Abstract
The newly developed multireceptor somatostatin analogs pasireotide (SOM230), octreotide and somatoprim (DG3173) have primarily been characterized according to their binding profiles. However, their ability to activate individual somatostatin receptor subtypes (sst) has not been directly assessed so far. Here, we transplanted the carboxyl-terminal phosphorylation motif of the sst2 receptor to other somatostatin receptors and assessed receptor activation using a set of three phosphosite-specific antibodies. Our comparative analysis revealed unexpected efficacy profiles for pasireotide, octreotide and somatoprim. Pasireotide was able to activate sst3 and sst5 receptors but was only a partial agonist at the sst2 receptor. Octreotide exhibited potent agonistic properties at the sst2 receptor but produced very little sst5 receptor activation. Like octreotide, somatoprim was a full agonist at the sst2 receptor. Unlike octreotide, somatoprim was also a potent agonist at the sst5 receptor. Together, we propose the application of a phosphorylation probe for direct assessment of G protein-coupled receptor activation and demonstrate its utility in the pharmacological characterization of novel somatostatin analogs.
Introduction
The development of novel multireceptor somatostatin analogs has primarily focused on the discovery of compounds with nanomolar binding affinities to more than one of the five somatostatin receptors (sst1–sst5). It is not clear, however, whether these compounds exhibit full or partial agonistic properties at individual somatostatin receptor subtypes. This lack of knowledge is due to the limited availability of methods allowing a direct assessment of G protein-coupled receptor (GPCR) activation.
In clinical practice, octreotide and lanreotide are used as first choice medical treatment of neuroendocrine tumors such as GH-secreting adenomas and carcinoids [1], [2]. Octreotide and lanreotide bind with high sub-nanomolar affinity to sst2 only, have moderate affinity to sst3 and sst5 and show very low or absent binding to sst1 and sst4. Recently, the novel multireceptor somatostatin analog, pasireotide (SOM230), has been synthesized [3]. Pasireotide is a cyclohexapeptide, which binds with high affinity to all somatostatin receptors except to sst4 [4]. In contrast to octreotide, pasireotide exhibits particular high sub-nanomolar affinity to sst5 [5]. Pasireotide is currently under clinical evaluation for treatment of acromegaly, Cushing’s disease and octreotide-resistant carcinoid tumors [6], [7], [8]. In addition to pasireotide, the novel pan-somatostatin analog somatoprim (DG3173) is currently under clinical and preclinical evaluation. Somatoprim exhibits a unique binding profile in that binds with high affinity to sst2, sst4 and sst5 but not to sst1 or sst3.
We have recently uncovered agonist-selective and species-specific patterns of sst2A receptor phosphorylation and trafficking [9]. Whereas octreotide, in a manner similar to that observed with somatostatin, stimulates the phosphorylation of a number of carboxyl-terminal phosphate acceptor sites in both rat and human sst2 receptors, pasireotide fails to promote any detectable phosphorylation or internalization of the rat sst2A receptor. In contrast, pasireotide is able to trigger a partial internalization of the human sst2 receptor. At present it is unclear whether the agonist-selective regulation of the sst2 receptor observed for pasireotide is a general property of all pan-somatostatin analogs, and whether such functional selectivity may exist for other clinically-relevant somatostatin receptors including sst5 and sst3.
In the present study, we addressed this problem by using the carboxyl-terminal tail of the sst2 receptor as transplantable phosphorylation probe to directly sense the activation of other somatostatin receptors. This approach was possible due to our recent success in generating a set of three phosphosite-specific antibodies for the sst2 receptor which allowed us to determine distinct patterns of phosphorylation induced by different agonists. Our assay utilizes the unique ability of G protein-coupled receptor kinases (GRKs) to detect only active conformations of GPCRs. Different phosphorylation patterns may hence reflect distinct receptor conformations.
Materials and Methods
Reagents and Antibodies
Pasireotide and octreotide were provided by Dr. Herbert Schmid (Novartis, Basel, Switzerland). Somatoprim was provided by Dr. Ursula Hoffmann (DeveloGen, Göttingen, Germany). Somatostatin (SS-14) was obtained from Bachem (Weil am Rhein, Germany). The phosphorylation-independent rabbit monoclonal anti-sst2 {UMB-1}, anti-sst3 {UMB-5} or anti-sst5 {UMB-4} antibodies were obtained from Epitomics (Burlingame, CA). The rabbit polyclonal phosphosite-specific sst2 antibodies anti-pT353/pT354 {0521}, anti-pT356/pT359 {0522}, and anti-pS341/pS343 {3155} were generated and extensively characterized previously [9], [10].
Generation of Mutant Somatostatin Receptors
A chimera of the human sst5 receptor with the carboxyl-terminal tail of the human sst2 receptor (hsst5-sst2CT) was generated by DNA synthesis by imaGenes (Berlin, Germany). A chimera of the rat sst3 receptor with the carboxyl-terminal tail of the rat sst2 receptor (rsst3-sst2ACT) was generated by exchange of the entire carboxyl-terminal tail using the FLS Motive present in the DNA sequence of both receptors in the seventh transmembrane domain. The fragments were cloned into pcDNA3.1(+) using HindIII and XbaI cloning sites.
Cell Culture and Transfection
Human embryonic kidney HEK293 cells were obtained from the German Resource Centre for Biological Material (DSMZ, Braunschweig, Germany). HEK293 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum in a humidified atmosphere containing 10% CO2. Cells were transfected with plasmids encoding for wild-type or mutant somatostatin receptors using jetPEI™ according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA). Stable transfectants were selected in the presence of 400 µg/ml G418. HEK293 cells stably expressing somatostatin receptors were characterized using radioligand-binding assays, Western blot analysis, and immunocytochemistry as described previously. The level of somatostatin receptor expression was ∼900 fmol/mg membrane protein. All chimeras and mutants tested were present at the cell surface and expressed similar amounts of receptor protein. The IC50 values of SS-14, octreotide and pasireotide for ligand binding affinities are given in Table S1.
Immunocytochemistry
Cells were grown on poly-L-lysine-coated coverslips overnight. After the appropriate treatment with SS-14, octreotide, pasireotide or somatoprim, cells were fixed with 4% paraformaldehyde and 0.2% picric acid in phosphate buffer (pH 6.9) for 30 min at room temperature and washed several times. Specimens were permeabilized and then incubated with anti-sst2 {UMB-1}, anti-sst3 {UMB-5} or anti-sst5 {UMB-4} antibodies followed by Alexa488-conjugated secondary antibodies (Amersham, Braunschweig, Germany). Specimens were mounted and examined using a Zeiss LSM510 META laser scanning confocal microscope [11].
Western Blot Analysis
Stably transfected HEK293 cells were plated onto poly-L-lysine-coated 60-mm dishes and grown to 80% confluence. After the appropriate treatment with SS-14, octreotide, pasireotide or somatoprim, cells were lysed in detergent buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 10 mM disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.2 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 1 µg/ml pepstatin A, 1 µg/ml aprotinin, and 10 µg/ml bacitracin). Glycosylated proteins were partially enriched using wheat germ lectin-agarose beads as described [12], [13], [14]. Proteins were eluted from the beads using SDS-sample buffer for 20 min at 60°C and then resolved on 10% SDS-polyacrylamide gels. After electroblotting, membranes were incubated with the phosphosite-specific antibodies anti-pS341/pS343 {3157}, anti-pT353/pT354 {0521} or anti-pT356/pT359 {0522} at a concentration of 0.1 µg/ml followed by detection using enhanced chemiluminescence (Amersham). Blots were subsequently stripped and reprobed with anti-sst2 {UMB-1} to confirm equal loading of the gels.
Radioligand Binding Assay
Competition binding assays were performed on membrane preparations from stable transfected cells as described above. Cells were harvested into PBS and stored at –80°C. After thawing, cells were centrifuged at 20,000×g for 10 min at 4°C and then homogenized in lysis buffer (50 mM Tris-HCl, 3 mM EGTA, 5 mM EDTA, pH 7.4). Cell membranes were pelleted by centrifugation at 50,000×g for 15 min at 4°C, washed twice with washing buffer (50 mM Tris-HCl, ph 7.4), and resuspended in binding buffer (10 mM HEPES, 5 mM MgCl2, 5 µg/ml bacitracin, pH 7.5). For competition binding assay, aliquots of the membrane preparations containing 30 µg of protein were incubated with 0.05 nM [125J-Tyr11]-SS-14 (specific activity: 74 TBq/mmol, PerkinElmer, USA) in the present or absence of either SS-14, octreotide, pasireotide or somatoprim in concentrations ranging from 10−12 to 10−6 M. The experiment for each concentration was performed in triplicate. Assays were performed in 96-well polypropylene plates in a final volume of 200 µl for 45 min at room temperature. Specific binding was calculated by subtracting non-specific binding – defined as that seen in the presence of 1 µM SS-14, octreotide, pasireotide or somatoprim – from total binding obtained with radioligand alone. The incubation was terminated by addition of ice-cold buffer and rapid vacuum filtration through glass fiber filters presoaked in 0.3% polyethyleneimine using an Inotech cell harvester (Dittikon, Switzerland). Filters were rinsed twice with washing buffer and air-dried. Bound radioactivity was determined using a γ-counter (COBRAII, Packard, USA). Data from ligand binding and IC50 were analyzed by curve fitting using GraphPad Prism 4.0 software [15].
GTPγS Binding Assays
Cells were harvested and lysed as described above except that a lysis buffer containing 50 mM Tris, 10 mM EDTA and 1 mM EGTA (pH 7.4) was used. The resulting pellet was resuspended in assay buffer (20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, pH 7.4). Aliquots containing 30 µg of protein were incubated with 3 µM GDP and 0.05 nM [35S]GTPγS (Specific activity –43.3 TBq/mmol, PerkinElmer USA) in the presence or absence of either SS-14, octreotide, pasireotide or somatoprim in concentrations ranging from 10−12 to 10−6 M. Assays were carried out in a final volume of 1 ml for 30 min at 30°C under continuous agitation. Nonspecific binding was determined in the presence of 10 µM unlabeled GTPγS. The incubation was terminated by the addtion of ice-cold buffer and rapid vacuum filtration through glass fiber filters as described above. Filters were rinsed twice with washing buffer (50 mM Tris-HCl, pH 7.4) and dried. A scintillation mixture was added, and radioactivity was determined unsing a β-counter (1600 TR, Packard, USA) [15].
Results
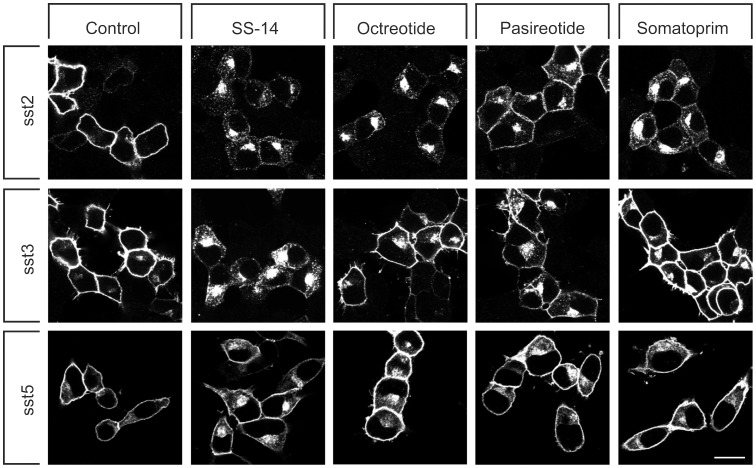
We have recently shown that pasireotide exhibits partial agonistic properties at the sst2 receptor. While binding with high affinity it triggers only a partial internalization of the human sst2 receptor. To test the possibility that this behavior would be a general property of all multireceptor somatostatin analogs, we evaluated the internalization profile of somatoprim in comparison to pasireotide and octreotide. First, we examined human sst2, sst3 and sst5 receptors expressed in HEK293 cells by confocal microscopy revealing that in the absence of agonist all three somatostatin receptor subtypes were almost exclusively confined to the plasma membrane (Figure 1, left panel ). As shown in Figure 1 (upper panel), octreotide and somatoprim were able to stimulate a robust endocytosis of human sst2 receptors similar to that seen after SS-14 exposure. In contrast, a saturating concentration of pasireotide induced only a very limited internalization of sst2 receptors. Conversely, examination of sst3-expressing cells revealed that pasireotide promoted a more pronounced receptor sequestration than octreotide, whereas somatoprim failed to stimulate any detectable sst3 internalization (Figure 1, middle panel). When sst5-expressing cells were exposed to SS-14, octreotide, pasireotide or somatoprim only the endogenous ligand SS-14 was able to stimulate a clearly detectable receptor endocytosis (Figure 1, lower panel).
Figure 1. Agonist-selective internalization of human somatostatin receptors.
HEK293 cells stably expressing sst2, sst3 or sst5 receptors were treated with either 1 µM SS-14, octreotide, pasireotide or somatoprim for 0 or 30 min. Cells were fixed, immunoflurescently stained with anti-sst2 {UMB-1}, anti-sst3 {UMB-5} or anti-sst5 {UMB-4} antibodies, and examined by confocal microscopy. Shown are representative images from one of three independent experiments performed in duplicate. Scale bar, 20 µm.
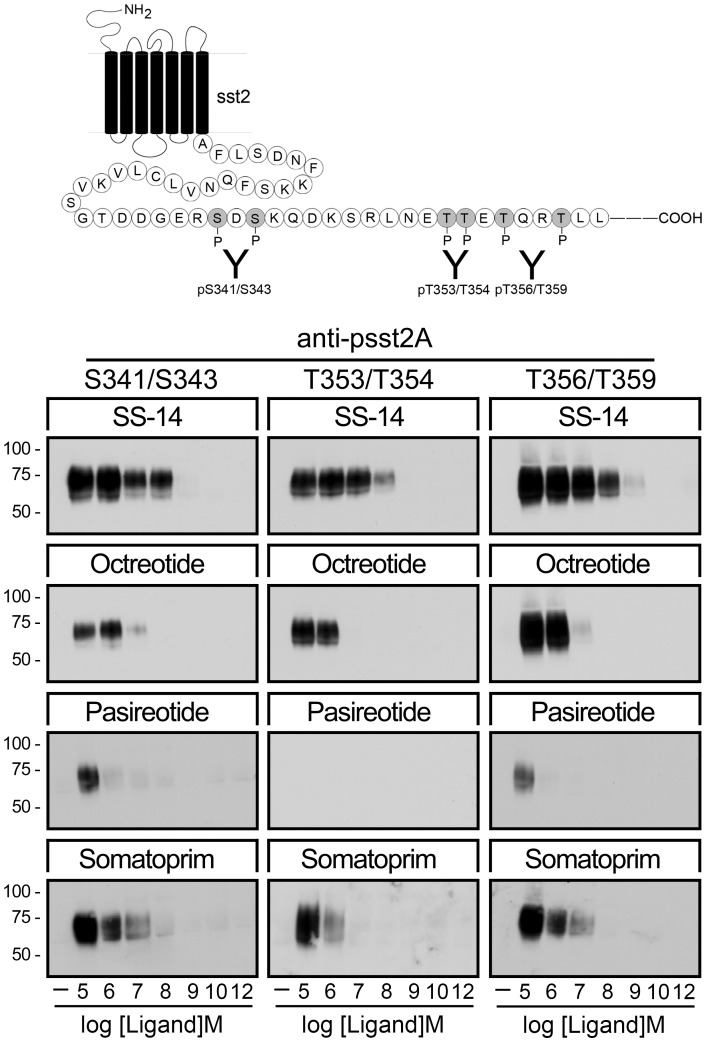
Recently, we have generated a set of three phosphosite-specific antibodies, which allowed us to detect selectively the S341/S343-, the T353/T354- and the T356/T359-phosphorylated forms of the sst2 receptor [16], . When HEK293 cells stably expressing the human sst2 receptor were exposed for 5 min to SS-14, octreotide, pasireotide or somatoprim in concentrations ranging from 10−12 to 10−5 M, SS-14, octreotide and somatoprim were able to promote a robust dose-dependent phosphorylation of all three sites (Figure 2). In contrast, pasireotide stimulated only at saturating concentration a detectable phosphorylation of S341/S343 and T356/T359 but not of T353/T354. Considering the high binding affinity of pasireotide this result was unexpected and indicates that, in contrast to octreotide and somatoprim, pasireotide is a partial agonist at the human sst2 receptor. It also shows that the sst2 receptor can exist in distinct active conformations, which favor different patterns of GRK-mediated phosphorylation. Thus, considerable differences may exist between the binding and efficacy profiles of pan-somatostatin analogs. It would therefore be desirable to know the patterns of phosphorylation induced by multireceptor ligands at the level of individual somatostatin receptors. However, at present phosphosite-specific antibodies are only available for the sst2 receptor.
Figure 2. Agonist-selective phosphorylation of the human sst2 receptor.
(Top) Schematic representation of the human sst2 receptor indicating the phosphate acceptor sites S341/343, T353/354 and T356/359 within its carboxyl-terminal tail. (Bottom) HEK293 cells stably expressing the sst2 receptor were either not exposed or exposed for 5 min to SS-14, octreotide, pasireotide or somatoprim in concentrations ranging from 10−12 to 10−5 M. The levels of phosphorylated sst2 receptors were then determined using the phosphosite-specific antibodies anti-pS341/pS343 {3157}, anti-pT353/pT354 {0521} and anti-pT356/pT359 {0522}. Western blots shown are representative of three to five independent experiments for each condition. The positions of the molecular mass markers are indicated on the left (in kDa).
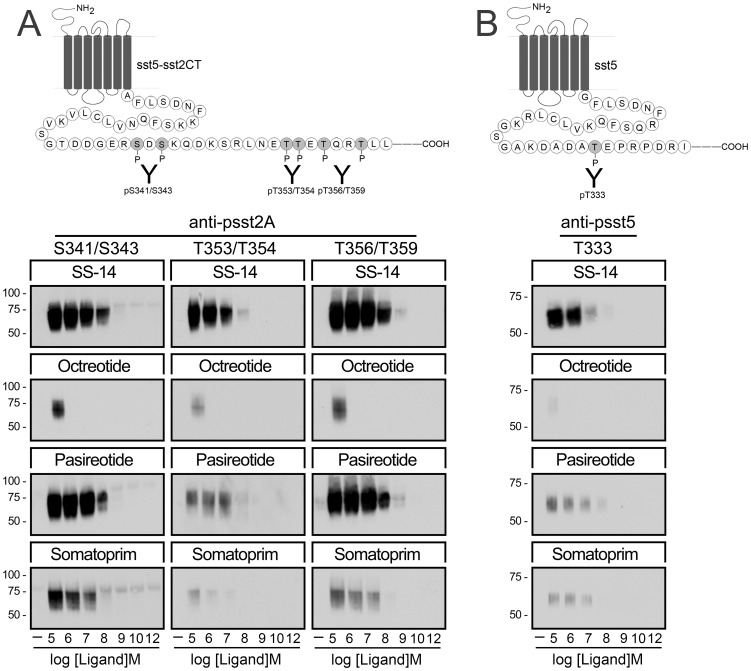
We therefore elucidated whether the carboxyl-terminal tail of the sst2 receptor can be used as probe to sense the activation of other somatostatin receptors. Consequently, we transplanted the carboxyl-terminal phosphorylation motif of the sst2 receptor to other clinically-relevant somatostatin receptors and assessed their patterns of activation using our set of three phosphosite-specific antibodies. Examination of cells expressing a sst5-sst2CT chimeric receptor revealed that SS-14 stimulated the most pronounced phosphorylation of all three sites (Figure 3 A). Pasireotide and somatoprim also promoted a robust phosphorylation of S341/S343 and T356/T359 (Figure 3 A). In contrast, octreotide induced only at saturating concentration a detectable phosphorylation of S341/S343 and T356/T359 (Figure 3 A). Interestingly, similar results were obtained at the wild-type sst5 receptor using a recently generated phosphosite-specific antibody to T333 validating our approach to study receptor activation (Figure 3 B). Analysis of ligand binding properties of the sst5-sst2CT chimera indicated that the transfer of the sst2 carboxyl-terminal tail to sst5 did not substantially affect the affinities for SS-14, octreotide, pasireotide or somatoprim (Table 1).
Figure 3. Agonist-selective phosphorylation of the human sst5 and sst5-sst2CT chimera.
(A,Top panel) Schematic representation of the sst5-sst2CT receptor indicating the phosphate acceptor sites S341/343, T353/354 and T356/359 within the carboxyl-terminal tail. (A, Bottom) HEK293 cells stably expressing the sst5-sst2ACT receptor were either not exposed or exposed for 5 min to SS-14, octreotide, pasireotide or somatoprim in concentrations ranging from 10−12 to 10−5 M. The levels of phosphorylated sst5-sst2ACT receptors were then determined using the phosphosite-specific antibodies anti-pS341/pS343 {3157}, anti-pT353/pT354 {0521} and anti-pT356/pT359 {0522}. (B,Top panel) Schematic representation of the human sst5 receptor indicating the phosphate acceptor site T333 within its carboxyl-terminal tail. (B, Bottom) HEK293 cells stably expressing the human sst5 receptors were either not exposed or exposed for 5 min to SS-14, octreotide, pasireotide or somatoprim in concentrations ranging from 10−12 to 10−5 M. The levels of phosphorylated sst5 receptors were then determined using the phosphosite-specific antibodies anti-pT333 {3567}. Western blots shown are representative of three to five independent experiments for each condition. The positions of the molecular mass markers are indicated on the left (in kDa).
Table 1. Ligand binding properties of sst5-sst2CT receptors.
| Ligand | Ligand binding affinity IC50 (nM) | ||
| human sst2 | human sst5 | human sst5-2CT | |
| SS-14 | 5.7±1.1 | 15.5±2.6 | 30.3±5.0 |
| Octreotide | 1.4±0.3 | 28.9±4.2 | 38.8±11.7 |
| Pasireotide | 21.3±5.7 | 3.6±1.5 | 10.8±5.3 |
| Somatoprim | 4.7±0.6 | 5.6±2.2 | 5.4±1.6 |
Ligand binding assays were carried out as described under “Materials and Methods”. The half-maximal inhibitory concentrations (IC50) were analyzed by nonlinear regression curve fitting using the computer program GraphPad Prism. Data are presented as the mean of three independent experiments performed in triplicate.
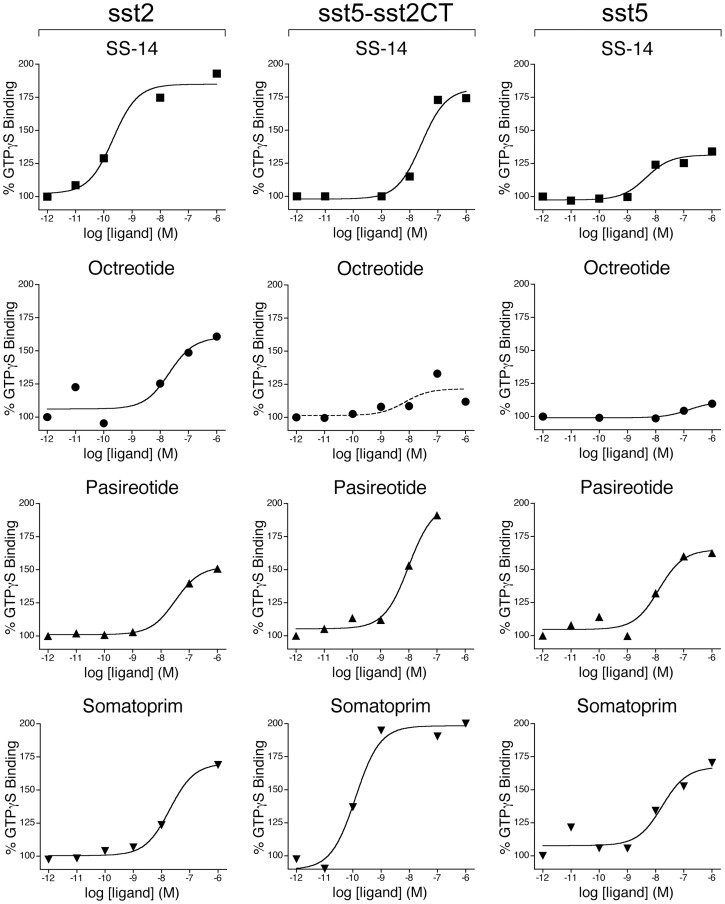
We then examined the capacity of these compounds to stimulate GTPγS binding in membrane preparations from the same cells (Figure 4). Unlike that seen in sst2 receptor phosphorylation assays, pasireotide was able stimulate GTPγS binding to a similar degree as octreotide or somatoprim suggesting that pasireotide is a G protein-biased ligand. In contrast, octreotide stimulated GTPγS binding in both sst5- and sst5-sst2CT-expressing cells to a much lesser extend than pasireotide or somatoprim suggesting it is indeed a weak partial agonist at the sst5 receptor. Again similar results were obtained with the wild-type sst5 and the sst5-sst2CT receptor.
Figure 4. Agonist-stimulated 35S-GTPγS binding.
Stimulation of [35S]GTPγS binding by SS-14, Octreotide, Pasireotide and Somatoprim in the concentration range of 10−12 to 10−6 M. Membranes wer prepared from HEK293 cells stably expressing either the human sst2, sst5 and sst5-sst2ACT or the rat sst3-sst2ACT receptor. Values represent means of triplicate determinations. SE values were smaller than 15%. Three replicate experiments gave similar results.
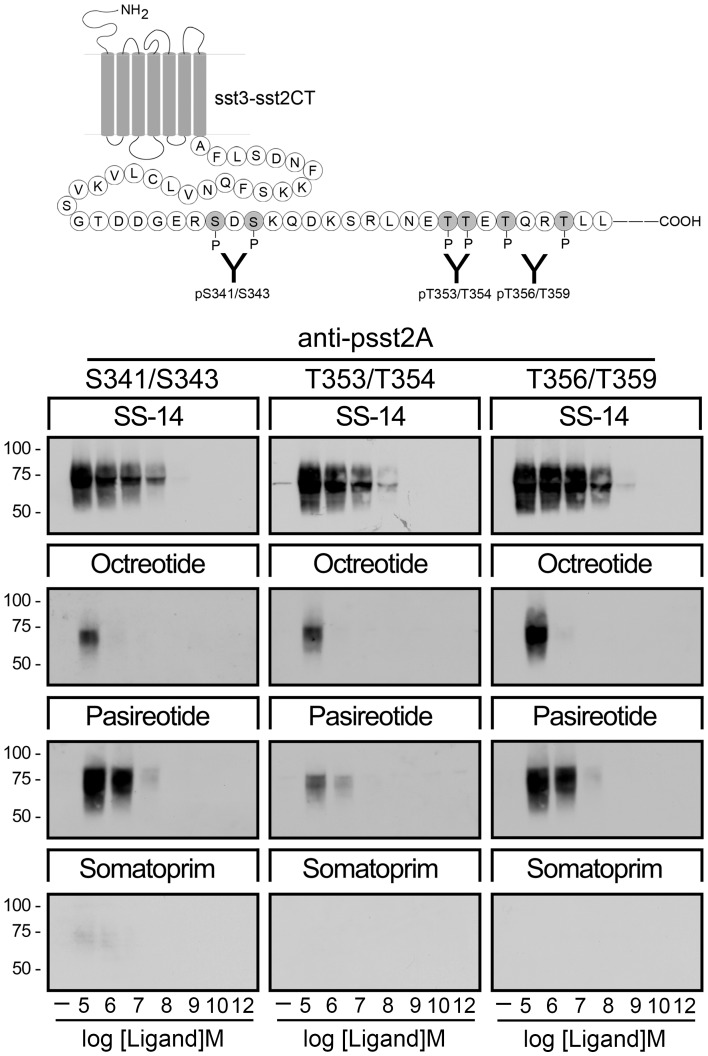
To elucidate whether our approach can be used to directly assess the activation of a wide variety of G protein-coupled receptors, we next examined a sst3-sst2CT chimera. Examination of HEK293 cells stably expressing sst3-sst2CT receptors revealed that only pasireotide but not octreotide was able to promote a robust phosphorylation of S341/S343 and T356/T359 (Figure 5). In contrast, somatoprim failed to induce any detectable phosphorylation. Pasireotide is less potent than octreotide in inducing internalization of the sst2 receptor but more potent than octreotide in inducing internalization of the sst3 receptor. Thus, the patterns of phosphorylation of the rsst3-sst2CT chimera correlates very well with pattern internalization of the wild-type sst3 receptor (Figure 1). Nevertheless, it should be noted that these results were obtained with rat sst3-sst2CT receptor construct. Given the recently observed species differences for the sst2 receptor, these results need to be reproduced with the human sst3 receptor.
Figure 5. Agonist-selective phosphorylation of the sst3-sst2CT chimera.
(Top) Schematic representation of the rat sst3-sst2CT chimera indicating the phosphate acceptor sites S341/343, T353/354 and T356/359 within the carboxyl-terminal tail. (Bottom) HEK293 cells stably expressing the rat sst3-sst2ACT receptor were either not exposed or exposed to concentrations of 10−12 to 10−5 M SS-14, octreotide, pasireotide or somatoprim for 5 min. The levels of phosphorylated rsst3-sst2ACT receptors were then determined using anti-pS341/pS343 {3157}, anti-pT353/pT354 {0521} or anti-pT356/pT359 {0522}. Western blots shown are representative of three to five independent experiments for each condition. The positions of the molecular mass markers are indicated on the left (in kDa).
Discussion
The development of new drugs targeting GPCRs is primarily focused on the discovery of compounds with nanomolar and subnanomolar binding affinities. Then indirect methods mostly assessing G protein signaling are being used to determine whether a new compound is a full or partial agonist. Accumulating evidence suggests that more than one active conformation exists for many GPCRs and that many compounds selectively stimulate specific signaling pathways [20]. Thus, there is clearly a need for methods providing more direct information on receptor activation. However, structural information is only available for a few activated receptors, and none of these has been crystallized in more than one active conformation yet [21],[22]. Determination of receptor activation using biophysical methods requires insertion of bulky fluorescent proteins into the receptor which may itself affect receptor activation [23].
In the present study, we have used the phosphorylation motif of the sst2 receptor to probe GPCR activation. This approach was possible due to our recent success in generating a set of three phosphosite-specific antibodies for the sst2 receptor which allowed us to determine distinct patterns of phosphorylation induced by different agonists. Given the unique ability of GRKs to detect only active GPCRs these distinct conformations may reflect different receptor conformations. However, phosphosite-specific antibodies are notoriously difficult to generate and are only available for a few receptors. We therefore elucidated whether the carboxyl-terminal tail of the sst2 receptor can be used as probe to sense the activation of other somatostatin receptors. Perhaps the most convincing evidence that this might be a valid and useful approach comes from a sst5-sst2CT chimera. In fact, the results obtained with the sst5-sst2CT chimeric receptor and the wild-type sst5 receptor were very similar. We have also confirmed that insertion of the sst2 phosphorylation motif into other somatostatin receptors did not dramatically change their binding properties with regard to the compounds tested. In addition, construction of a sst3-sst2CT chimera was also successful indicating that this approach could be used to examine the activation of a wide variety of GPCRs. Our assay can be adapted to a quantitative ELISA method and thus be applied to screening of large numbers of ligands [24]. However, for other receptors it cannot be completely ruled out that transplantation of the sst2CT may alter receptor function. Therefore, a functional analysis of such chimeric receptors needs to be performed in each case.
Our study also yielded valuable and previously unappreciated information about the pan-somatostatin analogs currently under clinical and preclinical examination. Pasireotide exhibited potent agonistic activity at the sst5 receptor but only weak partial agonistic properties at the sst2 receptor. Consequently, pasireotide should be classified as sst5-preferring ligand. Octreotide is a full agonist at the sst2 receptor but exhibited virtually no agonistic activity at the sst5 receptor. Consequently, octreotide should be classified as sst2-preferring ligand. In contrast, somatoprim is unique in that it was a potent agonist at both sst2 and sst5 receptors. In fact, this may provide the molecular basis for the recent observation that somatoprim can inhibit GH release in cases, which did not respond to octreotide [25].
In conclusion, we describe the use of a phosphorylation probe for direct assessment of GPCR activation and demonstrate its utility in the pharmacological characterization of novel pan-somatostatin analogs.
Supporting Information
Ligand binding properties of rat, human and mutant somatostatin receptors. Ligand binding assays were carried out as described under “Materials and Methods”. The half-maximal inhibitory concentrations (IC50) were analyzed by nonlinear regression curve fitting using the computer program GraphPad Prism. Data are presented as the mean of three independent experiments performed in triplicate.
(DOC)
Acknowledgments
We thank Heidrun Guder and Heike Stadler for excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Deutsche Forschungsgemeinschaft grant SCHU924/10-3 and the Deutsche Krebshilfe grant 109952. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Donangelo I, Melmed S. Treatment of acromegaly: future. Endocrine. 2005;28:123–128. doi: 10.1385/ENDO:28:1:123. [DOI] [PubMed] [Google Scholar]
- 2.Oberg KE, Reubi JC, Kwekkeboom DJ, Krenning EP. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 139: 742–753, 753 e741. 2010. [DOI] [PubMed]
- 3.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–716. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 4.Lewis I, Bauer W, Albert R, Chandramouli N, Pless J, et al. A novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential. J Med Chem. 2003;46:2334–2344. doi: 10.1021/jm021093t. [DOI] [PubMed] [Google Scholar]
- 5.Ma P, Wang Y, van der Hoek J, Nedelman J, Schran H, et al. Pharmacokinetic-pharmacodynamic comparison of a novel multiligand somatostatin analog, SOM230, with octreotide in patients with acromegaly. Clin Pharmacol Ther. 2005;78:69–80. doi: 10.1016/j.clpt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, et al. Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab. 2009;94:115–122. doi: 10.1210/jc.2008-1008. [DOI] [PubMed] [Google Scholar]
- 7.Pedroncelli AM. Medical treatment of Cushing’s disease: somatostatin analogues and pasireotide. Neuroendocrinology. 2010;92:120–124. doi: 10.1159/000314352. [DOI] [PubMed] [Google Scholar]
- 8.Petersenn S, Schopohl J, Barkan A, Mohideen P, Colao A, et al. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2009;95:2781–2789. doi: 10.1210/jc.2009-2272. [DOI] [PubMed] [Google Scholar]
- 9.Poll F, Lehmann D, Illing S, Ginj M, Jacobs S, et al. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol. 2010;24:436–446. doi: 10.1210/me.2009-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, et al. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab. 2008;93:4519–4524. doi: 10.1210/jc.2008-1063. [DOI] [PubMed] [Google Scholar]
- 11.Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab. 2009;94:654–661. doi: 10.1210/jc.2008-1919. [DOI] [PubMed] [Google Scholar]
- 12.Mundschenk J, Unger N, Schulz S, Hollt V, Schulz S, et al. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab. 2003;88:5150–5157. doi: 10.1210/jc.2003-030262. [DOI] [PubMed] [Google Scholar]
- 13.Plockinger U, Albrecht S, Mawrin C, Saeger W, Buchfelder M, et al. Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. J Clin Endocrinol Metab. 2008;93:1203–1210. doi: 10.1210/jc.2007-1986. [DOI] [PubMed] [Google Scholar]
- 14.Schulz S, Pauli SU, Schulz S, Handel M, Dietzmann K, et al. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin Cancer Res. 2000;6:1865–1874. [PubMed] [Google Scholar]
- 15.Pfeiffer M, Koch T, Schroder H, Klutzny M, Kirscht S, et al. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A). J Biol Chem. 2001;276:14027–14036. doi: 10.1074/jbc.M006084200. [DOI] [PubMed] [Google Scholar]
- 16.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Bee MS, Schonbrunn A. Site specificity of agonist and second messenger-activated kinases for somatostatin receptor subtype 2A (Sst2A) phosphorylation. Mol Pharmacol. 2009;76:68–80. doi: 10.1124/mol.108.054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wess J, Han SJ, Kim SK, Jacobson KA, Li JH. Conformational changes involved in G-protein-coupled-receptor activation. Trends Pharmacol Sci. 2008;29:616–625. doi: 10.1016/j.tips.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular Mechanism of ?-Arrestin-Biased Agonism at Seven-Transmembrane Receptors. Annu Rev Pharmacol Toxicol. 2011. [DOI] [PMC free article] [PubMed]
- 21.Kobilka BK. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol Sci. 2011;32:213–218. doi: 10.1016/j.tips.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrosio M, Zurn A, Lohse MJ. Sensing G protein-coupled receptor activation. Neuropharmacology. 2011;60:45–51. doi: 10.1016/j.neuropharm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M, Schonbrunn A. Differential temporal and spatial regulation of somatostatin receptor phosphorylation and dephosphorylation. J Biol Chem. 2011;286:13561–13573. doi: 10.1074/jbc.M110.215723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plockinger U, Hoffmann U, Geese M, Lupp A, Buchfelder M, et al. DG3173 (somatoprim), a unique somatostatin receptor subtypes 2-, 4- and 5-selective analogue, effectively reduces GH secretion in human GH-secreting pituitary adenomas even in Octreotide non-responsive tumours. Eur J Endocrinol. 2012;166:223–234. doi: 10.1530/EJE-11-0737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ligand binding properties of rat, human and mutant somatostatin receptors. Ligand binding assays were carried out as described under “Materials and Methods”. The half-maximal inhibitory concentrations (IC50) were analyzed by nonlinear regression curve fitting using the computer program GraphPad Prism. Data are presented as the mean of three independent experiments performed in triplicate.
(DOC)