Abstract
A previous meta-analysis determined that the effects of steroids during sepsis were dose-dependent; since then, additional trials have been published. The current analysis updates our previous analysis examining the effects of steroids during sepsis. A literature search from 2004 to 2008 identified seven randomized controlled trials in adult patients; these were added to 14 previously identified trials. The effects of steroids on mortality were highly variable among the 21 trials (p <0.001, I2 = 60%). In trials published before 1989, which involved short courses of high-dose steroids, steroids increased mortality (n = 8, I2 = 14%, OR of death 1.39 (95% CI 1.04–1.86), p 0.03). In trials published after 1997, which involved longer courses of lower-dose steroids, steroids consistently improved shock reversal (n = 7, I2 = 0%, OR of shock reversal 1.66 [95% CI 1.25–2.20), p <0.001), but demonstrated a more heterogeneous beneficial effect on mortality (n = 12, I2 = 25%, OR of death 0.64 (95% CI 0.45–0.93), p 0.02). An inverse linear relationship between severity of illness and the effects of steroids on mortality was identified across all trials (p 0.03) and within the subgroup of trials published after 1997 (p 0.03); steroids were harmful in less severely ill patient populations and beneficial in more severely ill patient populations. There was no effect of response to adrenocorticotrophic hormone (ACTH) stimulation testing concerning the effects of steroids and no increase in steroid-associated adverse events. Low-dose steroids appear to improve mortality rates in patients with septic shock who are at high risk of death; however, additional trials in this subpopulation are necessary to definitively determine the role of low-dose steroids during sepsis.
Keywords: Corticosteroids, glucocorticoids, meta-analysis, review, sepsis, septic shock, steroids
Introduction
Septic shock is one of the most common causes of death in the intensive-care unit. Despite effective therapies directed at the infecting agent (appropriate antibiotics, drainage procedures, etc.) and aggressive cardiovascular support (fluid resuscitation, vasopressors, etc.), mortality due to septic shock remains unacceptably high, at 30–60% [1–4]. Multiple adjunctive therapies for septic shock have been developed and studied in clinical trials, including agents directed against specific bacterial toxins, anti-inflammatory agents, and antithrombotic agents. Despite promising preclinical results, few therapies have demonstrated improved survival in controlled clinical trials [5]. Moreover, these beneficial effects have not been consistent in confirmatory trials. The varying treatment effects of these agents can be explained, in part, by methodological differences among the clinical trials; specifically, when the trials have been compared, several factors that may alter the efficacy of these agents have been identified [5–7]. For example, a meta-analysis of clinical trials of mediator-specific anti-inflammatory agents in sepsis demonstrated a significant relationship between severity of illness and treatment effect, characterized by beneficial effects in severely ill patient populations and no effect, or potentially harmful effects, in less severely ill patient populations [6]. Furthermore, a meta-analysis of trials of glucocorticoids in the treatment of sepsis demonstrated a significant relationship between steroid dose and treatment effect, with high doses having harmful effects and low doses having potentially beneficial effects [7]. In addition, others have reported that the beneficial effects of steroids in sepsis trials are dependent on a blunted response to a corticotropin stimulation test [8]. On the basis of these analyses, the influence of factors that might alter the efficacy of a therapy in clinical sepsis trials need to be evaluated when attempting to define the role of an adjunctive therapy in the treatment of sepsis.
Glucocorticoids have been studied extensively as adjunctive therapy in septic patients for over 40 years. Our previous meta-analysis examining the effects of steroid dose in sepsis demonstrated that early studies (published before 1989), which involved predominantly short courses of high-dose steroids, reported increased mortality and worsened secondary infections [7]. In contrast, more recent trials (published after 1997) involved longer courses of lower-dose steroids and demonstrated beneficial treatment effects, including improved shock reversal and survival [7]. Since the publication of this meta-analysis, multiple trials (including a large, multicentre, randomized controlled trial) have been performed. Therefore, with the current meta-analysis, we aimed to update our previous analysis and to determine whether severity of illness, drug dose, and response to corticotropin stimulation testing influence the treatment effects of steroids during sepsis [9].
Methods
The medical literature from January 2004 to December 2008 was searched using the following keywords in MEDLINE, EMBASE and the Cochrane library: ‘steroids and sepsis’, ‘steroids and septic shock’, ‘glucocorticoids and sepsis’, ‘glucocorticoids and septic shock’, ‘corticosteroids and sepsis’, and ‘corticosteroids and septic shock’. In addition, abstracts from national and international critical-care conferences and published meta-analyses were reviewed to identify other potentially relevant studies and outcomes not previously identified. Consistent with our previous analysis, clinical trials were included if they fulfilled all of the following criteria: a prospective randomized controlled trial design comparing glucocorticoid treatment with a control group (with or without placebo treatment), enrolment of adult patients who met the criteria for sepsis or septic shock, and a primary endpoint including either shock reversal or survival. Within each study, criteria for sepsis or septic shock needed to be defined and consistent with the ACCP/SCCM consensus definitions for sepsis, severe sepsis, and septic shock [10]. In addition, similar treatments must have been administered to both the steroid and control groups, with the exception of the steroid treatment regimen being investigated.
Each included trial was independently reviewed by two investigators using a standardized protocol and data collection form, with discrepancies being resolved by a third party. Abstracted data on patient characteristics, study characteristics, treatment interventions and treatment outcomes were collected, including: the presence of sepsis, severe sepsis, or septic shock; type, dose and duration of steroid administered; incidence and severity of complications of therapy (including secondary infections, gastrointestinal bleeding, and neuropathy); response to corticotropin stimulation testing; proportion of patients with shock reversal; and mortality. Trial quality was assessed by evaluating the method of randomization, use of blinding, and withdrawal/dropout reporting, based on the methods of Jadad [11]. To account for the different steroid regimens used in the trials, all doses were converted to hydrocortisone equivalents [12].
Statistical analysis
The OR of death following treatment, as compared with control, was estimated using a random-effects model (Comprehensive Meta-Analysis; Biostat Inc., Englewood, NJ, USA). Heterogeneity across studies was assessed using a Mantel–Haenszel derived Cochran Q statistic, and reported with an I2-value. [13,14] As previously, trials were partitioned and excluded when significant heterogeneity or influence was present, to decrease the heterogeneity within a group of studies, and to maximize differences among groups of studies [7]. Variables previously evaluated to explain the heterogeneity in the treatment effects were again explored, using metaregression techniques (Comprehensive Meta-analysis, SAS v 9.2; SAS Institute, Cary, NC, USA) [7]. Potential bias was assessed by creating a funnel plot, and formally tested using Egger’s regression method [15]. In addition, a jack-knife sensitivity analysis of the late studies was performed by sequentially removing each study to detect the studies that contributed most to the remaining heterogeneity within this group of trials [16]. All pooled ORs of death are reported with associated 95% CIs, using random-effects models. Significant differences in characteristics between early and late studies were assessed using an ANOVA, when a weighted analysis was needed, or a two-sample Wilcoxon test, when an unweighted analysis was performed.
Results
Study flow and quality
The current literature search identified seven new prospective randomized controlled trials, published since 2003, which met our inclusion criteria and were included in the current analysis (Fig. 1) [17–23]. Characteristics of the 21 trials included in this analysis are depicted in Table 1 [8,17–36]. All included studies were prospective randomized trials that involved similar co-interventions in each study group, with the exception of the steroid regimen being investigated. Blinding, randomization schemes and patient follow-up were adequate in most trials, with all but two studies having a Jadad quality score of three or higher (Table 1) [23,31]. Among the 21 trials, three have been published only in abstract form [23,29,35].
FIG. 1.
Study selection flow diagram.
TABLE 1.
Randomized controlled trials of steroids in sepsis
| Author | Year published |
Treatment | Baseline differences reported |
Study design |
Co-interventions reported |
Endpoints available for meta-analysisa |
Jadad score |
|---|---|---|---|---|---|---|---|
| Bennett et al. [25] | 1963 | Hydrocortisone 300 mg × 1, then decreased by 50 mg/day |
Yes | Double-blind | Antibiotics, vasopressors |
Hospital mortality, complications of treatment |
4 |
| Klastersky et al. [30] | 1971 | Betamethasone 1 mg/kg/day for 3 days |
No | Double-blind | Antibiotics, vasopressors, fluids |
20-day mortality, complications of treatment |
4 |
| Schumer [33] | 1976 | Dexamethasone 3 mg/kg or methylprednisolone 30 mg/kg; may be repeated × 1 |
No | Double-blind | Antibiotics | 28-day mortality, complications of treatment |
4 |
| Thompson et al. [35] | 1976 | Methylprednisolone 30 mg/ kg × 1, and then repeated up to three times within 24 h if in shock |
No | Double-blind | Antibiotics | Hospital mortality, toxicities of treatment |
3 |
| Lucas et al. [31] | 1984 | Dexamethasone 2 mg/kg bolus followed by 2 mg/kg/24 h in first 48 h |
No | Open-label | Antibiotics, digoxin, fluids, diuresis |
14-day mortality, complications of treatment |
1 |
| Sprung et al. [34] | 1984 | Methylprednisolone 30 mg/kg or dexamethasone 6 mg/kg; repeat × 1 at 4 h if still in shock |
No | Open-label | Antibiotics, vasopressors, fluids |
Hospital mortality, shock reversal, complications of treatment |
3 |
| Bone et al. [27] | 1987 | Methylprednisolone 30 mg/ kg × 4 |
Yes | Double-blind | Standard therapy | 14-day mortality, shock reversal, complications of treatment |
5 |
| Veterans Administration [24] |
1987 | Methylprednisolone 30 mg/kg bolus followed by 5 mg/kg/h for 9 h |
Yes | Double-blind | Antibiotics, fluids | 14-day mortality, complications of treatment |
5 |
| Luce et al. [32] | 1988 | Methylprednisolone 30 mg/ kg × 4 over 24 h |
No | Double-blind | Antibiotics, standard care |
Hospital mortality, ARDS, complications of treatment |
5 |
| Bollaert et al. [26] | 1998 | Hydrocortisone 100 mg every 8 h × 5 days, and then 6-day taper |
No | Double-blind | Antibiotics, vasopressors, fluids |
28-day mortality, shock reversal, complications of treatment |
5 |
| Briegel et al. [28] | 1999 | Hydrocortisone 100 mg × 1, and then 0.18 mg/kg/h until off pressors, and then 6-day taper |
No | Double-blind | Antibiotics, vasopressors, fluids |
30-day mortality, shock reversal, complications of treatment |
5 |
| Chawla et al. [29,37] | 1999 | Hydrocortisone 100 mg every 8 h × 3 days, and then tapered over 4 days |
No | Double-blind | Not reported | Shock reversal; 28-day mortality, complications of treatment |
3 |
| Yildiz et al. [36] | 2002 | Prednisolone 5 mg every morning and 2.5 mg every afternoon × 10 days |
No | Double-blind | Antibiotics, vasopressors, fluids |
28-day mortality, complications of treatment |
5 |
| Annane et al. [8] | 2002 | Hydrocortisone 50 mg every 6h + fludrocortisone 50 μg every day × 7 days |
No | Double-blind | Antibiotics, vasopressors, fluids |
28-day mortality, shock reversal, complications of treatment |
5 |
| Confalonieri et al. [18] | 2005 | Hydrocortisone 200 mg × 1, and then 10 mg/h × 7 days |
Yes | Double-blind | Antibiotics | Shock reversal, hospital and 60-day mortality, complications of treatment |
5 |
| Mussack et al. [19] | 2005 | Hydrocortisone 100 mg × 1, and then 0.18 mg/kg/h until off pressors, and then 6-day taper |
No | Double-blind | Antibiotics, vasopressors, fluids |
Shock reversal; 28-day mortality |
4 |
| Oppert et al. [20] | 2005 | Hydrocortisone 50 mg × 1, and then 0.18 mg/kg/h until off pressors, and then 3-day taper |
No | Double-blind | Antibiotics, vasopressors, fluids |
Shock reversal; 28-day mortality |
5 |
| Tandan et al. [23] | 2005 | Low-dose steroids (regimen not specified) |
Not specified | Not specified | Not specified | Shock reversal; 28-day mortality |
1 |
| Rinaldi et al. [21] | 2006 | Hydrocortisone 12.5 mg/ h × 6 days, and then tapered |
No | Open-label | Antibiotics, fluids | Hospital mortality | 3 |
| Cicarelli et al. [17] | 2007 | Dexamethasone 0.2 mg/kg every 36 h × 3 |
Yes | Double-blind | Antibiotics, vasopressors, fluids |
28-day mortality, complications of treatment |
5 |
| CORTICUS [22] | 2008 | Hydrocortisone 50 mg every 6 h × 5 days, and then tapered over 6 days |
No | Double-blind | Antibiotics, vasopressors, fluids |
Shock reversal; 28-day mortality, complications of treatment |
5 |
ARDS, adult respiratory distress syndrome.
Effects of steroids on mortality
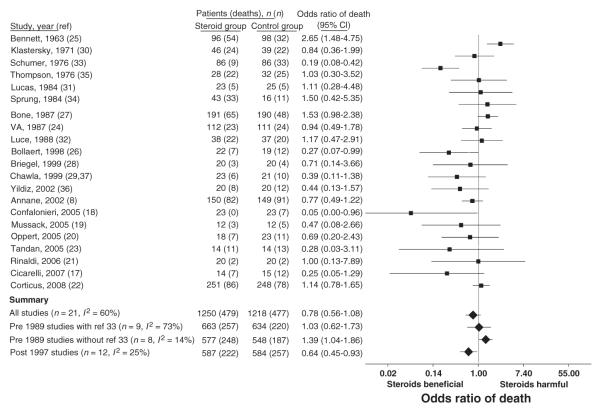
The effects of steroids on mortality were highly variable among the 21 trials (p <0.001, I2 = 60%), with an overall non-significant effect on mortality (Fig. 2). Consistent with our previous analysis, heterogeneity decreased when the 21 trials were partitioned into studies published before 1989 [24,25,27,30–35] and published after 1997 [8,17–23,26,28,29,36]. As the group of trials published before 1989 was identical to our previous analysis, they were analysed in a similar fashion, with subsequent exclusion of a single outlier study from subsequent analyses (Fig. 2) [33]. Similar to the findings of our previous meta-analysis, the treatment effects of steroids were significantly different in trials published before 1989 than in trials published after 1997 (p 0.001). Steroids increased mortality in trials published before 1989 (n = 8, I2 = 14%, OR of death 1.39, 95% CI 1.04–1.86, p 0.03), and decreased mortality in trials published from 1997 through 2008 (n = 12, I2 = 25%, OR of death 0.64, 95% CI 0.45–0.93, p 0.02).
FIG. 2.
Effects of steroids on mortality in randomized controlled sepsis trials. The OR of death and 95% CI with steroid therapy is shown. Analysis of all 21 trials demonstrated variability in the effects of steroids on mortality (I2 = 60%, p <0.001). As previously, studies were partitioned into those published before 1989 and after 1997 to decrease heterogeneity. In the 12 trials published after 1997, there remained a moderate amount of heterogeneity in the effects of steroids on mortality (I2 = 25%, p 0.20), with an overall decrease in the OR of death with steroid therapy (p 0.02). Significant heterogeneity remained in the treatment effects of steroids in the nine trials published before 1989 (I2 = 73%, p <0.001). Removal of a single trial that was a significant outlier [33] decreased the heterogeneity in these trials (I2 = 14%, p 0.33). In the remaining eight trials published before 1989, steroids significantly increased the OR of mortality (p 0.03).
Influence of steroid regimen and control mortality on the treatment effects of steroids
As compared with the trials published before 1989, trials published from 1997 through 2008 involved lower total doses of steroids over a longer duration, and were more likely to taper the steroids (p 0.007, p 0.01, and p 0.01, respectively) (Table 2). Mean control mortality rates, indications for therapy, and percentage of enrolled patients with shock or patients receiving vasopressors were similar between the two groups of studies.
TABLE 2.
Characteristics of trials published before 1989 vs. after 1997
| Author | Years in which patients were enrolled |
Control mortality rate (%) |
Patients with shock (%) |
Patients receiving vasopressors (%) |
Total steroid dose (mg of hydrocortisone equivalents)a,b |
Length of therapyc |
Steroid taper |
Indication for therapy |
|---|---|---|---|---|---|---|---|---|
| Bennett et al. [25] | 1959–1963 | 33 | NR | NR | 1050 | 6 days | Nod | Severe infection |
| Klastersky et al. [30] | NR | 56 | NR | NR | 7000 | 3 days | No | Severe infection |
| Thompson et al. [35] | NR | 78 | 100 | NR | 42 000 | 24 h | No | Shock |
| Lucas et al. [31] | 1978–1980 | 20 | 100 | NR | 11 200 | 48 h | No | Shock |
| Sprung et al. [34] | 1979–1982 | 69 | 100 | 93 | 21 700 | 4 h | No | Shock |
| Bone et al. [27] | 1982–1985 | 25 | 38 | NR | 42 000 | 24 h | No | Severe sepsis |
| Veterans Administration [24] |
1983–1986 | 22 | NR | NR | 26 250 | 9 h | No | Sepsis |
| Luce et al. [32] | 1983–1986 | 54 | 100 | 44 | 42 000 | 24 h | No | Shock |
| Bollaert et al. [26] | NR | 63 | 100 | 100 | 1500 | 5 days | Yes | Vasopressor-dependent shock |
| Briegel et al. [28] | 1993–1996 | 20 | 100 | 100 | 1209 | 6 days | Yes | Vasopressor-dependent shock |
| Chawla et al. [29,37] | NR | 48 | 100 | 100 | 900 | 3 days | Yes | Vasopressor-dependent shock |
| Yildiz et al. [36] | 1997–1999 | 60 | 23 | NR | 300 | 10 days | No | Severe sepsis |
| Annane et al. [8] | 1995–1999 | 61 | 100 | 100 | 1400 | 7 days | No | Vasopressor-dependent shock |
| Confalonieri et al. [18] | 2000–2003 | 30 | 7 | 7 | 1880 | 7 days | No | Pneumonia |
| Mussack et al. [19] | NR | 42 | 100 | 100 | 1209 | 6 days | Yes | Vasopressor-dependent shock |
| Oppert et al. [20] | NR | 48 | 100 | 100 | 453 | 2 days | Yes | Vasopressor-dependent shock |
| Tandan et al. [23] | NR | 93 | 100 | NR | NR | NR | NR | Septic shock |
| Rinaldi et al. [21] | NR | 10 | 0 | 0 | 1800 | 6 days | Yes | Severe sepsis |
| Cicarelli et al. [17] | 2004–2005 | 80 | 100 | 100 | 1119 | 3 days | No | Vasopressor-dependent shock |
| CORTICUS [22] | 2002–2005 | 31 | 100 | 99 | 1000 | 5 days | Yes | Vasopressor-dependent shock |
| Summary | Value | Weighted mean (%) |
Weighted mean (%) |
Weighted mean (%) |
Median | Median (days) | Rate | Median |
| Before 1989 | 35 | 63 | 65 | 23 975 | 1 | 0/8 | Shock | |
| After 1997 | 44 | 90 | 89 | 1209 | 6 | 7/11 | Vasopressor-dependent shock |
NR, not reported.
Total possible dose of steroid that could have been received by a patient in a trial before beginning a taper; if two drugs were used in the trial, the average total dose was used.
Dose based on a 70-kg patient.
Length of steroid therapy before taper was begun.
Decreasing doses of steroids over 6 days were considered to constitute a steroid treatment regimen and not a taper in the original report and all previous meta-analyses.
Meta-regression analysis of all of the trials revealed a significant direct linear relationship between steroid dose and the OR of death (p 0.03), characterized by beneficial effects at lower doses and harmful effects at higher doses (Fig. 3). In addition, meta-regression analysis demonstrated a significant inverse linear relationship between severity of illness of the patient population (measured by control odds of death) and the OR of death (p 0.02), indicating that, in less severely ill patients, steroids were harmful and, as severity of illness increased, steroids became beneficial (Fig. 3). As in our previous analysis, a single overly influential trial was identified and removed from the meta-regression analyses [25]. The results of the current meta-regression analyses remain essentially unchanged, whether the influential study is included or excluded.
FIG. 3.
Influence of steroid dose and severity of illness on the effects of steroids on mortality. (a) There is a significant linear relationship between the dose of steroids administered in the first 24 h after study enrolment and the OR of death across the steroid sepsis trials (p 0.03); steroids decreased mortality at lower doses, and increased mortality as steroid dose increased. (b) A significant inverse linear relationship between the control odds of death (a measure of severity of illness) and the OR of death also exists across the steroid sepsis trials (p 0.03); steroids increase mortality in patient populations with a low control odds of death (less severely ill), and decrease mortality in patient populations with a high control odds of death (more severely ill). As in our previous analysis, a single overly influential trial with methodological differences was identified and removed from these meta-regression analyses [25]. (c) In the subgroup of trials published after 1997 that administered only lower-dose steroids (range in hydrocortisone equivalents: 30–420 mg over first 24 h after enrolment), a similar significant inverse linear relationship between the control odds of death and the OR of death exists (p 0.03), suggesting that low-dose steroids increase mortality in less severely ill patient populations and decrease mortality in more severely ill patient populations.
A subgroup analysis of the trials published after 1997 using meta-regression analysis demonstrated a significant inverse linear relationship between severity of illness of the patient population and the OR of death (p 0.03), similar to the relationship demonstrated among all of the trials combined (Fig. 3). As expected, because the doses of steroids administered in the trial published after 1997 were similar (range in hydrocortisone equivalents: 30–420 mg over first 24 h after enrolment), there was no relationship between steroid dose and the OR of death within the group of trials published after 1997 (p not significant; data not shown).
Influence of response to adrenocorticotropic hormone (ACTH) stimulation testing on the treatment effects of steroids
Five of the trials published after 1997 report mortality results based on patient response to a 250-μg ACTH stimulation test (Fig. 4) [8,20,22,26,36]. Four of the studies defined a non-responder according to a change in cortisol level of <9 μg/dL from baseline [8,20,22,36]; the fifth study defined a non-responder according to a change in cortisol level of <6 μg/dL from baseline [26]. The treatment effects of steroids on mortality, based on response to ACTH stimulation testing, were similar (p 0.86), and consistent within each subgroup and overall (non-responder, I2 = 0%; responder, I2 = 14%; combined, I2 = 0%). Steroid therapy led to similar non-significant decreases in mortality in both non-responders (OR of death 0.84, 95% CI 0.59–1.12, p 0.33) and responders (OR of death 0.83, 95% CI 0.50–1.39, p 0.49). It is of note that there was no difference in the control mortality rate based on response to ACTH stimulation testing (p 0.26; non-responder control mortality 50%, 95% CI 31–69%; responder control mortality 38%, 95% CI 19–57%).
FIG. 4.
Effects of low-dose steroids on mortality based on response to adrenocorticotropic hormone (ACTH) stimulation testing and the effects of steroids on shock reversal. (a) The OR of death and 95% CI with steroid therapy based on patient response to a 250-μg ACTH stimulation test are shown for the five trials published after 1997 that reported mortality results using these subgroups [8,20,22,26,36]. The treatment effects of steroids on mortality based on response to ACTH stimulation testing were similar (p 0.86) and consistent within both subgroups and combined groups (non-responder, I2 = 0%; responder, I2 = 14%; combined, I2 = 0%). Low-dose steroids demonstrated similar non-significant decreases in mortality in both non-responders (OR of death 0.84, 95% CI 0.59–1.12, p 0.33) and responders (OR of death 0.83, 95% CI 0.50– 1.39, p 0.49) to ACTH stimulation testing. (b) The OR of 28-day shock reversal and 95% CI with steroid therapy is displayed for the seven trials published after 1997 reporting this outcome. Low-dose steroids consistently improved 28-day shock reversal (p 0.70, I2 = 0%), with a significant increase in shock reversal in patients treated with steroids as compared with controls (OR of shock reversal 1.66, 95% CI 1.25–2.20, p <0.001).
Effects of steroids on shock reversal
Seven trials published after 1997 reported a consistent effect of steroids on 28-day shock reversal (p 0.70, I2 = 0%), with an overall significant increase in shock reversal in patients treated with steroids as compared with controls (OR of shock reversal 1.66, 95% CI 1.25–2.20, p <0.001) (Fig. 4) [8,19,22,23,26,28,29]. Severity of illness did not influence the effects of steroids on shock reversal across these trials (data not shown, p 0.43). Two trials published before 1989 reported opposite effects of high-dose steroids on shock reversal (data not shown, p not significant within each trial) [27,34].
Incidence of treatment-related complications in trials published after 1997
Several trials published after 1997 reported the incidence of treatment-related complications. As compared with controls, there was no increase in the incidence of secondary infections (n = 8, I2 = 19%, OR of secondary infection 0.96, 95% CI 0.63–1.45, p 0.84) [8,17,18,22,26,28,29,36,37], gastrointestinal bleeding (n = 8, I2 = 0%, OR of gastrointestinal bleeding 1.10, 95% CI 0.65–1.88, p 0.72) [8,17,18,22,26,28,29,36,37] or neuropathy (n = 2, I2 = 0%, OR of neuropathy 0.35, 95% CI 0.08–1.55, p 0.17) with steroid therapy [18,22].
Publication bias and sensitivity analysis in trials published after 1997
Visual examination of a funnel plot of the trials published after 1997 suggests potential publication bias, with a disproportionately high number of the published small trials demonstrating beneficial treatment effects of steroids (Fig. 5). Formal testing for publication bias, using Egger’s regression method, statistically supports the presence of publication bias in the studies published after 1997 (p 0.002). A jack-knife sensitivity analysis revealed that eight studies increased heterogeneity (I2 increased) and four studies decreased heterogeneity (I2 decreased) when the individual study was removed (Fig. 5). It is of note that the two studies with largest effect on the heterogeneity among these 12 trials (I2 = 25%) were the largest randomized controlled trial (I2 = 0% if removed) [22] and the small trial with the largest beneficial treatment effect of steroids (I2 = 11% if removed) [18].
FIG. 5.
Publication bias and jackknife sensitivity analysis in the steroid trials published after 1997. (a) A funnel plot of precision versus the log odds ratio of death with steroid therapy for the trials published after 1997 is presented; visual inspection of the plot suggests potential publication bias. There are a disproportionately high number of published small trials demonstrating beneficial treatment effects of steroids (demonstrated by the solid circles with negative log odds ratios of death on the lower left of the vertical line in the middle of the graph) than published small trials demonstrating harmful effects of steroids (reflected by paucity of solid circles with positive log odds ratios of death on the lower right of the vertical line in the middle of the graph). (b) A jackknife sensitivity analysis examining the heterogeneity of the treatment effects of steroids on mortality in the group of trials published after 1997 is displayed. The individual trials are ordered on the X-axis by the change in I2 in when each trial is removed one at time. This analysis identified that eight studies increased heterogeneity (I2 increased; represented by the corresponding circle with the study name to the right of the vertical line at zero) and four studies decreased heterogeneity (I2 decreased; represented by the corresponding circle with the study name to the left of the vertical line at zero) when the individual study was removed. The two studies with the largest effects on the heterogeneity among these 12 trials are on the far left of the figure. When the CORTICUS trial [22] is removed, the I2 goes from 25% to 0%; when the trial Confalanieri et al. [18] is removed, I2 goes from 25% to 11%. Of note, these trials represent the largest randomized controlled trial and the trial with the largest beneficial treatment effect of steroids respectively. Circle size is proportional to overall study size.
Discussion
Since 2003, seven prospective randomized controlled trials have reported the effects of low-dose steroids on either shock reversal or mortality during sepsis. Previously, we identified five trials (published from 1997 to 2003) reporting the effects of low-dose steroids on either shock reversal or mortality during sepsis; these five trials demonstrated a consistent beneficial effect of low-dose steroids on both shock reversal and mortality [8,26,28,29,36,37]. Combined analysis of all 12 low-dose steroid trials published after 1997 demonstrated beneficial effects of low-dose steroids during sepsis. Similar to the findings of our previous analysis, low-dose steroids demonstrated a consistent improvement in shock reversal across the trials reporting this outcome. In contrast, heterogeneity in the effects of low-dose steroids on mortality increased to moderate levels in the current analysis. The differing effects of steroids on mortality across the 12 low-dose steroid trials may be explained, in part, by the influence of severity of illness on the treatment effects of steroids. An inverse linear relationship between severity of illness of the studied patient population and the effects of steroids on mortality was identified; low-dose steroids appear to increase mortality or have no effect in less severely ill patient populations, and to decrease mortality in more severely ill patient populations. Our current meta-analysis suggests that low-dose steroid therapy may reverse shock in most patients with septic shock, but may decrease mortality only in patients with more severe septic shock who are at high risk of death.
Previously, we identified nine trials examining the effects of steroids during sepsis published before 1989 [7]. Overall, these trials demonstrated a harmful effect of steroids on mortality after exclusion of a single outlier trial [33]. In our previous analysis, the different effects of steroids in trials published before 1989, as compared with trials published after 1997, could be explained, in part, by a linear relationship between steroid dose and treatment effect [7]. Our current analysis demonstrated similar differences between the nine trials published before 1989 and the 12 trials published after 1997, and confirmed the relationship between steroid dose and treatment effect. In the trials published before 1989, high-dose steroids were administered to decrease the excessive inflammation associated with sepsis. These earlier trials involved significantly shorter courses of higher-dose steroids, which may have led to harmful immunosuppression. In contrast, the trials published after 1997 involved longer courses of steroids, in a dose range equivalent to the amount of steroids produced by the body during a stressful state (c. 300 mg of cortisol per day), to treat critical illness-related corticosteroid insufficiency [38]. The lower doses of steroids administered in the more recent trials do not appear to be immunosuppressive, as evidenced by similar rates of secondary infections in the treatment and control groups in these trials. In addition, low-dose steroids did not lead to higher rates of other steroid-related adverse events, including gastrointestinal bleeding and neuropathy.
In our earlier meta-analysis, we were unable to identify a relationship between severity of illness and treatment effects [7]. At that time, we noted that this relationship may exist, but may not have been identified, secondary to the influence of drug dose, a limited range of severity of illness, or a lack of power. In the current analysis, there was no relationship between severity of illness and the effects of steroids on shock reversal; however, there was a relationship between severity of illness and the effects of steroids on mortality, both within the group of the trials published after 1997 and when examining all of the trials combined. The increased power and range of severity of illness provided by the addition of the seven studies published since 2003 allowed for the identification of this relationship in the current analysis. The relationship between severity of illness and treatment effect identified in this analysis is consistent with the reported effects of severity of illness on the treatment effects of other adjunctive therapies for sepsis, including mediator-specific anti-inflammatory agents and recombinant activated protein C, in both preclinical and clinical trials [6,39]. However, this relationship could not be reproduced prospectively in an antibiotic-treated mouse pneumonia model, which was used to investigate the effects of hydrocortisone over a wide range of risk of death [40].
Potential mechanisms responsible for the beneficial effects of low-dose steroids include an increase in vascular smooth muscle sensitivity to both exogenous and endogenous vasopressors, treatment of critical illness-related corticosteroid insufficiency, and attenuation of dysregulated inflammation and coagulation associated with sepsis [41,42]. The steroid-associated increase in vascular smooth muscle sensitivity may lead to improved shock reversal in patients with septic shock, regardless of their risk of death. However, similar to what occurs with other adjunctive therapies, the potential anti-inflammatory effects of low-dose steroids may explain their effects on mortality [6,39]. In severely ill patients who are likely to die from sepsis, attenuation of sepsis-associated dysregulated inflammation by low-dose steroids may decrease mortality. In contrast, in septic patients for whom the likelihood of survival is high, the potential anti-inflammatory and immunosuppressive effects of low-dose steroids may impair host defence mechanisms and lead to worse outcome.
Some authors have suggested that a patient’s response to ACTH stimulation testing should be used to determine which septic patients should receive steroids, on the basis of reports that only patients with a blunted response to ACTH stimulation testing derived benefit from steroid therapy [8]. However, other authors have challenged the reliability and reproducibility of the results of ACTH stimulation testing [43–46]. The current analysis demonstrates no difference in the effects of steroids on mortality according to the results of ACTH stimulation testing, and therefore does not support the use of ACTH stimulation testing to determine which septic patients should receive steroid therapy. This recommendation is consistent with a recent consensus statement for the use of steroids in sepsis from the American College of Critical Care Medicine, stating that “ACTH stimulation testing should not be used to identify those patients with septic shock … who should receive glucocorticoids” [38].
Conclusions regarding the benefits of low-dose steroids in sepsis derived from this analysis are limited by the potential influence of publication bias and by increased heterogeneity among the trials. The funnel plot and Egger’s regression suggest that a disproportionately high number of the published small trials demonstrated beneficial effects of steroids on mortality and may be influencing the estimate of the overall effect of low-dose steroids on mortality. In addition, the jack-knife sensitivity analysis suggests that the small trial by Confalonieri et al. disproportionately increases the heterogeneity within the group of low-dose trials [18]. This trial reported the largest decrease in mortality with steroids, and is fundamentally different from the others, considering that the enrolment criterion was pneumonia and not, specifically, sepsis. Removal of this trial does not substantially change the overall relationship between severity of illness and the effects of steroids on mortality.
The jack-knife sensitivity analysis also suggests that CORTICUS [22], the largest and most recent randomized controlled trial of low-dose steroids in sepsis, disproportionately increases the heterogeneity within the group of low-dose trials. This multicentre trial is the only study to report increased mortality with low-dose steroids during sepsis. Multiple issues surrounding the interpretation of the CORTICUS [22] results have been raised. First, according to the authors, this trial was terminated early, “due to a combination of slow enrollment, termination of funding, and time expiry of the drug”. The early termination decreased the power of the study to detect a treatment effect of steroids on mortality to <35% [47]. In addition, the power of this trial was also limited by a lower than expected control mortality rate (expected, 40%; actual, 31%). Furthermore, the slow enrolment and less severely ill patient population may have been due to a loss of equipoise during the study, secondary to the publication of treatment guidelines recommending low-dose steroid therapy for patients with vasopressor-dependent septic shock [48]. It is possible that clinicians chose to treat more severely ill septic patients with steroids rather than allow them to participate in the randomized controlled trial; this could account for the lower than expected control mortality rate in the trial, the slow enrolment, and an undetectable treatment effect. It is of note that the results of the CORTICUS trial are consistent with the relationship between severity of illness and the effects of steroids on mortality demonstrated in our analysis. In addition, removal of this trial does not substantially change the overall relationship between severity of illness and the effects of steroids on mortality. Interpreted together, these results suggest that low-dose steroids may improve survival in severely ill septic patients and that further clinical trials of low-dose steroids in septic patients with high mortality rates are warranted.
Conclusions
The effects of steroids in sepsis are dependent on both steroid dose and severity of illness. High-dose steroids during sepsis increase mortality. Low-dose steroids improve shock reversal during sepsis, independently of severity of illness; however, the effects of low-dose steroids on mortality appear to be dependent on severity of illness, with low-dose steroids decreasing mortality in more severely ill patients. Additional clinical trials with severely ill patients with refractory septic shock who require high-dose vasopressors are necessary to definitively determine the role of low-dose steroids during sepsis. Until such data are available, the decision to administer low-dose steroid therapy to a septic patient must be made on an individual basis, taking into account the patient’s severity of illness and the risk of adverse events due to therapy. ACTH stimulation testing should not be used to determine which patients should receive steroid therapy. The currently available data suggest that a 5–7-day course of hydrocortisone (200–300 mg daily, either in divided doses or as a continuous infusion), followed by a taper over 3–5 days, may reduce mortality in patients with septic shock who are at high risk of death (i.e. patients with refractory septic shock requiring high-dose vasopressors).
Footnotes
Transparency Declaration The authors have no conflicts of interest to report.
References
- 1.Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 4.Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney DA, Danner RL, Eichacker PQ, Natanson C. Once is not enough: clinical trials in sepsis. Intensive Care Med. 2008;34:1955–1960. doi: 10.1007/s00134-008-1274-6. [DOI] [PubMed] [Google Scholar]
- 6.Eichacker PQ, Parent C, Kalil A, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 7.Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141:47–56. doi: 10.7326/0003-4819-141-1-200407060-00014. [DOI] [PubMed] [Google Scholar]
- 8.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 9.Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998;351:47–52. doi: 10.1016/S0140-6736(97)08461-4. [DOI] [PubMed] [Google Scholar]
- 10.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 12.Garber EK, Targoff C, Paulus HE. Glucocorticoid preparations. In: Paulus HE, Furst DE, Droomgoole SH, editors. Drugs for rheumatic diseases. Churchill Livingstone; New York: 1987. p. 446. [Google Scholar]
- 13.Breslow NE, Day NE. The analysis of case–control studies. In: Davis W, editor. Statistical methods in cancer research. IARC Scientific Publications; Lyon, France: 1980. [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007;5:32–44. doi: 10.1186/1741-7015-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicarelli DD, Vieira JE, Bensenor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007;125:237–241. doi: 10.1590/S1516-31802007000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 19.Mussack T, Briegel J, Schelling G, Biberthaler P, Jochum M. Effect of stress doses of hydrocortisone on S-100B vs. interleukin-8 and polymorphonuclear elastase levels in human septic shock. Clin Chem Lab Med. 2005;43:259–268. doi: 10.1515/CCLM.2005.044. [DOI] [PubMed] [Google Scholar]
- 20.Oppert M, Schindler R, Husung C, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33:2457–2464. doi: 10.1097/01.ccm.0000186370.78639.23. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-dose hydrocortisone during severe sepsis: effects on microalbuminuria. Crit Care Med. 2006;34:2334–2339. doi: 10.1097/01.CCM.0000233872.04706.BB. [DOI] [PubMed] [Google Scholar]
- 22.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 23.Tandan SM, Guleria R, Gupta N. Low dose steroids and adrenocortical insufficiency in septic shock: a double-blind randomized controlled trial from India. American Thoracic Society Meeting. 2005;2 [Google Scholar]
- 24.Hinshaw L, Peduzzi P, Young E, et al. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. The Veterans Administration Systemic Sepsis Cooperative Study Group. N Engl J Med. 1987;317:659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 25.Bennett IL, Finland M, Hamburger M, Kass EH, Lepper M, Waisbren BA. The effectiveness of hydrocortisone in the management of severe infection. JAMA. 1963;183:462–465. [PubMed] [Google Scholar]
- 26.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–650. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 28.Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723–732. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Chawla K, Kupfer Y, Goldman I. Hydrocortisone reverses refractory septic shock. Crit Care Med. 1999;27:A33. [Google Scholar]
- 30.Klastersky J, Cappel R, Debusscher L. Effectiveness of betamethasone in management of severe infections. A double-blind study. N Engl J Med. 1971;284:1248–1250. doi: 10.1056/NEJM197106032842206. [DOI] [PubMed] [Google Scholar]
- 31.Lucas CE, Ledgerwood AM. The cardiopulmonary response to massive doses of steroids in patients with septic shock. Arch Surg. 1984;119:537–541. doi: 10.1001/archsurg.1984.01390170037008. [DOI] [PubMed] [Google Scholar]
- 32.Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 33.Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 35.Thompson WL, Gurley HT, Lutz BA, Jackson DL, Kyols LK, Morris IA. Inefficacy of glucocorticoids in shock (double-blind study) Clin Res. 1976;24:258A. [Google Scholar]
- 36.Yildiz O, Doganay M, Aygen B, Guven M, Keleutimur F, Tutuu A. Physiological-dose steroid therapy in sepsis [ISRCTN36253388] Crit Care. 2002;6:251–259. doi: 10.1186/cc1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004;329:480. doi: 10.1136/bmj.38181.482222.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 39.Eichacker PQ, Natanson C. Recombinant human activated protein C in sepsis: inconsistent trial results, an unclear mechanism of action, and safety concerns resulted in labeling restrictions and the need for phase IV trials. Crit Care Med. 2003;31:S94–S96. doi: 10.1097/00003246-200301001-00013. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Cui X, Li X, et al. Risk of death does not alter the efficacy of hydrocortisone therapy in a mouse E. coli pneumonia model: risk and corticosteroids in sepsis. Intensive Care Med. 2008;34:568–577. doi: 10.1007/s00134-007-0921-7. [DOI] [PubMed] [Google Scholar]
- 41.Collins S, Caron MG, Lefkowitz RJ. Beta-adrenergic receptors in hamster smooth muscle cells are transcriptionally regulated by glucocorticoids. J Biol Chem. 1988;263:9067–9070. [PubMed] [Google Scholar]
- 42.Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the alpha 1B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest. 1991;88:385–389. doi: 10.1172/JCI115315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725–3745. doi: 10.1210/jc.2006-0674. [DOI] [PubMed] [Google Scholar]
- 44.Bouachour G, Roy PM, Guiraud MP. The repetitive short corticotropin stimulation test in patients with septic shock. Ann Intern Med. 1995;123:962–963. doi: 10.7326/0003-4819-123-12-199512150-00018. [DOI] [PubMed] [Google Scholar]
- 45.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 46.Loisa P, Uusaro A, Ruokonen E. A single adrenocorticotropic hormone stimulation test does not reveal adrenal insufficiency in septic shock. Anesth Analg. 2005;101:1792–1798. doi: 10.1213/01.ANE.0000184042.91452.48. [DOI] [PubMed] [Google Scholar]
- 47.Finfer S. Corticosteroids in septic shock. N Engl J Med. 2008;358:188–190. doi: 10.1056/NEJMe0708098. [DOI] [PubMed] [Google Scholar]
- 48.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]