Abstract
Enrichment of polyunsaturated fatty acids, particularly docosahexaenoic acid (DHA, 22:6n–3), in the brain is known to be critical for optimal brain development and function. Mechanisms for DHA’s beneficial effects in the nervous system are not clearly understood at present. DHA is incorporated into the phospholipids in neuronal membranes, which in turn can influence not only the membrane chemical and physical properties but also the cell signaling involved in neuronal survival, proliferation and differentiation. Our studies have indicated that DHA supplementation promotes phosphatidylserine (PS) accumulation and inhibits neuronal cell death under challenged conditions, supporting a notion that DHA is an important neuroprotective agent. This article summarizes our findings on the DHA-mediated membrane-related signaling mechanisms that might explain some of the beneficial effects of DHA, particularly on neuronal survival.
1. Introduction
Mammalian brain is highly enriched with docosahexaenoic acid (DHA, 22:6n–3) [1,2]. It is well-recognized that DHA is important for proper neurodevelopment and function. Early dietary supply of DHA improves cognitive and visual development in human infants [3,4], and memory-related learning ability in young rats [5]. In contrast, inadequate support of brain DHA level during development induces cognitive deficits such as memory loss and learning disability in experimental animals [6,7]. Growing evidence also suggests that low levels of DHA in the brain are associated with psychiatric and neurodegenerative conditions such as bipolar disorder [8], generalized peroxisomal disorders [9] and Alzheimer’s disease [10]. Moreover, it has been demonstrated that in some patients with peroxisomal disorders, DHA supplementation reduces the severity of the disease [11]. Also, a meta-analysis of the outcome from clinical trials of omega-3 fatty acids indicated a significant improvement in bipolar disorder and major depression patients [12]. Similarly, DHA has been shown to prevent dendritic pathology in a mouse model of Alzheimer’s disease [13] and improve injury outcome in experimental brain ischemia [14]. At the cellular level, DHA supplementation promotes neuronal survival [15–17], neurogenesis [18], neurite development [19,20], neuronal cell migration [21] and synaptogenesis [20]. Signaling mechanisms underlying these beneficial effects of DHA are beginning to unfold. We found that DHA promotes membrane-targeted signaling events involved in cell survival at least in part through phosphatidylserine (PS) accumulation in neuronal membranes. Considering that neuronal cells are difficult to regenerate, DHA’s role in promoting survival signaling, thus preventing inappropriate neuronal cell death under adverse conditions, may be of critical importance for supporting proper neurodevelopment and function.
2. DHA-mediated phosphatidylserine accumulation in neuronal cells
Phosphatidylserine (PS) is the major acidic phospholipid class in eukaryotic biomembranes and is especially enriched in neuronal membranes [2]. In mammalian cells, PS biosynthesis occurs in the endoplasmic reticulum exclusively through the serine base exchange reaction, i.e., replacement of the choline moiety of phosphatidylcholine (PC) or the ethanolamine moiety of phosphatidylethanolamine (PE) with L-serine [2,22,23]. The enzymes phosphatidylserine synthase (PSS) I and II have been identified for catalyzing the serine base exchange reaction in mammalian cells using PC and PE as substrates, respectively [24–26] (Fig. 1A). Conversely, PS can be converted to PE by phosphatidylserine decarboxylase (PSD) in mitochondria [2,23,27]. Consequently, PS levels in cell membranes are influenced by both PS biosynthesis and decarboxylation.
Fig. 1.
DHA supplementation increases PS accumulation in neuronal cells; (A) PS biosynthesis and degradation scheme. PSS, phosphatidylserine synthase; and PSD, phosphatidylserine decarboxylase; (B) DHA among other fatty acids increases PS most effectively in neuro 2A cells and serine depletion inhibits this effect (from Refs. [15–17,32]); (C) PS increase by DHA is specific to neuronal cells (from Ref. [33]); and (D) the decrease of PS observed in CGC neurons during culture can be prevented by DHA supplementation (Kim, Akbar and Kim, unpublished data). ***, p<0.001; **, p<0.01; and *, p<0.05 vs. non-enriched control (B,C) or cerebella PS level (D).
Although cellular phospholipids levels are rigorously regulated, the PS content in neuronal membranes can be altered according to the membrane DHA status. We have previously shown that DHA containing phospholipids serve as the best substrate for PS biosynthesis by brain microsomes followed by phospholipid species containing docosapentaenoic acid (DPAn–6, 22:5n–6) [28]. We have also demonstrated that in vivo DHA depletion by n–3 fatty acid deficiency decreases the total PS level in neural tissues [29–31], despite the well-established compensatory replacement of DHA with DPAn–6 [32]. Incomplete support of PS levels in n–3 fatty acid deficiency is in agreement with the fact that DPAn–6 containing phospholipid species are not as effective substrates as DHA containing species for PS biosynthesis [28].
The PS level can be significantly increased by DHA supplementation of neuronal cells in culture [15–17,31,33], which are DHA-depleted under the usual culture conditions. DPAn–6 can also increase the PS content, although to a lesser extent in comparison to DHA. However, oleic acid (OA, 18:1n–9) does not have a significant effect on the PS level (Fig. 1B). As PS synthesis requires serine, the PS accumulation induced by DHA or DPAn–6 was reduced when cells were supplemented in the absence of serine (Fig. 1B), although cellular incorporation of these fatty acids was unaltered. Accordingly, this approach provides a useful tool for testing the involvement of PS in biological effects of DHA. The DHA-mediated PS accumulation appears to be specific in neuronal cells [33] as the PS accumulation remained unaltered after DHA enrichment in non-neuronal cells, such as CHO-K1, NIH-3T3, and HEK-293 cells (Fig. 1C). DHA supplementation raised the level of 18:0, 22:6-PS species significantly in both neuronal and non-neuronal cells, contributing to the substantial increase of total PS in neuronal cells. In non-neuronal cells, however, the increase of 18:0, 22:6-PS species was compensated by a considerable decrease in other species such as 18:0, 18:1- and 18:1, 18:1-PS [33]. Consequently, the total PS level remained unchanged in these non-neuronal cells.
We found that DHA supplementation is important in order to maintain PS levels in primary neuronal cells in culture. During the course of in vitro culture, these primary cells are known to be depleted with polyunsaturated fatty acids such as DHA and arachidonic acid (AA, 20:4n–6) [18,34]. We found that PS levels also declined along with DHA loss (Fig. 1D). In freshly isolated (0 DIV) cerebellar granular cells (CGC neurons), the level of total PS decreased considerably (~23%) in comparison to those in rat cerebella obtained just after dissection. During isolation both AA- and DHA-PS species decreased most prominently. The level of total PS in CGC neurons further decreased (~52%) after 7 days in vitro (DIV) culture. The observed loss of PS was prevented by 1 μM DHA supplementation during 7 days of culture. At the individual PS molecular species level, the significant decrease of 18:0, 22:6-PS (~43%) during culture from 0 to 7 DIV was completely restored with DHA supplementation.
These examples demonstrate that the membrane PS levels are dependent on the DHA status in neuronal cells. Neuronal membrane PS levels can be increased by DHA supplementation, whereas PS levels can be decreased in vivo by lowering DHA in neural tissues by dietary n–3 fatty acid depletion [29–31]. The PS levels cannot be altered significantly using other approaches such as direct supplementation of PS, or over-expression or silencing the genes encoding PSS enzymes [17,33], suggesting that the levels of PS in mammalian cells are indeed difficult to perturb. In this regard, DHA enrichment may represent a unique way of expanding the PS pool in mammalian membranes, although this phenomenon is limited to neuronal cells.
3. DHA-mediated protection of neuronal cell death
We have previously demonstrated that DHA supplementation results in inhibition of neuronal apoptosis induced by serum starvation [15,17] or staurosporine (ST, a general PKC inhibitor) treatment [16] in a PS-dependent manner. Activation of caspase-3, a member of cysteine protease family that produces apoptotic cell death in mammalian cells, was significantly inhibited in cells enriched with DHA in both apoptotic models (Fig. 2A). We found that the PS accumulation by DHA is at least in part responsible for the protective effect of DHA. Enrichment of neuro 2A cells with DPAn–6, which accumulates less PS than with DHA enrichment, produced less protection, while OA enrichment, which does not alter PS levels did not have any effects on neuronal cell death (Figs. 1B, 2B). When the DHA- or DPAn–6-induced PS accumulation was reduced by supplementing cells in the absence of serine (Fig. 1B), the protective effect of these fatty acids was significantly diminished in comparison to the cells supplemented with these fatty acids in the presence of serine (Fig. 2B). It is well-established that CGC neurons undergo apoptosis when they are cultured in media containing physiological KCl concentrations (5 mM, low KCl) while depolarizing KCl concentrations (25 mM, high KCl) protects CGC neurons from apoptotic cell death [35]. In this primary neuronal culture model, the protective effect of DHA was also apparent. The DHA-supplemented culture showed significantly less TUNEL (TdT-mediated dUTP-biotin nick end-labeling) positive apoptotic cells and contained reduced level of active caspase-3 (Fig. 2C). The protective effects also appear to correlate with the PS accumulation in this model, as the CGC neurons supplemented with DHA contained higher PS levels in comparison to the non-supplemented neurons (Fig. 1D). Similarly, when DHA and PS levels were lowered in vivo by dietary n–3 fatty acid depletion, the hippocampal neuronal cell death observed in culture was significantly increased, particularly when the trophic factor was removed [17]. These data consistently support the notion that DHA’s anti-apoptotic protective effect is at least in part mediated through PS accumulation.
Fig. 2.
DHA prevents neuronal cell death through PS accumulation. (A) DHA supplementation reduces apoptotic cell death induced by serum deprivation or staurosporine (ST) treatment in neuro 2A cells (from Refs. [15–17,32]); (B) the protective effect of DHA is PS-dependent (from Refs [15,17]); and (C) apoptosis of CGC neurons induced by low KCl (LKCl) is significantly reduced by DHA supplementation as indicated by TUNEL assay and the Western blot analysis of caspase-3 (Akbar and Kim, unpublished data).*, p<0.05; and ***, p<0.001.
4. Kinase signaling modulated by DHA
It has been demonstrated that PS participates in signaling events of key kinases such as protein kinase C (PKC) [36–38], Raf-1 kinase [15,39,40] and Akt [17], which are known to translocate to the membrane for their activation. Therefore, translocation of these kinases may be target signaling events affected by the DHA-mediated neuronal specific increase of PS. Indeed, we found that DHA has significant impact on the activation of these kinases in neuronal cells.
Protein kinase B/Akt
The serine/threonine protein kinase Akt is a critical enzyme for cell survival [41]. It has been well-established that Akt is activated through two essential steps involving membrane translocation and phosphorylation [42,43]. The binding of growth factors to a receptor tyrosine kinase activates phosphoinositide-3 kinase (PI3K) that transforms 4,5-phosphatidylinositol bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the plasma membrane. PIP3 formation triggers Akt translocation from the cytosol to the plasma membrane where it is activated through phosphorylation at T308 and S473 by phosphoinositide-dependent protein kinase 1 (PDK1) and mTORC2, respectively [41–44].
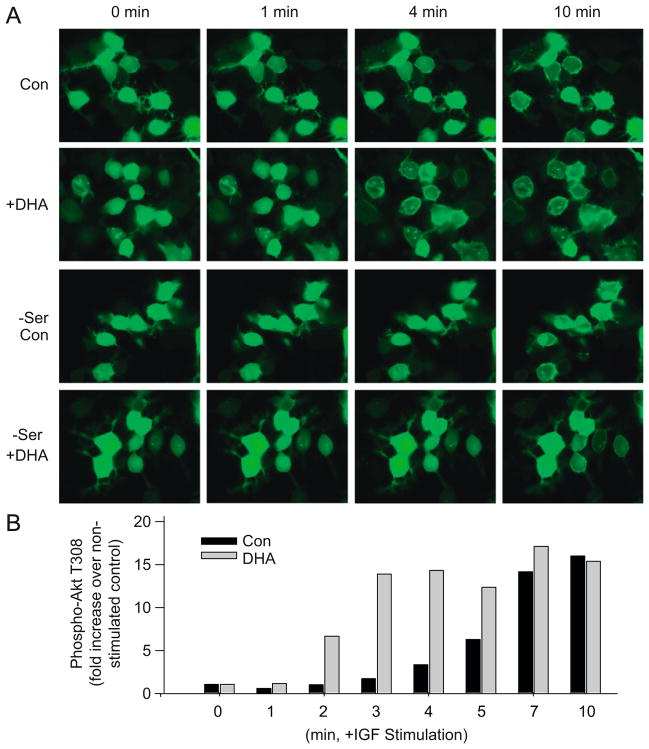
Our findings indicated that PI3K/Akt pathway is important in mediating the protective effects of DHA on neuronal survival [16,17]. Serum deprivation or ST-treatment decreased Akt phosphorylation at both Thr-308 and Ser-473, which was reflected by the decreased Akt activity and increased cell death under these conditions [16,17]. The reduction in Akt phosphorylation and activity was partly prevented by DHA enrichment, thus rescuing cells from apoptotic cell death. However, in the presence of PI3K inhibitors such as wortmanin (WM) and LY-294002 (LY), which prevent the formation of PIP3, DHA was no longer effective in restoring Akt phosphorylation. Accordingly, the protective effect of DHA on neuronal survival was no longer apparent [16,17]. Moreover, DHA enrichment did not affect the PI3 kinase activity [17], suggesting that DHA exerts its effects on Akt activation at the membrane interaction stage. Indeed, DHA enrichment facilitated the membrane translocation of the Akt-PH domain and phosphorylation at T308 in neuro 2A cells upon IGF stimulation (Fig. 3A and B). The DHA-mediated facilitation of Akt translocation was not observed when cells were supplemented with DHA under a serine-depleted condition, indicating that PS accumulation is an important aspect for this effect [17]. These data consistently indicate that Akt translocation/activation is a target for DHA’s anti-apoptotic protective effect under adverse conditions.
Fig. 3.
PI3 kinase/Akt signaling is facilitated by DHA supplementation as evidenced by faster Akt translocation to the plasma membrane (A) and phosphorylation (B) upon stimulation with IGF-1 in neuro 2A cells (from Ref. [17]).
Raf-1 kinase
Raf kinase signaling is also essential for regulating cell proliferation, differentiation and survival [45]. The Raf family consists of three isoforms of serine/threonine kinase, A-Raf, B-Raf and Raf-1, and deletion of each results in distinctive phenotypes. Growth factor receptor stimulation and subsequent Ras-GTP formation activates Raf kinases and the downstream MEK/ERK pathway [46], leading to a variety of cellular responses [45]. It is well-established that Ras-dependent traslocation of Raf-1 to the plasma membrane is a critical event for Raf-1 activation [47,48]. Phosphorylation of S338 and Y341 is necessary for Raf-1 activation [49]. Because Raf-1 contains distinct binding domains for acidic phospholipids [39,40], Raf 1 membrane interaction may be another target affected by the DHA-induced PS increase.
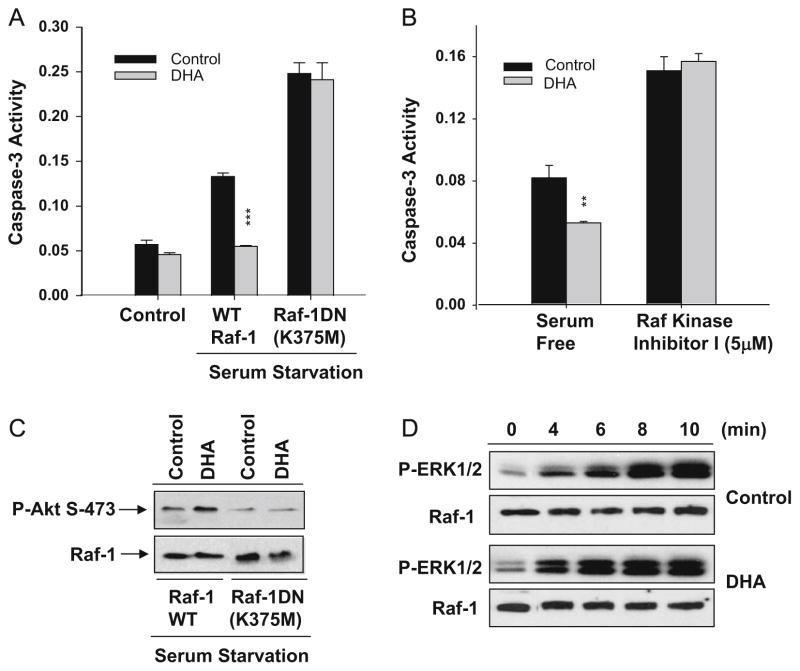
We have previously shown that DHA enrichment promotes membrane localization of Raf-1 upon growth factor stimulation [15]. The role of Raf-1 in neuronal survival was tested using neuro 2A cells transfected with a kinase inactive dominant negative Raf-1 (K375M) mutant (Raf-1DN). As in the case of non-transfected control cells, DHA supplementation decreased caspase-3 activity induced by serum starvation in cells transfected with WT Raf-1. With the expression of Raf-1DN, caspase-3 activation due to serum starvation was potentiated and the protective effect of DHA was abolished (Fig. 4A). Although the importance of Raf-1/MEK/ERK signaling is well-recognized in cell survival [50], it has been also reported that anti-apoptotic effects of Raf-1 may not necessarily require kinase activity [51]. Nevertheless, our study indicated that Raf-1 activation is important for maintaining survival signaling as indicated by the decreased Akt phosphorylation in cells expressing kinase inactive Raf-1DN (Fig. 4A). Consistently, cell death was increased and the protective effect of DHA was abolished when Raf-1 activity was inhibited using a Raf-1 kinase inhibitor (5 μM) (Fig. 4B). These data support the importance of Raf-1 activity for the protective effects of DHA on neuronal survival. As Raf-1 kinase contains distinct binding domains for acidic phospholipids [39,40], the membrane localization of Raf-1 may be dependent on the membrane concentration of PS. Indeed, an in vitro binding assay indicated that the interaction of Raf-1 with the membrane correlates with the PS content of the membrane [15]. The fatty acyl composition of PS also influences the Raf-1 membrane interaction. The DHA-PS species interacts more effectively with Raf-1 than the PS species containing oleic acid [32]. These results support the notion that DHA’s ability to increase DHA containing PS levels in neuronal membranes has significant impact on Raf-1 activation through facilitated interaction of Raf-1 with PS-enriched plasma membranes. Coincidentally, the IGF-1 induced phosphorylation of ERK1/2, a downstream event of Raf-1 activation, was found to be faster in DHA-enriched cells in comparison to non-enriched cells (Fig. 4C). It has been reported that PI3K functions upstream of Ras, which in turn activates Raf/MEK/ERK pathway [52]. Since DHA enrichment has little effects on PI3K activity [17], it is likely that the observed faster phosphorylation of ERK1/2 is due to facilitated Raf-1 activation. These findings collectively suggest that DHA-induced PS enrichment is a facilitating factor for Raf-1 activation, possibly contributing to the observed improvement in neuronal survival by DHA under adverse conditions. However, the precise relationship between Raf-1 and Akt signaling affected by DHA enrichment has yet to be defined.
Fig. 4.
Raf-1 kinase signaling is also important in DHA-mediated neuronal survival. Expression of dominant negative (DN) Raf-1 (K375M) (A) or treatment with Raf kinase inhibitor 1 (5 μM) (B) inhibits protective effect of DHA in neuro 2A cells; and (C) DHA facilitates Raf-1 activation as evidenced by the faster time course of downstream ERK 1/2 phosphorylation in response to IGF-1 (25 ng/mL) in neuro 2A cells (Akbar and Kim, unpublished data).
Protein kinase C
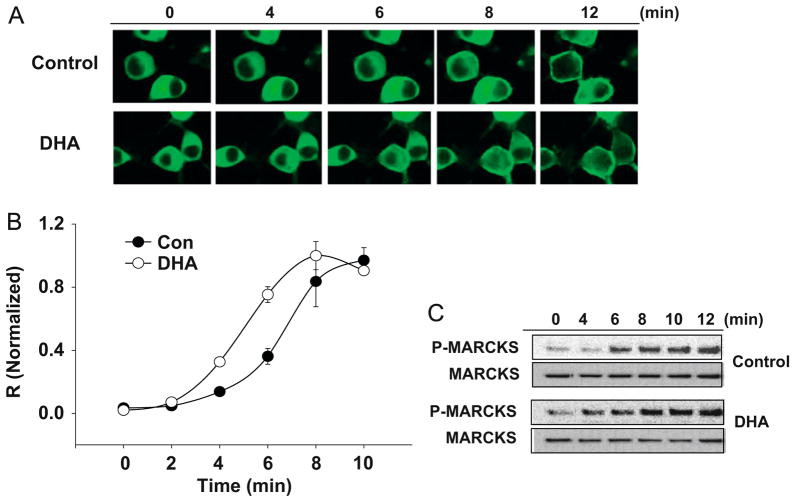
Protein kinase C (PKC), another Ser/Thr kinase family, comprises three distinct groups of isozymes; the calcium-, phospholipid- and diacylglycerol-dependent conventional PKC (cPKC, α, βI, βII, and γ), the calcium-independent novel PKC (nPKC, δ, ε, η, and θ) and the atypical PKC (aPKC, ζ, λ and μ), which requires neither calcium nor diacylglycerol (DAG) [53]. PKC isozymes are expressed in a cell- and tissue-specific manner [54] and have been shown to exert isotype-specific effects on cell proliferation, survival and differentiation [55–57]. Similar to Akt and Raf-1, PKC activation requires its translocation to the plasma membrane [58]. Activation of PKC is initiated by hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) into DAG and inositol-1,4,5-trisphosphate (IP3). The IP3 increases the intracellular Ca2+ through interaction with a calcium channel in the endoplasmic reticulum (ER). Binding of DAG and Ca2+ to the C1 and C2 domains, respectively, results in PKC translocation to the plasma membrane. In addition to Ca2+ and DAG, activation of PKC also requires binding to PS in the membrane, [36–38,58,59]. The binding occurs through the C2 domain via a calcium bridge [60] and specificity for PS binding has been shown to be conferred by the C1 domain [61]. Despite structural variability, PKC isozymes preserve the affinity of C1 domains for the anionic phospholipid, PS [61]. Accordingly, PKC may be another signaling molecule under the influence of DHA-mediated PS increase. It appears to be the case as the PMA-induced PKC translocation to the plasma membrane, which was visualized by GFP-PKCα expressed in neuro 2A cells, was faster in DHA-supplemented cells in comparison to control cells (Fig. 5A and B). In agreement with faster PKC translocation, the phosphorylation of MARCKS, a substrate of PKC, was accelerated in DHA-enriched cells in comparison to non-enriched control cells (Fig. 5C). These findings suggest possible contribution of PKC signaling to the PS-dependent protective effects of DHA.
Fig. 5.
DHA supplementation also facilitates PKCα activation in neuro 2A cells. (A, B) Time course of GFP–PKCα translocation from the cytoplasm to the plasma membrane upon PMA (100 nM) stimulation is faster in DHA-supplemented cells as indicated by the fluorescence micrographs (A) and quantification results using averaged relative R values [R=(plasma membrane fluorescence−cytosolic fluorescence)/cytosolic fluorescence] normalized to the plateau R values (B); and (C) MARCKS phosphorylation, a downstream event of PKCα activation, is also faster in DHA-supplemented cells (Akbar and Kim, unpublished data).
5. Conclusion
The effect of DHA on neuronal survival is an important aspect of the neuroprotective function of DHA. Preferential accumulation of DHA and unique metabolism to PS in neural cells are at least in part responsible for the DHA effect on neuronal survival. While DHA can increase the PS pool specifically in neuronal cells in culture, depletion of DHA in vivo reduces the PS content in the brain regions. Such modulation of PS in neuronal membranes has significant impact on neuronal survival. DHA and PS enrichments in neuronal membranes result in facilitated activation of PS interacting kinases, preventing inappropriate cell death and supporting neuronal survival under challenged conditions. In this regard, the loss of DHA and PS in pathological states or by nutritional deprivation of n–3 fatty acids may diminish protective capacity in the central nervous system with significant implications in neuronal dysfunction.
Although underlying mechanisms are not completely understood, our findings suggest that DHA mediates its protective effect in neuronal cells at least in part through the accumulation of PS, which in turn modulates the activities of a few key protein kinases. At least three kinase pathways involving PI3K/Akt, Raf-1 and PKC are identified as targets for DHA’s neuroprotective effects (Fig. 6). Common features of these kinases include requirements of membrane translocation for their activation, interaction with PS and influence on vital cellular function such as survival, proliferation and differentiation. Accordingly, the biochemical ability of DHA to increase PS accumulation in neuronal membranes is an important underpinning of the neurotrophic function of DHA. Specifically, Akt, Raf-1 and PKCα translocation/activation evoked by stimuli is facilitated by the high concentration of PS in neuronal membranes. The PS-dependent acceleration of Akt translocation is particularly important under sub-optimal conditions where generation of survival signals such as PIP3 is not adequate. Likewise, the Raf-1 and PKCα translocation facilitated by DHA may also contribute to neuronal survival, which is a downstream consequence of the activation of these kinases. It is not clear at present whether crosstalk among these signaling pathways has a role in neuronal survival and DHA-mediated neuroprotection. The Raf/MEK/ERK pathway is known to crosstalk with PI3K/Akt, although regulated in a concentration- and ligand-dependent manner [62]. For example, Akt has been reported to phosphorylate S259 of Raf-1 in the resting state [63,64]. Our data suggest a role of Raf-1 in Akt phosphorylation, but it is not clear where Akt and Raf-1 signaling converges for the observed effect of DHA. Similarly, phosphorylation of Raf-1 inhibitory proteins by PKC has been implicated in Raf-1 activation [65], and regulation of Akt activity by PKC through phosphorylation of Akt at T34 has been demonstrated [66]. Nevertheless, the significance of the interaction of these kinases in DHA-mediated effects has not been explored. Besides these pathways, other DHA- and/or PS-dependent signaling events may also exist for the neuroprotective effects of DHA. Elucidating new signaling events as well as the nature of their interaction for specific target effects of DHA may lead to better understanding of the protective function of DHA in the nervous system.
Fig. 6.
Signaling pathways affected by DHA enrichment, contributing to neuroprotective effects of DHA. DHA increases PS levels in the plasma membrane, which in turn facilitates the membrane translocation and activation of Akt, Raf and PKC in response to stimuli. The PS-dependent membrane interaction of these signaling molecules is particularly important for neuronal survival under adverse conditions where the survival signal such as PIP3 is limited.
References
- 1.Salem N., Jr . Omega-3 Fatty Acids: Molecular and Biochemical Aspects. Alan R. Liss; New York: 1989. pp. 109–228. [Google Scholar]
- 2.Kim HY. Novel metabolism of docosahexaenoic acid in neural cell. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 3.Willatts P, Forsyth JS, Di Modugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352:688–691. doi: 10.1016/s0140-6736(97)11374-5. [DOI] [PubMed] [Google Scholar]
- 4.Birch EE, Garfield S, Hoffman DR, Uauy RD, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 5.Gamoh S, Hashimoto M, Sugioka K, Shahdat Hossain M, Hata N, Misawa Y, Masumura S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 6.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 7.Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N., Jr Cognitive deficits in docosahexaenoic acid-deficient rats. Behav Neurosci. 2002;116:1022–1031. doi: 10.1037//0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- 8.McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford SK. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Martinez M. Severe deficiency of docosahexaenoic acid in peroxisomal disordersa defect of delta 4 desaturation? Neurology. 1990;40:1292–1298. doi: 10.1212/wnl.40.8.1292. [DOI] [PubMed] [Google Scholar]
- 10.Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 11.Martinez M, Vazquez E, Garcia-Silva MT, Manzanares J, Bertran JM, Castello F, Mougan I. Therapeutic effects of docosahexaenoic acid ethylester in patients with generalized peroxisomal disorders. Am J Clin Nutr. 2000;71:376S. doi: 10.1093/ajcn/71.1.376s. [DOI] [PubMed] [Google Scholar]
- 12.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 13.Calon F, Lim GP, Yang F, Morihara T, Teter B, Obeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada M, Amamoto T, Tomonaga M, Kawachi A, Yazawa K, Mine K, Fujiwara M. The chronic administration of docosahexaenoic acid reduces the spatial cognitive deficit following transient forebrain ischemia in rats. Neuroscience. 1996;71:17–25. doi: 10.1016/0306-4522(95)00427-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3): role of phosphatidylserine in anti-apoptotic effect. J Biol Chem. 2000;275:35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 16.Akbar M, Kim HY. Protective effects of docosahexaenoic acid (DHA) in staurosporine-induced apoptosis: involvement of wortmanin-sensitive pathway. J Neurochem. 2002;82:655–665. doi: 10.1046/j.1471-4159.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 17.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Calderon F, Kim HK. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yavin E, Himovichi E, Eilam R. Delayed cell migration in the developing rat brain following maternal Omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162:1011–1022. doi: 10.1016/j.neuroscience.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Kanfer JN. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980;5:81370–81380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- 23.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44:207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Kuge O, Nishijima M. Phosphatidylserine synthase I and II of mammalian cells. Biochim Biophys Acta. 1997;1348:151–156. doi: 10.1016/s0005-2760(97)00137-9. [DOI] [PubMed] [Google Scholar]
- 25.Kuge O, Saito K, Nishijima M. Cloning of a Chinese hamster ovary (CHO) cDNA encoding phosphatidylserine synthase (PSS) II, overexpression of which suppresses the phosphatidylserine biosynthetic defect of a PSS I-lacking mutant of CHO-K1 cells. J Biol Chem. 1997;272:19133–19139. doi: 10.1074/jbc.272.31.19133. [DOI] [PubMed] [Google Scholar]
- 26.Stone J, Cui Z, Vance JE. Cloning and expression of mouse liver phosphatidylserine synthase-1 cDNA. Overexpression in rat hepatoma cells inhibits the CDP–ethanolamine pathway for phosphatidylethanolamine biosynthesis. J Biol Chem. 1998;273:7293–7302. doi: 10.1074/jbc.273.13.7293. [DOI] [PubMed] [Google Scholar]
- 27.Voelker DR. Phosphatidylserine decarboxylase. Biochim Biophys Acta. 1997;1348:236–244. doi: 10.1016/s0005-2760(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim HY, Bigelow J, Kevala JH. Substrate preference in phosphatidylserine biosynthesis for docosahexanoic acid containing species. Biochemistry. 2004;43:1030–1036. doi: 10.1021/bi035197x. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton J, Greiner R, Salem N, Jr, Kim HY. N-3 fatty acid deficiency decrease phosphatidylserine accumulation selectively in neuronal tissues. Lipids. 2000;35:863–869. doi: 10.1007/s11745-000-0595-x. [DOI] [PubMed] [Google Scholar]
- 30.Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Jr, Kim HY. Differential effects of n-3 fatty acid deficiency on phospholipids molecular species composition in the rat hippocampus. J Lipid Res. 2002;43:611–617. [PubMed] [Google Scholar]
- 31.Kim HY, Akbar M, Lau A. Effects of docosapentaenoic acid on neuronal apoptosis. Lipids. 2003;38:453–457. doi: 10.1007/s11745-003-1083-z. [DOI] [PubMed] [Google Scholar]
- 32.Galli C, Trzeciak HI, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: Reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochim Biophys Acta (BBA)—Lipids Lipid Metab. 1971;248:449–454. [Google Scholar]
- 33.Guo M, Stockert L, Akbar M, Kim HY. Neuronal specific increase of phosphatidylserine by docosahexaenoic acid. J Mol Neurosci. 2007;33:67–73. doi: 10.1007/s12031-007-0046-z. [DOI] [PubMed] [Google Scholar]
- 34.Bourre JM, Faivre A, Dumont O, Nouvelot A, Loudes C, Puymirat J, Tixier-Vidal A. Effect of polyunsaturated fatty acids on fetal mouse brain cells in culture in a chemically defined medium. J Neurochem. 1983;41:1234–1242. doi: 10.1111/j.1471-4159.1983.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 35.Contestabile A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum. 2002;1:41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- 36.Boni LT, Rando RR. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985;260:10819–10825. [PubMed] [Google Scholar]
- 37.Newton AC, Keranen LM. Phophatidyl-L-serine is necessary for protein kinase C’s high-affinity interaction with diacylglycerol-containing membranes. Biochemistry. 1994;33:6651–6658. doi: 10.1021/bi00187a035. [DOI] [PubMed] [Google Scholar]
- 38.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calphα directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Improta-Brears T, Ghosh S, Bell RM. Mutational analysis of Raf-1 cysteine rich domain: requirement for a cluster of basic aminoacids for interaction with phosphatidylserine. Mol Cell Biochem. 1999;198:171–178. doi: 10.1023/a:1006981411691. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. R. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J Biol Chem. 1996;271:8472–8480. doi: 10.1074/jbc.271.14.8472. [DOI] [PubMed] [Google Scholar]
- 41.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to Follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 42.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 43.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 44.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTor complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 45.Thiel G, Ekici M, Rossler OG. Regulation of cellular proliferation, differentiation and cell death by activated Raf. Cell Commun Signal. 2009;7:8. doi: 10.1186/1478-811X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellbock C, Karasarides M, Marais R. The Raf proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 47.Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science 264 (1994) 1463–1467; Correction. Science. 1994;266:1792–1793. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 48.Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 49.King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 50.Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- 51.Huser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R, Pritchard C. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 2001;20:1940–1951. doi: 10.1093/emboj/20.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu L, Xie LY, Lou L. PI3K is required for insulin-stimulated but not EGF-stimulated ERK1/2 activation. Eur J Cell Biol. 2006;85:367–374. doi: 10.1016/j.ejcb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 54.Goodnight JA, Mischak H, Kolch W, Mushinski JF. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIN 3T3 fibroblasts: isoform-specific association with microfilaments, Golgi endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 55.Gutcher I, Webb PR, Anderson NG. The isoform specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci. 2003;60:1061–1070. doi: 10.1007/s00018-003-2281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodnight JA, Mischak H, Mushinski JF. Selective involvement of protein kinase C isozymes in differentiation and neoplastic transformation. Adv Cancer Res. 1994;64:159–209. doi: 10.1016/s0065-230x(08)60838-6. [DOI] [PubMed] [Google Scholar]
- 57.Papp H, Czifra G, Bodó E, Lázár J, Kovács I, Aleksza M, Juhász I, Ács P, Sipka S, Kovács L, Blumberg PM, Bíró T. Opposite roles of protein kinase C isoforms in proliferation, differentiation, apoptosis, and tumorigenicity of human HaCaT keratinocytes. Cell Mol Life Sci. 2004;61:1095–1105. doi: 10.1007/s00018-004-4014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newton AC. Lipid activation of protein kinases. J Lipid Res. 2009;50:S266–S271. doi: 10.1194/jlr.R800064-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosior M, Newton AC. Mechanism of apparent cooperativity in the interaction of protein kinase C with phosphatidylserine. Biochemistry. 1998;37:17271–17279. doi: 10.1021/bi981344t. [DOI] [PubMed] [Google Scholar]
- 60.Verdaguer N, Corbalan-Garcia1 S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca2+ bridges the C2 membrane-binding domain of protein kinase Cα directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson JE, Giorgione J, Newton AC. The C1, C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry. 2000;39:11360–11369. doi: 10.1021/bi000902c. [DOI] [PubMed] [Google Scholar]
- 62.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 64.Rommel C, Clarke BA, Zimmerman S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage specific inhibition of the Raf–MEK–ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 65.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003;278:13061–13068. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- 66.Weyrich P, Neuscheler D, Melzer M, Hennige AM, Häring HU, Lammers R. The Par6alpha/αPKC complex regulates Akt1 activity by phosphorylating Thr34 in the PH-domain. Mol Cell Endocrinol. 2007;268:30–36. doi: 10.1016/j.mce.2007.01.011. [DOI] [PubMed] [Google Scholar]