Abstract
We hypothesized that patterns of elevated stroke mortality among those born in the US Stroke Belt (SB) states also prevailed for mortality related to all-cause dementia or Alzheimer Disease (AD). Cause specific mortality (contributing cause of death, including underlying cause cases) rates in 2000 for US-born African-Americans and whites aged 65–89 were calculated by linking national mortality records with population data based on race, sex, age, and birth state or state of residence in 2000. Birth in a SB state (North Carolina, South Carolina, Georgia, Tennessee, Arkansas, Mississippi, or Alabama) was cross-classified against SB residence at the 2000 Census. Compared to those who were not born in the SB, odds of all cause dementia mortality were significantly elevated by 29% for African-Americans and 19% for whites born in the SB. These patterns prevailed among individuals who no longer lived in the SB at death. Patterns were similar for AD-related mortality. Some non-SB states were also associated with significant elevations in dementia-related mortality. Dementia mortality rates follow geographic patterns similar to stroke mortality, with elevated rates among those born in the SB. This suggests important roles for geographically patterned childhood exposures in establishing cognitive reserve.
Keywords: Dementia, cerebrovascular disease/stroke, geography, Stroke Belt, lifecourse, racial disparities
Introduction
The prominent role of cerebrovascular disease in the development of dementia suggests that lifecourse risk factors influencing stroke also contribute to dementia. This is consistent with evidence that both stroke and dementia risk in late life are predicted by social or individual risk factors much earlier in life.1–3 Stroke incidence and mortality is strongly geographically patterned.4, 5 A band of elevated risk has been documented for decades in the southern United States, a region often called the nation’s Stroke Belt (SB).4, 6 It has previously been shown that stroke rates are elevated for people who spent their early lives in the SB, regardless of their subsequent migration patterns.6, 7 It has also been shown that states with high stroke mortality rates also tend to have higher Alzheimer Disease (AD) mortality rates, but the role of early life residence in explaining this pattern has not been examined.8 Although many individuals remain in the same geographic region throughout their lives, many migrate away, thus region of current residence is an imperfect marker for region of childhood residence. Furthermore, mortality differences based on place of residence at death might be attributable solely to differences in cause-of-death coding patterns rather than differences in disease etiology.8 Given the role of early life factors in predicting cerebrovascular disease, we hypothesized that mortality related to dementia would be higher in individuals who were born in the SB compared to those born in other regions of the country. Cerebrovascular disease may also exacerbate the functional consequences of Alzheimer pathology, so we also hypothesized that AD mortality would follow a similar geographic pattern. We compared the geographic patterns in all-cause dementia and AD mortality rates to those for stroke.
Methods
Data sources
The 2000 US Census Public Use Microsample data (the 5% sample who completed the long form of the Census questionnaire), upweighted to represent the actual US population, was used to define the at-risk population.9 Samples were restricted to individuals who were: born in the District of Columbia (DC) or any of 49 US states (Hawaii was excluded because the small population made matching difficult); ages 65–89 on the census date; and self-reporting race as African-American or white. The Census reports are based on the reports of the householder who completed the Census form.
Mortality records for 2000 were obtained from the National Center for Health Statistics multiple cause of death files.10 We considered the geographic patterns of three causes of death: AD (ICD-10: G30); all-cause dementia, including AD (F00-F03, G30); and stroke or transient ischemic attack (I60-I69, G458, G459). Deaths were considered as “related” to AD, all cause dementia, or stroke if the corresponding ICD-10 code was listed as a contributing cause of death (the list of contributing causes includes the underlying cause) in the death certificate record.11 Results were qualitatively similar when deaths were classified exclusively based on the “underlying” cause of death code.
Death records were included only if the decedent would have met criteria for census sample inclusion if living: born in DC or a US state other than Hawaii and resided in DC or a US state other than Hawaii at death; age 65–89 in the year 2000; and reported race as African-American or white. Death certificate data are only accurate to the extent that the proxy informant accurately reported the age, race, sex, and birthplace of the decedent.
Cause-specific mortality rates were calculated for each possible combination of the covariates, including the geographic exposures (described below for each analysis) and key covariates including race, sex, and year of birth. In other words, for each subgroup defined by all possible combinations of the covariates, we calculated the number of deaths from the death certificates and the number at risk from the Census sample. For analyses comparing SB-birth to SB-adult residence, this generated 400 strata (2 race categories × 2 sex categories × 25 birth year categories × 2 SB-birth categories × 2 SB adult residence categories), and for analyses based on state of birth, this generated 5000 possible combinations (2 race × 2 sex × 25 birth years × 50 birth states including DC). After calculating the dementia-related mortality rate for each combination, the data from each of the birth year, sex, and region subgroups were pooled, so we could adjust for continuous age in the regression models.
Data collection and measurements
We defined seven states (North Carolina, South Carolina, Georgia, Tennessee, Arkansas, Mississippi, and Alabama) as comprising the SB, corresponding to the US Department of Health & Human Services Stroke Belt Elimination Initiative12 and our previous work.6, 7 We cross-classified rates based on place of birth (SB versus non-SB) and place of residence in the year 2000 (SB versus non-SB) to create four SB exposure categories: born in the SB and residing in the SB in 2000; born in the SB but did not live there in 2000; born outside the SB but resided in the SB in 2000; or neither born in the SB nor lived there in 2000. Place of residence between birth and the year 2000 is not documented, so we do not know for example how long SB-born children remained there. Evidence from prior Census records suggest that most individuals reside in their state of birth into at least late teen years. For example, among 1940 Census 1% Microsample respondents who were aged 15 at the time of the Census, 88% reported that they resided in their state of birth.9
Analyses
We calculated mortality rates within the four SB exposure categories adjusted for each covariate by linking mortality and population data within each combination defined by covariates (age, race, and sex) and SB exposure.
We first compared crude mortality rates for whites and African-Americans in the four cross-classified SB exposure categories. We then used logistic regression (weighted to represent the eligible US population) to estimate race-stratified odds ratios for cause specific mortality for those born in the SB compared to all others and those residing in the SB in 2000 compared to all others. After examining SB birth and SB residence in 2000 in separate regression models, we conducted the logistic regressions simultaneously adjusting for SB residence at birth or in 2000. All models were adjusted for sex, age, and age-squared in order to account for possible non-linearities in the association between age and mortality rates.
To assess the possibility that SB birth and SB adult residence may have interactive effects, we used logistic regression to compare those who did not live in the SB at birth or in 2000 to the three groups of people with known exposure to the SB (those born in the SB who no longer lived there in 2000; those born outside the SB but who lived there in 2000; and those who were born in the SB and also lived there in 2000).
Finally, to examine a finer level of geographic resolution than the SB, we estimated odds ratios for AD and all-cause dementia related mortality associated with birth in each of the 49 states plus DC. We used race-stratified logistic regressions with random effects (SAS proc glimmix) for each state, adjusted for sex, age, and age-squared. Empirical Bayes (shrinkage) estimates of the odds ratios (OR) associated with each state of birth were mapped. We used the empirical Bayes estimates to account for uncertainty in coefficients for states with small populations (this was especially relevant for estimating ORs among African-Americans). This approach assumes that state-specific logistic regression coefficients are normally distributed around a national average; the empirical Bayes estimate “shrinks” the predicted value for each state towards the national average, in inverse proportion to the variance of the effect estimate.13, 14 Analyses were conducted using SAS 9.2.
Results
Approximately 13% (179,736/1,393,733) of eligible death records listed stroke as a contributing cause of death (Table 1). All-cause dementia was listed as a contributing cause of death on 13% of death certificates that included stroke, but only 8% of the deaths that were not related to stroke. AD was listed as a contributing cause on 3% of the stroke-related deaths and 4% of deaths that did not include stroke as a contributing cause.
Table 1.
Deaths in 2000, by Contributing Cause from Stroke, Alzheimer’s Disease, and All Cause Dementia
| Additional Contributing Causes of Death | Stroke a Contributing Cause of Death
|
|||
|---|---|---|---|---|
| Yes | No | |||
| n | % | n | % | |
|
|
|
|||
| All Cause Dementia | 23,980 | 13% | 102,084 | 8% |
| Alzheimer Disease | 5,567 | 3% | 48,703 | 4% |
| Neither Listed | 155,756 | 87% | 1,063,210 | 88% |
| Total Fatalities | 179,736 | 100% | 1,213,997 | 100% |
Cause Dementia category. Contributing cause of death includes both those listed as the underlying cause and other contributing causes on the death certificate.
As of 2000, the majority of older white Americans (87%) were born outside of the SB, whereas the majority of older African-Americans (53%) were born in the SB (Table 2). Only 4% of whites who resided outside of the SB in 2000 were born inside the SB. In contrast, 37% of African Americans who lived outside of the SB in 2000 were born inside the SB. In general, older African Americans were more likely to be inter-state migrants: 58.3% of whites lived at census date in their state of birth, and 49.2% of blacks lived at census date in their state of birth (results not shown).
Table 2.
Crude mortality rates by contributing cause of death and race, Stroke Belt birth, and Stroke Belt residence in 2000.
| Total Population
|
Born in the Stroke Belt
|
Not Born in the Stroke Belt
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resided in the Stroke Belt in 2000
|
Did Not Reside in the Stroke Belt in 2000
|
Resided in the Stroke Belt in 2000
|
Did Not Reside in the Stroke Belt in 2000
|
|||||||
| n/deaths | Rate per 100,000 Popl’n | n/deaths | Rate per 100,000 Popl’n | n/deaths | Rate per 100,000 Popl’n | n/deaths | Rate per 100,000 Popl’n | n/deaths | Rate per 100,000 Popl’n | |
|
|
|
|
|
|
||||||
| Whites | ||||||||||
| Population at Risk (in 1,000s) | 26,856 | 2,533 | - | 849 | - | 751 | - | 22,722 | - | |
| All Cause Dementia Deaths | 115,727 | 431 | 12,285 | 485 | 4,316 | 508 | 2,987 | 397 | 96,139 | 423 |
| Alzheimer Disease Deaths | 50,676 | 189 | 5,714 | 226 | 1,944 | 229 | 1,414 | 188 | 41,604 | 183 |
| Stroke Deaths | 159,255 | 593 | 17,805 | 703 | 5,842 | 688 | 4,244 | 565 | 131,364 | 578 |
| African-Americans | ||||||||||
| Population at Risk (in 1,000s) | 2,534 | 645 | - | 684 | - | 34 | - | 1,170 | - | |
| All Cause Dementia Deaths | 10,337 | 408 | 2,967 | 460 | 3,154 | 461 | 107 | 313 | 4,109 | 351 |
| Alzheimer Disease Deaths | 3,594 | 142 | 1,085 | 168 | 1,065 | 156 | 30 | 88 | 1,414 | 121 |
| Stroke Deaths | 20,481 | 808 | 6,057 | 939 | 5,807 | 849 | 249 | 727 | 8,368 | 715 |
Restricted to US residents ages 65–89 born in the United States (excluding Hawaii)
All causes of death include all fatalities with the condition listed as the underlying or a contributing cause of death.
Among whites born in the SB, the crude all-cause dementia-related mortality rate for those who resided in the SB in the year 2000 was 485 per 100,000 people. Among the SB born who no longer resided in the SB in 2000, the crude dementia mortality rate was slightly higher: 508. By comparison, among individuals who were neither born in the SB nor lived there in the year 2000, the dementia mortality rate was 423, and the rate among those who were not born in the SB but lived there in 2000 was 397 (Table 2). Among African-Americans, all-cause dementia mortality rates were elevated among those born in the SB, regardless of whether they resided inside the SB (460 vs 313) or outside the SB (461 vs 351) in 2000. SB birth was also associated with elevated mortality rates from AD and stroke among whites and African-Americans.
After adjusting for age, age-squared and sex (Table 3), SB birth remained a significant predictor of all cause dementia mortality for both whites (OR=1.23; 95% CI: 1.21, 1.25) and African-Americans (OR=1.25; 1.20, 1.30). SB adult residence was also associated with all cause dementia mortality for whites (OR=1.19; 1.17, 1.21) and African-Americans (OR=1.08; 1.03, 1.12) (Table 3). Patterns were similar for AD mortality, which was predicted by SB birth among whites (OR= 1.30, 95% CI: 1.27, 1.33) and African-Americans (OR=1.28; 1.19, 1.36). The magnitude of associations between SB exposure and dementia and AD related mortality were similar to those for stroke-related mortality.
Table 3.
Age and sex adjusted odds ratios for cause specific mortality, by Stroke Belt birth or residence in 2000.
| Birth and Adulthood Modeled Separately
|
Birth and Adulthood Modeled Together
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Stroke Belt Birth* | Stroke Belt Residence in 2000^ | Stroke Belt Birth* | Stroke Belt Residence in 2000^ | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
|
|
|
|
||||||
| Whites | ||||||||
| All cause Dementia | 1.23 | (1.21, 1.25) | 1.19 | (1.17, 1.21) | 1.19 | (1.16, 1.22) | 1.04 | (1.02, 1.07) |
| Alzheimer Disease | 1.30 | (1.27, 1.33) | 1.28 | (1.25, 1.31) | 1.21 | (1.16, 1.25) | 1.11 | (1.06, 1.15) |
| Stroke | 1.27 | (1.25, 1.29) | 1.24 | (1.22, 1.25) | 1.21 | (1.18, 1.23) | 1.07 | (1.05, 1.09) |
| African-Americans | ||||||||
| All cause Dementia | 1.25 | (1.20, 1.30) | 1.08 | (1.03, 1.12) | 1.29 | (1.24, 1.35) | 0.93 | (0.89, 0.98) |
| Alzheimer Disease | 1.28 | (1.19, 1.36) | 1.14 | (1.06, 1.22) | 1.28 | (1.19, 1.39) | 0.99 | (0.91, 1.07) |
| Stroke | 1.22 | (1.18, 1.25) | 1.17 | (1.14, 1.21) | 1.18 | (1.14, 1.22) | 1.07 | (1.03, 1.11) |
All causes of death include all fatalities with the condition listed as an underlying or contributing cause of death. All models adjusted for age, age-squared, and sex. Restricted to US residents ages 65–89 born in the United States (excluding Hawaii).
Odds ratios are in comparison to individuals born outside the Stroke Belt.
Odds ratios are in comparison to individuals who lived outside the Stroke Belt in 2000.
When birth and adult SB residence were modeled together, SB birth remained an important predictor of all-cause dementia mortality for both whites (OR=1.19; 1.16, 1.22) and African-Americans (OR=1.29; 1.24, 1.35), but adult residence in the SB retained only a small association with all-cause dementia mortality among whites (OR=1.04; 1.02, 1.07) and the association was reversed for African-Americans (OR=0.93; 0.89, 0.98). The patterns for stroke and AD were nearly identical to those for all-cause dementia.
Compared to the reference group of individuals who were neither born in the SB nor lived in the SB in 2000, whites who were both born in the SB and lived there in 2000 had a 22% higher odds of death related to all-cause dementia in the year 2000 (Table 4). Whites who were born outside the SB but lived there in adulthood had a 12% higher odds of dementia related death, and whites who were born in the SB but subsequently left the SB had a 25% elevation in odds of all-cause dementia related mortality in the year 2000.
Table 4.
Age and sex adjusted odds ratios for cause-specific mortality associated with cross-classified Stroke Belt birth and Stroke Belt residence in 2000.
| Stroke Belt Birth; Stroke Belt in 2000 | Non-Stroke Belt Birth; Stroke Belt in 2000 | Stroke Belt Birth; Non-Stroke Belt in 2000 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
|
|
|
|
||||
| Whites | ||||||
| All cause Dementia | 1.22 | (1.20, 1.25) | 1.12 | (1.08, 1.16) | 1.25 | (1.22, 1.29) |
| Alzheimer Disease | 1.31 | (1.27, 1.35) | 1.23 | (1.16, 1.29) | 1.30 | (1.24, 1.36) |
| Stroke | 1.29 | (1.27, 1.31) | 1.10 | (1.07, 1.13) | 1.23 | (1.20, 1.27) |
| African-Americans | ||||||
| All cause Dementia | 1.21 | (1.15, 1.26) | 0.95 | (0.78, 1.15) | 1.29 | (1.24, 1.36) |
| Alzheimer Disease | 1.27 | (1.17, 1.37) | 0.78 | (0.54, 1.12) | 1.27 | (1.17, 1.37) |
| Stroke | 1.26 | (1.22, 1.30) | 1.05 | (0.92, 1.19) | 1.18 | (1.14, 1.22) |
Parameter estimates are compared to the reference group of people who were neither born in the Stroke Belt nor resided in the Stroke Belt in 2000 All models are adjusted for sex, age and age squared.
Mortality rates for each condition include all deaths for which the condition was listed as the underlying or a contributing cause of death.
Results were similar among African-Americans: compared to the reference group of individuals who were neither born in the SB nor lived in the SB in 2000, African-Americans who were both born in the SB and lived there in 2000 had a 21% higher odds of death related to all-cause dementia in the year 2000. The odds of dementia related death was similarly elevated (29%) for African-Americans born in the SB who no longer lived in the SB in 2000.
For both African-Americans and whites, the odds ratios for AD-related mortality associated with SB birth were at least as extreme as the odds ratios for all-cause dementia related mortality or stroke.
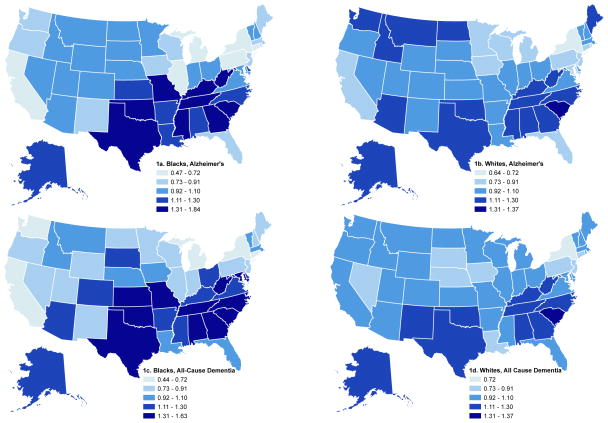
Considering each state of birth separately, patterns were very similar for all-cause dementia and AD, so we describe results for all-cause dementia (figure 1). All-cause dementia mortality odds ratios for both African-Americans and whites were elevated in classic SB states such as South Carolina (figure 1). However, dementia mortality was also elevated in some non-SB states such as Oklahoma and Texas (for both African-Americans and whites) and Kansas and Missouri (for African-Americans). Furthermore, African-Americans born in several states, including New York, Pennsylvania, Illinois, Michigan and California had significantly lower odds of dementia related mortality compared to the national average. Whites born in New York also experienced relatively lower odds of dementia related mortality than the national average. The variance in odds ratios associated with state of birth was smaller for whites than for African-American (i.e., state-specific coefficients were closer to the null among whites than African-Americans).
Figure 1.
Odds ratios for Alzheimer’s or all-cause dementia-related mortality in 2000, by race and state of birth, compared to the national average, based on empirical Bayes (shrinkage) random effect estimates from logistic models, US born blacks and whites ages 65–89. Cutpoints are identical for the maps, but the minimum and maximum values in the keys reflect actual minimum and maximum odds ratios observed for blacks or whites for each outcome.
Discussion
We find that Americans aged 65–89 who were born in a SB state experience higher rates of dementia related mortality compared to individuals of similar age, sex, and race who were born outside the SB. This pattern is true whether considering all cause dementia or AD, and in fact patterns were slightly stronger for AD than all-cause dementia. The geographic patterns of dementia related mortality were in the same direction and of roughly the same magnitude as the geographic patterns of stroke. The pattern was similar for whites and African-Americans, although whites who migrated into the SB but were not born in the SB also had a modest elevation of dementia related mortality. African-Americans born outside the SB who migrated into the SB did not have higher rates of dementia related mortality than African-Americans who were born outside the SB and resided outside the SB in 2000. The variance in risk associated with state of birth was larger for African-Americans than whites, suggesting that geographically patterned risk factors may have a greater effect on dementia risk among African-Americans than whites.
A key limitation in these analyses is the reliance on death certificate data. Geographic patterns in mortality rates do not necessarily correspond with geographic patterns in incidence rates, which are likely to provide more information on the etiology of dementia. Furthermore, there are two major potential sources of bias entailed in this study design: inaccuracies in death certificate coding and selective migration patterns. We presented several complementary analyses to help understand the extent to which each limitation might have biased the results. As described below, we believe the evidence strongly suggests that SB birth contributes to risk of dementia and AD related mortality, although this does not rule out possible consequences of SB adult residence
There are well documented inaccuracies in cause of death coding on death certificates.15 The patterns for AD merit special caution because of secular trends of increasing diagnosis rates. If the recognition of AD and increase in diagnosis rates occurred more quickly in the SB than elsewhere, this could have contributed to an apparent excess AD in the SB states. Based on current evidence on the prevalence of dementia in North America16 and the effect of dementia on mortality risk17, we estimate that the number of death certificates with dementia listed as a contributing cause is only approximately 60% of all the deaths of people with dementia (estimates available from authors). However, these estimates are subject to a great deal of uncertainty. Most importantly, the relevant sources of bias in this study arise from regional differences in death certificate coding, and specifically differences based on place of birth. For example, if coding in the SB tended to emphasize dementia more than death certificate coding in other regions of the country, this could have influenced apparent dementia mortality rates in the SB. Indeed, this was a concern in previous research documenting the overlap between geographic patterns of AD mortality and stroke mortality.8 For this reason, we examined results for migrants: people who were born in the SB but who lived outside the SB in 2000. If the regional differences in dementia mortality rates were attributable to differences in death certificate coding, we would expect migrants to show the same patterns of mortality rates as other residents of the region to which they migrated. However, we saw that dementia mortality rates were elevated among the SB-born even if they died outside the SB. Further, among African-Americans, immigrants to the SB had lower dementia mortality rates than African-Americans who were born outside the SB and also resided outside the SB in 2000, as well as lower rates compared to African-Americans born in the SB. This suggests that for African-Americans at least, the elevated dementia mortality rates for those born in the SB cannot be attributed strictly to differences in coding at the time of death. This argument rests on the assumption that individuals who left the SB did not differentially move to states where dementia coding on death certificates is elevated relative to other non-SB sates. We did not adjust for individual state fixed effects and therefore cannot evaluate this possibility.
It is also possible that birth in the SB may affect the likelihood of being diagnosed with AD or other causes of dementia, given the same level of underlying disease. For example, the SB born may perform worse on neuropsychological exams for reasons unrelated to dementia (e.g., lower quality of education due to historically lower schooling opportunities18, 19). We cannot evaluate this hypothesis with our data. Because of uncertainty in the criteria used to classify AD versus other types of dementia for the death certificates, we interpret the findings specific to AD cautiously. It is possible that the association between SB birth and AD is entirely attributable to misclassified cases of vascular dementia. However, because all AD deaths were also included in the all-cause dementia death category, the finding that the associations between SB exposure and AD are often stronger than the associations between SB exposure and all-cause dementia mortality provide at least some evidence that this is not an adequate explanation. We did not attempt to examine vascular dementia related deaths as a separate category.
A related concern is inaccuracy regarding place of birth recorded on the death certificate versus the census, which would typically be reported by different individuals. It is possible that the proxy interviewed for the death certificate would be unaware of the decedent’s place of birth. This might be especially true for migrants whose place of birth differed from their place of residence at death: i.e. some migrant decedents would be classified as non-migrants. Assuming the Census reports on place of birth are accurate, this type of misclassification on the death certificates would result in an overestimation of the mortality rates for non-migrants. As shown in table 2, most migration was from the SB to other regions of the country. Some decedents classified as individuals who were born and died outside the SB may actually have been people who were born in the SB and migrated out but whose place of birth was misreported. This type of misclassification, however, would be expected to reduce estimated mortality rates associated with SB birth. Misclassification of birth place is thus unlikely to explain our key findings, although a bias toward same-region birth responses among proxies outside the SB might have attenuated the estimated effects.
Although our results show lower rates of AD or dementia-related mortality for African-Americans than whites, this contrast is likely uninformative, given concerns about differences in diagnosis rates.20, 21 Our data cannot help evaluate this differential, so we do not interpret the racial differences in mortality rates.
The second major concern in this study design is selective migration. People who leave their region of birth may differ with respect to many demographic, socioeconomic, or psychological characteristics from people who do not migrate. This may be especially acute for migrants into the SB. The phenomenon of the “Great Migration”—in which millions of African Americans moved from southern states to northern industrial cities during the middle of the 20th century—was so large that the selection process was probably not as extreme. Prior research shows inconsistent results on this topic,22 but analyses from Census records suggest that African Americans who left the South averaged more education compared to African Americans who remained in the South. 23 In contrast, African Americans born in the north averaged higher education levels than those who migrated from the south..23
Because of the potential bias introduced by selective migration, it is important to examine the models in which region of birth is not cross-classified against region of adult residence (such as findings in Table 3). These models are not subject to bias due to selective migration, and they showed that SB birth strongly predicted dementia related mortality. Without cross-classification, we cannot tell if it was region of birth or region of adult residence that was important, because they are strongly related. However, we note that the parameter estimates for SB birth are larger than the parameter estimates for SB adult residence for all outcomes. These differences generally were not statistically significant, but do indicate that place of birth is at least as important as place of adult residence.
Our analyses cannot pinpoint the key etiologic period associated with elevated dementia risk, because we have measures of place of residence at only two points in the lifecourse. The results suggest modest contributions of current region of residence for risk of dementia mortality, but we cannot rule out an important role for earlier adult exposure. Our findings are most consistent with a role for early life in shaping long term risk of dementia-related mortality. Prior research suggests that childhood disadvantage and region of residence predict adult dementia risk.24, 25 Southern birth may be an indicator of childhood disadvantage, especially for older African-Americans. For example, as early as 1946, when approximately 14.2% of US white households had incomes under $1,500, 19.5% of white and 49% of non-white households in the South had incomes below this level.26 Region of birth might influence adult dementia risk via the same pathways as cerebrovascular disease, or through some additional mechanisms. The similar patterns observed for white and African American populations suggest that genetic explanations are unlikely.
SB pathways especially relevant to dementia may be those factors that contribute to clinical expression of disease, i.e. determinants of cognitive reserve.27–29 In general, SB birth is almost certainly associated with lower quantity and quality of education.18, 19, 30 Although it is well recognized that segregated schools for African Americans severely underserved the population, segregated white schools in the south were also generally of lower quality than schools in the north, with shorter term lengths and less qualified teachers.31, 32 Older southerners, whether white or black, completed less schooling on average than their non-southern counterparts. This lower quantity and quality of education may contribute to the southern disadvantage. Consistent with this interpretation, Missouri, a non-SB state with de jure racial segregation of schools, was associated with elevated dementia risk for African-Americans, comparable to the risk for SB-birth.
Nutritional factors may also contribute to these patterns. There is extensive evidence suggesting that severe poverty that affects nutritional adequacy -- for example manifested in anthropometric measures such as height or cranial size -- increases dementia risk.33–35 Elderly people born in the SB may have experienced greater poverty as children. We note however that adult education, income, and wealth do not appear to explain the relationship between SB residence and stroke incidence6, but we cannot rule out this hypothesis with respect to dementia.
Our results provide further evidence for common pathways leading to mortality related to cerebrovascular disease and dementia, including cases diagnosed as AD. Furthermore, many of these processes appear to originate in early life, even for conditions such as dementia that are not manifest until old age.
Acknowledgments
This research was supported in part with funding from the Harvard Program on the Global Demography of Aging, funded by the National Institute on Aging (National Institutes of Health).
Footnotes
Statistical analyses were conducted by Anna Kosheleva.
References
- 1.Rogers MAM, Plassman BL, Kabeto M, et al. Parental Education and Late-life Dementia in the United States. J Ger Psych Neurol. 2009;22:71. doi: 10.1177/0891988708328220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whalley LJ, Starr JM, Athawes R, et al. Childhood mental ability and dementia. Neurology. 2000;55:1455–9. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 3.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurology. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 4.Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. American Journal of the Medical Sciences. 1999;317:160–7. doi: 10.1097/00000441-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Feigin VL, Lawes CMM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. The Lancet Neurology. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 6.Glymour MM, Avendano MP, Berkman LF. Is the stroke belt worn from childhood? Risk of first stroke and state of residence in childhood and adulthood. Stroke. 2007;38:2415–21. doi: 10.1161/STROKEAHA.107.482059. [DOI] [PubMed] [Google Scholar]
- 7.Glymour M, Kosheleva A, Boden-Albala B. Birth and adult residence in the stroke belt independently predict stroke mortality. Neurology. 2009;73 doi: 10.1212/WNL.0b013e3181c47cad. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steenland K, MacNeil J, Vega I, et al. Recent Trends in Alzheimer Disease Mortality in the United States, 1999 to 2004. Alzheimer Disease & Associated Disorders. 2009;23:165. doi: 10.1097/wad.0b013e3181902c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggles S, Sobek M, Alexander T, et al. Integrated Public Use Microdata Series: Version 3.0 [Machine-readable database] Minnesota Population Center; Minneapolis, MN: 2004. ( http://www.ipums.org). [Google Scholar]
- 10.National Vital Statistics System NCHS. Multiple Cause-of-Death Mortality Data [Machine-readable database] Hyattsville, MD: National Bureau of Economic Research; 1959–2005. [Google Scholar]
- 11.Gillum RF, Bosworth HB. New Considerations in Analyzing Stroke and Heart Disease Mortality Trends: The Year 2000 Age Standard and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Stroke. 2002;33:1717–22. doi: 10.1161/01.str.0000016925.58848.ea. [DOI] [PubMed] [Google Scholar]
- 12.Release UHaHSN. HHS Announces Initiative to Reduce the Incidence of Stroke in Stroke Belt States. 2004 ( http://www.hhs.gov/news/press/2004pres/20040805.html)
- 13.Greenland S. Principles of multilevel modelling. International Journal of Epidemiology. 2000;29:158–67. doi: 10.1093/ije/29.1.158. [DOI] [PubMed] [Google Scholar]
- 14.Witte JS, Greenland S, Kim LL, et al. Multilevel modeling in epidemiology with GLIMMIX. Epidemiology. 2000;11:684–8. doi: 10.1097/00001648-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Goldacre MJ. Cause-Specific Mortality - Understanding Uncertain Tips of the Disease Iceberg. Journal of Epidemiology and Community Health. 1993;47:491–6. doi: 10.1136/jech.47.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. The Lancet. 2006;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostbye T, Hill G, Steenhuis R. Mortality in elderly Canadians with and without dementia: a 5-year follow-up. Neurology. 1999;53:521. doi: 10.1212/wnl.53.3.521. [DOI] [PubMed] [Google Scholar]
- 18.Goldin C. America’s graduation from high school: The evolution and spread of secondary schooling in the twentieth century. Journal of Economic History. 1998;58:345–74. [Google Scholar]
- 19.Goldin C, Katz LF. The shaping of higher education: The formative years in the United States, 1890 to 1940. Journal of Economic Perspectives. 1999;13:37–62. [Google Scholar]
- 20.Daker-White G, Beattie AM, Gilliard J, et al. Minority ethnic groups in dementia care: a review of service needs, service provision and models of good practice. Aging & Mental Health. 2002;6:101–8. doi: 10.1080/13607860220126835. [DOI] [PubMed] [Google Scholar]
- 21.Østbye T, Taylor DH, Jr, Clipp EC, et al. Identification of Dementia: Agreement among National Survey Data, Medicare Claims, and Death Certificates. Health Services Research. 2008;43:313–26. doi: 10.1111/j.1475-6773.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klineberg O. Race differences. New York: Harper & Brothers; 1935. [Google Scholar]
- 23.Tolnay SE. Educational selection in the migration of southern blacks, 1880–1990. Social Forces. 1998;77:487–514. [Google Scholar]
- 24.Moceri VM, Kukull WA, Emanuel I, et al. Early-life risk factors and the development of Alzheimer’s disease. Neurology. 2000;54:415–20. doi: 10.1212/wnl.54.2.415. [DOI] [PubMed] [Google Scholar]
- 25.Moceri VM, Kukull WA, Emanual I, et al. Using census data and birth certificates to reconstruct the early-life socioeconomic environment and the relation to the development of Alzheimer’s disease. Epidemiology. 2001;12:383–9. doi: 10.1097/00001648-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Consumer Income. Washington, DC: Bureau of the Census Department of Commerce; 1948. [Google Scholar]
- 27.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Disease & Associated Disorders. 2006;20:63. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- 28.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease & Associated Disorders. 2006;20:112–7. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 29.Glymour MM, Kawachi I, Jencks CS, et al. Does childhood schooling affect old age memory or mental status? Using state schooling laws as natural experiments. Journal of Epidemiology and Community Health. 2008;62:532–7. doi: 10.1136/jech.2006.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldin C, Katz LF. Education and income in the early twentieth century: Evidence from the prairies. Journal of Economic History. 2000;60:782–818. [Google Scholar]
- 31.Tyack DB, Cuban L. Tinkering toward utopia: A century of public school reform. Harvard Univ Pr; 1995. [Google Scholar]
- 32.Margo RA. Race and schooling in the South, 1880–1950: An economic history. University of Chicago Press; 1990. [Google Scholar]
- 33.Huang TL, Carlson M, Fitzpatrick AL, et al. Height, armspan and knee height: a reflection of early life environment and risk of dementia. Neurobiology of Aging. 2004;25:S385. [Google Scholar]
- 34.Abbott R, White L, Ross G, et al. Height as a marker of childhood development and late-life cognitive function: the Honolulu-Asia Aging Study. Pediatrics. 1998;102:602–9. doi: 10.1542/peds.102.3.602. [DOI] [PubMed] [Google Scholar]
- 35.Graves AB, Mortimer JA, Bowen JD, et al. Head circumference and incident Alzheimer’s disease - Modification by apolipoprotein E. Neurology. 2001;57:1453–60. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]