaching steps (Fig. 1B), indicating again that these molecules were tetramers with four GFP molecules. These results demonstrate that, under low-ionic-strength conditions, individual Cin8 molecules can move in the plus-end direction on MTs. While coupling between multiple motors could be an additional mechanism for switching, our results provide support for the existence of the motor-intrinsic switch model.
Figure 1. In low-ionic-strength conditions, single Cin8 motors move toward the plus end of MTs. (A) Kymographs of Cin8 moving away from the brightly labeled seed marking the minus end (-) toward the plus end (+) of the MT. Kymograph in the right bottom panel was captured in MB-30; the other two in MB-0 (exact buffer compositions are given below). Scale bars: horizontal: 10 sec; vertical: 3 µm. (B) Exemplary intensity traces of two motors landing on a MT and moving to the MT plus end (black and red) and of a motor landing on a MT and remaining immobile (green). The traces of the moving motors correspond to the two events shown in the left panel of (A). (C) Histogram of fluorescence intensities before and after final bleaching steps of immobile motors, summed from a 800 nm × 800 nm square of camera pixels containing the image of the motor (number of traces /motors: 8). A sum of two Gaussians was fitted to the histogram. The first narrower peak corresponds to the background [compare also to (B)]. The broader second peak represents the intensity of a single GFP. (D) Histogram of initial intensities of Cin8 molecules right after landing, measured in the same arbitrary units as in (C). The intensity of the first three frames (1.5 sec) after landing was averaged for both moving and immobile motors. The histogram has a maximum at 4 times the intensity of a single GFP [compare with (C)]. Materials and Methods: Single-molecule fluorescence assays were performed as described in detail in Gerson-Gurwitz et al.19 In short, the custom-built total-internal-reflection-fluorescence (TIRF) microscope consisted of a 473 nm laser (Viasho, USA) for excitation, a 100x objective (SFluor, NA 1.49, oil, Nikon, USA), and a CCD camera (Cascade 512B, Roper Scientific, USA) for detection. To observe several colors simultaneously, the fluorescence emission signal was split by dichroic mirrors and directed to separate areas on the CCD camera. Fluorescently Cin8-TEV-GFP-6HIS was overexpressed in S. cerevisiae and affinity purified using the his-tag and a Ni-NTA affinity column (Invitrogen, USA). The low-salt buffers for the motility assays were composed as follows: MB-0: 50 mM Tris/HCl, 30 mM PIPES/KOH, pH 7.2, 2 mM EDTA, 1 mM EGTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol. MB-30: the same as MB-0 but with 30 mM added NaCl. MTs were polymerized from tissue-purified porcine tubulin, essentially as described before,31 but with Atto-488 (Atto-Tec GmbH, Siegen, Germany) labeled seeds that also fluoresce in the green and in that way mark the minus end of the MTs.19 Kymographs were generated and analyzed with a custom-written LabView (National Instruments, USA) routine. The fluorescence intensity emitted by single proteins was summed over an area of 5 × 5 camera pixels, corresponding to an area of 800 nm × 800 nm in the specimen plane and was analyzed with ImageJ software (NIH, USA), and histograms and fits were done with OriginPro software (OriginLab Corporation, USA).
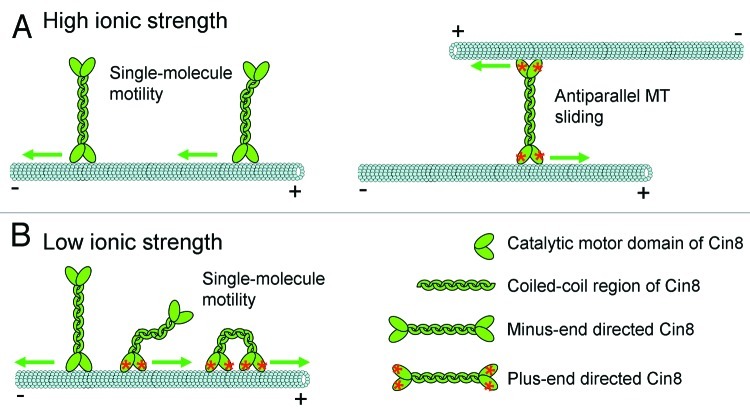
Since ionic strength generally modulates electrostatic interactions, the unphysiologically low-ionic-strength conditions might mimic the effects of phosphorylation or binding of accessory proteins or of binding geometry to the MTs. A qualitatively similar ionic-strength effect has been reported in the context of cargo regulation of other kinesins.13,21,22 Binding of a cargo vesicle to kinesin-1 or of a second MT to kinesin-5 Eg5 activates these motors, but this activation also occurs spontaneously (i.e., without cargo) at low ionic strength in both cases. Tail-head interaction is mediating this regulation in both cases. It is thus tempting to speculate that for Cin8, cargo regulation is also the physiological switch mechanism. In the case of Cin8, a mechanism detecting the binding of a second MT might not just turn the motor on or off, but lead to the observed switching of directionality. Consistent with this hypothesis, we observed plus-end directed antiparallel sliding of MTs by Cin8 when they entered the overlap zone between antiparallel MTs under high-ionic-strength conditions, while motors on single MTs in the same sample were still minus-end directed.19 Similarly, it has been previously demonstrated that, while the vertebrate Eg5 does neither bind to nor move on single MTs under high-ionic-strength conditions, binding between two antiparallel MTs activates MT sliding, driven by plus-end directed Eg5 motility.13 A similar activation effect might also occur in multi-motor MT surface-gliding assays, in which surface-attached Cin8 was also demonstrated to be plus-end directed.20 In MT sliding assays, Cin8 obviously exerts force in the plus-end direction which is reflected in the relative sliding of the MTs, but individual motors between the coupled MTs move on rather erratic tracks such that clear plus-end-directed periods cannot be detected.19 Cin8 behaves very differently in single-molecule fluorescence experiments between parallel MTs. For the most part, motors continue minus-end motion, apparently not interacting with the second MT.19 The capability to distinguish relative orientation of bound MTs is consistent with the reported preference of Drosophila kinesin-5 Klp61f for bundling antiparallel MTs.13 For this kinesin-5, a preferred orientation was due to the ATP-independent binding sites in the C-terminal tails of the molecules. A similar binding mechanism appears to also exist for Cin8 because full-length Cin8 diffusively slides along MTs in ADP buffer.19 For Xenopus laevis kinesin-5 Eg5 it was found that all MT binding sites in the C-terminal tails were necessary for motor engagement between MTs.23 It still remains unclear exactly why and how low ionic strength mimics cargo binding. Taking into account the fact that MT attachment of the two pairs of motor domains triggers plus-end directed motility (Fig. 2A), a speculative possibility is that under low-ionic-strength conditions, Cin8 can flex in such a way that the two pairs of catalytic domains interact with the same MT (Fig. 2B) and thus trigger plus-end directed motility. Alternatively, low-ionic-strength conditions could modify tail–head interactions as in kinesin-1 to trigger plus-end directed motility.
Figure 2. Proposed model for the directionality switch of Cin8. A microtubule is sketched in light blue, with plus and minus end indicated; Cin8 is shown in green; catalytic motor domain and coiled-coil regions are indicated in the legend. Arrows indicate the direction of movement of Cin8 on the MT to which it is attached. (A) On a single MT, in high-ionic-strength conditions, Cin8 is minus-end directed. Binding between two antiparallel MTs activates Cin8 to move in the plus-end directions of the MTs, causing their antiparallel sliding. (B) Under low-ionic-strength conditions on a single MT, a modified interaction between tails and catalytic domains or flexing of the whole tetramer triggers Cin8 to move in the plus-end direction of the MT. If the tetramer can flex enough, plus-end directed motility of single molecules of Cin8 could be triggered by binding of the two heads of Cin8 to the same MT.
One further piece of evidence in favor of a motor-intrinsic mechanism for directional switching is the regulatory influence of the 99 amino acid insert in loop 8 of the Cin8 motor domain, deletion of which does not abolish the switch of directionality, but shifts the switch to lower ionic strength.19 The mechanism by which phosphorylation in the catalytic domain of Cin8 regulates its in vivo function is likely to include the modulation of interactions with the midzone-organizing protein Ase1,8,24, or with kinetochore proteins. The fact that a deletion construct (Cin8Δ99) and a loop 8 Cdk1 phosphorylation-deficient construct (Cin8-2A) exhibit reduced motility toward the midzone in vivo,19 suggests that one of the roles of Cin8 phosphorylation in the 99aa insert is to mediate the switch to plus-end directed motility of Cin8 on the mitotic spindle.
The question remains how its exceptional motile properties aid Cin8 in performing its roles in mitosis. One can speculate on the basis of the localization of Cin8 in the various stages of mitosis. The ionic strength in S. cerevisiae cells is ~300 mM salt.25,26 At this ionic strength, Cin8 is minus-end directed in vitro.19 Before spindle elongation in anaphase, Cin8 is known to be involved in the positioning of the chromosome kinetochores near the spindle pole bodies.27-29 Cin8 could function at that stage by crosslinking of kinetochore MTs (kMTs)27 and by aiding the disassembly of long kMTs.30 Since in S. cerevisiae cells each kinetochore is attached to the plus end of a single MT, motion of Cin8 in the minus-end direction of the kMTs might be a part of kinetochore positioning. Cin8 also shows plus-end directed motility in vivo in anaphase spindles,19 even on single MTs or on parallel MT bundles, which implies a further mode of regulation not seen in the in vitro experiments. Regulated bi-directional motility might be important to distribute Cin8 motors between the different locations where they are known to accumulate, i.e., near the spindle poles and in the midzone.
In summary, Cin8 has rather unexpectedly extended the spectrum of known kinesin capabilities. It is the first known kinesin that is truly bi-directional and processive in both directions. Found in a low eukaryote, this function might have evolved early and might have been lost in higher eukaryotes. The exact molecular mechanism remains to be clarified, but seems likely to be related to cargo switching known for other kinesins. Our results indicate a role of electrostatic interactions and possibly phosphorylation, and, most importantly, binding geometry between pairs of MTs.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/20395
References
- 1.Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996;379:270–2. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon DM, Roof DM. The kinesin-related protein Kip1p of Saccharomyces cerevisiae is bipolar. J Biol Chem. 1999;274:28779–86. doi: 10.1074/jbc.274.40.28779. [DOI] [PubMed] [Google Scholar]
- 3.Saunders WS, Hoyt MA. Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell. 1992;70:451–8. doi: 10.1016/0092-8674(92)90169-D. [DOI] [PubMed] [Google Scholar]
- 4.Blangy A, Lane HA, d’Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–69. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 5.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–4. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 6.Saunders WS, Koshland D, Eshel D, Gibbons IR, Hoyt MA. Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J Cell Biol. 1995;128:617–24. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp DJ, McDonald KL, Brown HM, Matthies HJ, Walczak C, Vale RD, et al. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J Cell Biol. 1999;144:125–38. doi: 10.1083/jcb.144.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avunie-Masala R, Movshovich N, Nissenkorn Y, Gerson-Gurwitz A, Fridman V, Kõivomägi M, et al. Phospho-regulation of kinesin-5 during anaphase spindle elongation. J Cell Sci. 2011;124:873–8. doi: 10.1242/jcs.077396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridman V, Gerson-Gurwitz A, Movshovich N, Kupiec M, Gheber L. Midzone organization restricts interpolar microtubule plus-end dynamics during spindle elongation. EMBO Rep. 2009;10:387–93. doi: 10.1038/embor.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerson-Gurwitz A, Movshovich N, Avunie R, Fridman V, Moyal K, Katz B, et al. Mid-anaphase arrest in S. cerevisiae cells eliminated for the function of Cin8 and dynein. Cell Mol Life Sci. 2009;66:301–13. doi: 10.1007/s00018-008-8479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Movshovich N, Fridman V, Gerson-Gurwitz A, Shumacher I, Gertsberg I, Fich A, et al. Slk19-dependent mid-anaphase pause in kinesin-5-mutated cells. J Cell Sci. 2008;121:2529–39. doi: 10.1242/jcs.022996. [DOI] [PubMed] [Google Scholar]
- 12.Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–8. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 13.Kapitein LC, Kwok BH, Weinger JS, Schmidt CF, Kapoor TM, Peterman EJG. Microtubule cross-linking triggers the directional motility of kinesin-5. J Cell Biol. 2008;182:421–8. doi: 10.1083/jcb.200801145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagenbach EM, Endow SA. A new kinesin tree. J Cell Sci. 2004;117:3–7. doi: 10.1242/jcs.00875. [DOI] [PubMed] [Google Scholar]
- 15.deCastro MJ, Fondecave RM, Clarke LA, Schmidt CF, Stewart RJ. Working strokes by single molecules of the kinesin-related microtubule motor ncd. Nat Cell Biol. 2000;2:724–9. doi: 10.1038/35036357. [DOI] [PubMed] [Google Scholar]
- 16.McDonald HB, Stewart RJ, Goldstein LS. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell. 1990;63:1159–65. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- 17.Walker RA, Salmon ED, Endow SA. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature. 1990;347:780–2. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- 18.Roostalu J, Hentrich C, Bieling P, Telley IA, Schiebel E, Surrey T. Directional switching of the kinesin Cin8 through motor coupling. Science. 2011;332:94–9. doi: 10.1126/science.1199945. [DOI] [PubMed] [Google Scholar]
- 19.Gerson-Gurwitz A, Thiede C, Movshovich N, Fridman V, Podolskaya M, Danieli T, et al. Directionality of individual kinesin-5 Cin8 motors is modulated by loop 8, ionic strength and microtubule geometry. EMBO J. 2011;30:4942–54. doi: 10.1038/emboj.2011.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheber L, Kuo SC, Hoyt MA. Motile properties of the kinesin-related Cin8p spindle motor extracted from Saccharomyces cerevisiae cells. J Biol Chem. 1999;274:9564–72. doi: 10.1074/jbc.274.14.9564. [DOI] [PubMed] [Google Scholar]
- 21.Seiler S, Kirchner J, Horn C, Kallipolitou A, Woehlke G, Schliwa M. Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat Cell Biol. 2000;2:333–8. doi: 10.1038/35014022. [DOI] [PubMed] [Google Scholar]
- 22.Hackney DD, Baek N, Snyder AC. Half-site inhibition of dimeric kinesin head domains by monomeric tail domains. Biochemistry. 2009;48:3448–56. doi: 10.1021/bi8022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinger JS, Qiu M, Yang G, Kapoor TM. A nonmotor microtubule binding site in kinesin-5 is required for filament crosslinking and sliding. Curr Biol. 2011;21:154–60. doi: 10.1016/j.cub.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khmelinskii A, Roostalu J, Roque H, Antony C, Schiebel E. Phosphorylation-dependent protein interactions at the spindle midzone mediate cell cycle regulation of spindle elongation. Dev Cell. 2009;17:244–56. doi: 10.1016/j.devcel.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Olz R, Larsson K, Adler L, Gustafsson LO. Energy flux and osmoregulation of Saccharomyces cerevisiae grown in chemostats under NaCl stress. J Bacteriol. 1993;175:2205–13. doi: 10.1128/jb.175.8.2205-2213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Eunen K, Bouwman J, Daran-Lapujade P, Postmus J, Canelas AB, Mensonides FI, et al. Measuring enzyme activities under standardized in vivo-like conditions for systems biology. FEBS J. 2010;277:749–60. doi: 10.1111/j.1742-4658.2009.07524.x. [DOI] [PubMed] [Google Scholar]
- 27.Tytell JD, Sorger PK. Analysis of kinesin motor function at budding yeast kinetochores. J Cell Biol. 2006;172:861–74. doi: 10.1083/jcb.200509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner MK, Bouck DC, Paliulis LV, Meehl JB, O’Toole ET, Haase J, et al. Chromosome congression by Kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell. 2008;135:894–906. doi: 10.1016/j.cell.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wargacki MM, Tay JC, Muller EG, Asbury CL, Davis TN. Kip3, the yeast kinesin-8, is required for clustering of kinetochores at metaphase. Cell Cycle. 2010;9:2581–8. doi: 10.4161/cc.9.13.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner MK, Haase J, Mythreye K, Molk JN, Anderson M, Joglekar AP, et al. The microtubule-based motor Kar3 and plus end-binding protein Bim1 provide structural support for the anaphase spindle. J Cell Biol. 2008;180:91–100. doi: 10.1083/jcb.200710164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakämper S, Thiede C, Düselder A, Reiter S, Korneev MJ, Kapitein LC, et al. The effect of monastrol on the processive motility of a dimeric kinesin-5 head/kinesin-1 stalk chimera. J Mol Biol. 2010;399:1–8. doi: 10.1016/j.jmb.2010.03.009. [DOI] [PubMed] [Google Scholar]