Abstract
Background
Exposure to pesticides has been reported to increase the risk of Parkinson disease (PD), but identification of the specific pesticides is lacking. Three studies have found elevated levels of organochlorine pesticides in postmortem PD brains.
Objective
To determine whether elevated levels of organochlorine pesticides are present in the serum of patients with PD.
Design
Case-control study.
Setting
An academic medical center.
Participants
Fifty patients with PD, 43 controls, and 20 patients with Alzheimer disease.
Main Outcome Measures
Levels of 16 organochlorine pesticides in serum samples.
Results
β-Hexachlorocyclohexane (β-HCH) was more often detectable in patients with PD (76%) compared with controls (40%) and patients with Alzheimer disease (30%). The median level of β-HCH was higher in patients with PD compared with controls and patients with Alzheimer disease. There were no marked differences in detection between controls and patients with PD concerning any of the other 15 organochlorine pesticides. Finally, we observed a significant odds ratio for the presence of β-HCH in serum to predict a diagnosis of PD vs control (odds ratio, 4.39; 95% confidence interval, 1.67–11.6) and PD vs Alzheimer disease (odds ratio, 5.20), which provides further evidence for the apparent association between serum β-HCH and PD.
Conclusions
These data suggest that β-HCH is associated with a diagnosis of PD. Further research is warranted regarding the potential role of β-HCH as a etiologic agent for some cases of PD.
Although Parkinson Disease (PD) may be caused by single gene mutations, genetic causes are rare, which suggests that the causation is complex and often involves environmental factors. The contention that factors other than genetics play a major role in the etiology of PD is further strengthened by twin studies1,2 that show that the concordance of PD is virtually identical in monozygotic and dizygotic twins. Epidemiologic studies3–8 further advance the potential role of environmental factors in PD by the identification of pesticide exposure as a risk factor. However, most of these studies have not identified specific pesticides that may contribute to PD.
Previous studies9–11 have reported an increased presence of organochlorine pesticides in the brain tissue of patients with PD. Two of the studies9,10 reported elevated levels of dieldrin in postmortem PD brain tissue vs that of controls, and 1 study11 reported elevated levels of lindane (γ-hexachlorocyclohexane [γ-HCH]) in the substantia nigra of patients with PD vs controls. Organochlorine pesticides were commonly used in the United States during the 1950s through 1970s before their use was restricted owing to concerns about bioaccumulation and toxic effects. Because of their high lipophilicity and resistance to degradation, these compounds are extremely persistent in the environment, which has resulted in the current contamination found worldwide.12 Organochlorine pesticides have also been demonstrated to be neurotoxic, to cause oxidative stress, and to damage the dopaminergic system in the rodent brain.13–17 The combination of their persistence in the environment and their potential to damage the dopamine system suggests that organochlorine pesticides may be significant contributors to the observed association between pesticide exposure and risk of PD. In this study, we sought to determine whether a diagnosis of PD is associated with the presence of organochlorine pesticides in the serum.
METHODS
STUDY PARTICIPANTS AND CLINICAL DEFINITION
Patients with PD were seen by a neurologist in the Clinical Center for Movement Disorders at the University of Texas Southwestern Medical Center (UTSWMC) between June 10,2002, and December 31, 2007. Patients had a diagnosis of probable PD based on the presence of 3 of the 4 cardinal features18 (bradykinesia, rigidity, resting tremor, and asymmetrical onset); the absence of atypical features, such as early dementia, falling or freezing, autonomic failure, supranuclear palsy, and hallucinations unrelated to medications; and the absence of conditions known to cause parkinsonism. Most (41 of 50) of the PD blood samples used for the present study were collected as part of another project, a prospective study19 of the effect of homocysteine on disease course in patients with early PD. The enrollment criteria for that study were a diagnosis of PD, as described previously; adequate cognitive capacity to provide informed consent, as judged by the physician investigator; the absence of neurologic or psychiatric comorbidities independent of PD that might significantly affect neuropsychological testing; and the ability to comprehend and participate in the English-language neuropsychometric testing. The sample size was increased by the use of a sample of patients seen at the Clinical Center for Movement Disorders at the UTSWMC who had PD according to the previous definition (n = 9; 18%). Scores on the Folstein Mini-Mental State Examination were available for 41 patients (median score, 28; range, 21–30). Eighteen patients with PD had blood samples collected 5 years apart, and these patients were evaluated for the persistence of pesticide levels across time.
Controls and patients with Alzheimer disease (AD) were evaluated in the Alzheimer Disease. Center at the UTSWMC. Blood samples were collected from patients with probable AD and control subjects routinely seen at the AD center. The AD and control blood samples were collected from individuals seen since 2002. Alzheimer disease was defined according to standard clinical diagnostic methods,20 and the Mini-Mental State Examination scores of these patients ranged from 1 to 29 (median, 23). For controls, the inclusion criteria were as follows: (1) a Mini-Mental State Examination score of 27 to 30 (median, 30), (2) no structural brain abnormalities indicated by magnetic resonance imaging or (3) normal general neurologic examination findings, (4) normal Consortium to Establish a Registry for Alzheimer’s Disease test battery findings, and (5) no PD-like symptoms based on the Unified Parkinson Disease Rating Scale. All the procedures were approved by the institutional review boards of the UTSWMC and the University of Medicine and Dentistry of New Jersey–Robert Wood Johnson Medical School.
SAMPLE COLLECTION AND EXPOSURE ANALYSIS
Blood samples were collected using standard venipuncture techniques. Serum was prepared by means of centrifugation at room temperature at 503 g for 10 minutes, aliquoted, labeled with a unique identification number, and stored at −80°C within 2 hours of phlebotomy and until used for the present study. All the samples were shipped frozen to the University of Medicine and Dentistry of New Jersey–Robert Wood Johnson Medical School and were analyzed in a masked manner regarding diagnosis.
The pesticides that were assayed were α-HCH; β-HCH; γ-HCH; δ-HCH; aldrin; dieldrin; endrin; endrin aldehyde; heptachlor; heptachlor epoxide; α-chlordane; γ-chlordane p,p′-DDE; 4,4′-DDD; p,p′-DDT; and methoxychlor. The compounds selected for assay were based on previous findings21 that those were the pesticides most commonly found in human samples and based on the compounds that the Environmental Protection Agency uses to assay organochlorines in biological samples. Serum pesticide levels were determined through the use of gas chromatography–mass spectrometry. Briefly, a ratio of 500 mg of urea to 1 mL of serum was added to serum samples (180–1400 µL), followed by sonication for 20 minutes. Serum samples were then passed through a solid-phase column to isolate the analytes. The eluate was passed through an Oasis HLB column (Waters Corporation; Milford, Massachusetts) for cleanup and preconcentration of the chlorinated analytes. The pesticides were eluted in methylene chloride, evaporated to dryness, and reconstituted in 50 µL of methylene chloride. Separation was performed using a gas chromatograph (Varian 3400; Varian Inc, Palo Alto, California) and a 30-m × 320-nm, 0.25-µm DB-XLB capillary column (Agilent Technologies; Foster City, California). Calibration curves containing the standards were run using the method for analyte quantitation that uses the integrated areas of the primary analyte ion (m/z = 181 for β-HCH and 246 for p,p′-DDE). A laboratory control sample extract from normal serum spiked with known concentrations of pesticide standards was run with every 10 samples to monitor for drift during the run (coefficient of variation = 7.8%; accuracy of the 16 measurements, ±2%–10%). Mass spectrometric analysis for unequivocal identification of the compounds was performed using a Varian 2000 ion trap mass spectrometer (Varian Inc) with MSn capabilities. We calculated the pesticide concentrations based on volume of serum, a criterion that has been demonstrated to be valid for studies involving human samples.22 We also performed a cholesterol assay on a subset of the samples and found no difference in the results when correcting using cholesterol values instead of volume of serum (data available on request), which suggests that differences in lipid status were unlikely to affect the results. The limit of detection for the pesticides assayed was 100 to 150 pg/mL.
STATISTICAL ANALYSIS
We used the χ2 test for analysis of categorical variables, including sex and pesticide detection with disease state, and Tukey-type multiple comparison tests were used for post hoc comparisons.23 Nonparametric analysis of variance (Kruskal-Wallis test) with post hoc nonparametric multiple comparison tests was used to compare disease states for pesticide levels. To ascertain the stability of the pesticide measurement across time (June 10, 2002, and December 31, 2007) in the same patients, we used the Wilcoxon signed rank test, and we assessed the correlation across time using the Spearman correlation coefficient. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated using logistic regression analysis, with β-HCH (pesticide present = 1), age, and sex (male = 1) as predictor variables for PD versus control status, and a similar analysis was used for PD versus AD status. Two analyses were used because we were interested in the ways that patients with PD compare with controls and patients with AD. To evaluate the fit of the model to the data, residuals were computed using a Hosmer-Lemeshow test. All analyses were performed using SAS version 9.1 (SAS Institute Inc; Cary, North Carolina).
RESULTS
Demographic characteristics of the participants are given in Table 1. There was no significant difference in the groups in terms of sex (χ2 = 4.3; P = .12); however, patients with PD were younger than those with AD and control subjects (H = 25.29; P < .001; nonparametric multiple comparison tests, P < .05).
Table 1.
Demographic Data for the 3 Study Groups
| PD Group (n = 50) |
AD Group (n = 20) |
Control Group (n = 43) |
|
|---|---|---|---|
| Age, mean (SD), y | 65.9 (8.5) | 76.1 (4.9) | 71.6 (7.4) |
| Sex, M/F, No. | 35/15 | 12/8 | 21/22 |
| Disease duration, mean (SD), y | 4.9 (3.7) | 5.6 (2.4) | NA |
Abbreviations: AD, Alzheimer disease; NA, not applicable; PD, Parkinson disease.
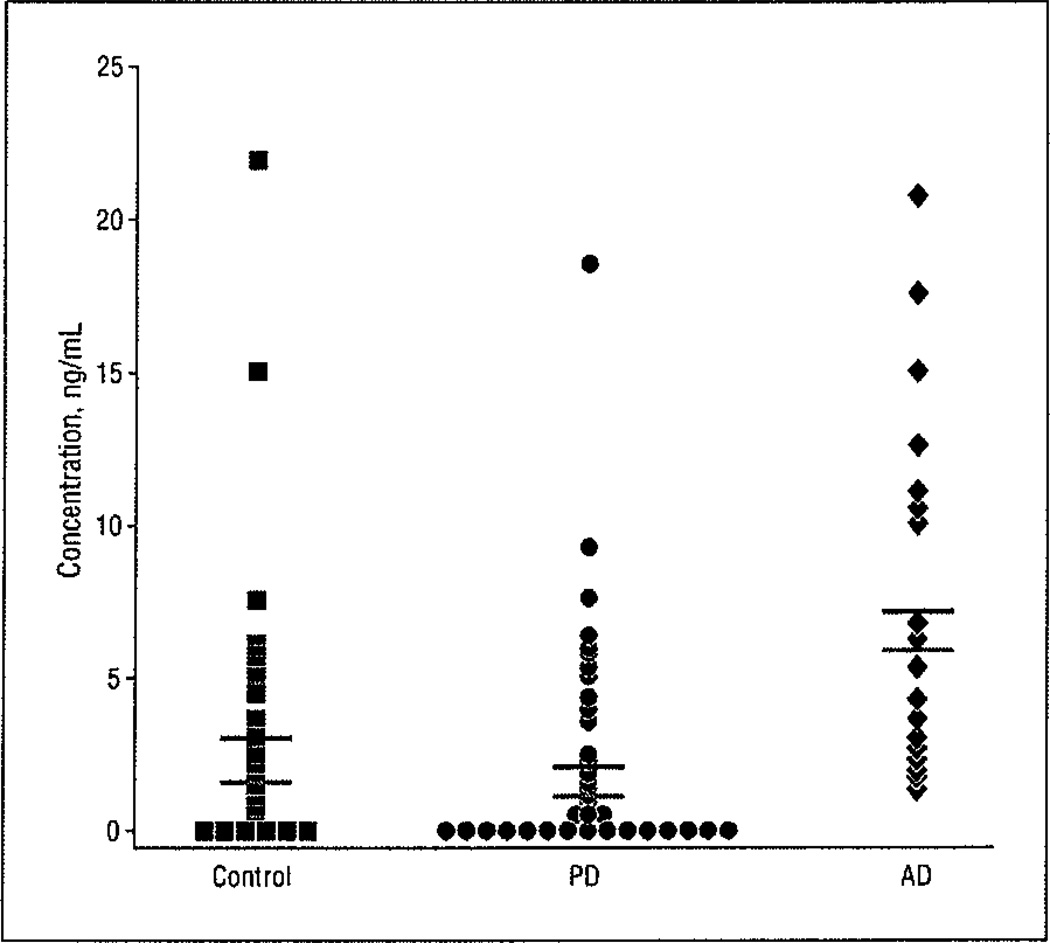
Only 9 of the 16 organochlorine pesticides were present in any of the samples (Table 2), and we chose to statistically analyze those that were detected in at least 50% of the patients with PD. The most frequently detected pesticide was p,p′-DDE; it was detected in 36 of 50 patients with PD (72%), in 37 of 43 controls (86%), and in all 20 patients with AD. The levels of p,p′-DDE were not the same in the 3 study groups (Kruskal-Wallis H = 21.31; P < .001), and nonparametric multiple comparison tests indicated that the pesticide level was higher in the AD group (median, 5.8 ng/mL; range, 1.29–20.74 ng/mL; mean [SEM], 7.1 [5.4] ng/mL) compared with the control group (median, 1.44 ng/mL; range, 0.2–21.85 ng/mL; mean [SEM], 2.66 [4.0] ng/mL) and the PD group (median, 1.06 ng/mL; range, 0.05–18.56 ng/mL; mean [SEM], 2.4 [4.6] ng/mL), with P < .05 for the 2 post hoc comparisons (Figure 1).
Table 2.
Detectable Serum Levels of Organochlorine Pesticides in the 3 Study Groups
| PD Group | Control Group | AD Group | ||||
|---|---|---|---|---|---|---|
| Patients, % | Detectable Range, ng/mL |
Patients, % | Detectable Range, ng/mL |
Patients, % | Detectable Range, ng/mL |
|
| β-HCH | 76 | 0.12–1.80 | 40 | 0.02–0.43 | 30 | 0.05–0.46 |
| p,p′-DDE | 72 | 0.05–18.56 | 86 | 0.2–21.85 | 100 | 1.29–20.74 |
| Methoxychlor | 26 | 0.14–1.62 | 23 | 0.09–0.59 | 0 | NA |
| α-HCH | 8 | 0.06–0.33 | 7 | 0.10–0.11 | 0 | NA |
| δ-HCH | 6 | 0.11–0.36 | 2 | 0.79 | 0 | NA |
| γ-HCH | 6 | 0.07–0.39 | 9 | 0.08–0.25 | 0 | NA |
| Heptachlor | 4 | 0.15–0.32 | 2 | 0.22 | 0 | NA |
| 4,4′-DDD | 4 | 0.12–0.47 | 5 | 0.25–0.59 | 35 | 0.88–2.39 |
| p,p′-DDT | 4 | 0.17–0.56 | 0 | NA | 0 | NA |
| Endrin aldehyde | 0 | NA | 0 | NA | 0 | NA |
| Endrin | 0 | NA | 0 | NA | 0 | NA |
| Dieldrin | 0 | NA | 0 | NA | 0 | NA |
| Heptachlor epoxide | 0 | NA | 0 | NA | 0 | NA |
| Aldrin | 0 | NA | 0 | NA | 0 | NA |
| γ-Chlordane | 0 | NA | 0 | NA | 0 | NA |
| α-Chlordane | 0 | NA | 0 | NA | 0 | NA |
Abbreviations: AD, Alzheimer disease; HCH, hexachlorocyclohexane; NA, not applicable; PD, Parkinson disease.
Figure 1.
Serum levels of p,p′-DDE are similar in controls and patients with Parkinson disease (PD) but are significantly higher in patients with Alzheimer disease (AD). Black bars indicate the mean values; red bars, the median values.
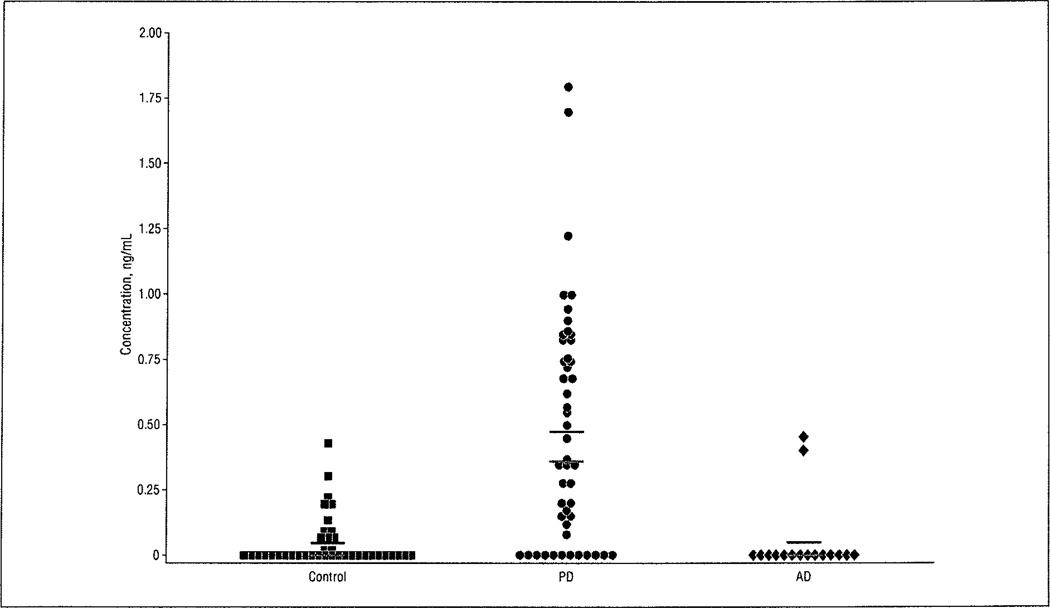
The organochlorine pesticide β-HCH was detected in the serum of patients with PD most often among the 3 groups; 38 of 50 patients with PD (76%) had detectable levels of β-HCH compared with 17 of 43 controls (40%) and 6 of 20 patients with AD (30%) (χ2 = 18.01; P < .001). Using Tukey-type multiple comparison tests, significantly more patients with PD had detectable β-HCH levels than either controls or patients with AD (P < .05 for each). In addition to a greater frequency of detection in patients with PD, β-HCH levels were not the same in the 3 groups (Kruskal-Wallis H = 32.13; P < .00.1) (Figure 2). The median level of β-HCH is markedly higher in patients with PD (median, 0.36 ng/mL; range, 0.12–1.80 ng/mL; mean [SEM], 0.47 [0.06] ng/mL) compared with controls (median, 0 ng/mL; range, 0.02–0.43 ng/mL; mean [SEM], 0.05 [0.02] ng/mL), and it is also significantly higher than in patients with AD (median, 0 ng/mL; range, 0.05–0.46 ng/mL; mean [SEM], 0.06 [0.13] ng/mL), but the levels in patients with AD and controls are not different (based on post hoc testing, P < .05), suggesting that elevated levels of β-HCH are specific to PD in this sample.
Figure 2.
Patients with Parkinson disease (PD) have higher serum β-hexachlorocyclohexane (β-HCH) levels than controls and patients with Alzheimer disease (AD). Levels of β-HCH in patients with AD are not significantly different from those in controls, which indicates that elevated levels of β-HCH are specific to PD in this sample. Black bars indicate the mean values; red bars, median values.
We also measured the levels of β-HCH in samples from 18 of the patients with PD taken at 2 different points in time, 5 years apart, to ascertain the stability of the pesticides across time in the same patients. Levels of β-HCH were not significantly different between the 2 sampling points (Wilcoxon t = 44.0; P > .05). However, the levels were not related in a systematic manner among the patients (Spearman rho = −0.072; P = .80).
We used logistic regression to determine whether β-HCH could differentiate between patients with PD and control subjects, adjusting for age and sex, and a separate analysis was performed between patients with PD and those with AD. For the PD vs control analysis, the OR estimates (95% CIs) for the 3 variables were as follows: β-HCH, 4.39 (1.67–11.6); sex, 3.52 (1.29–9.66); and age, 0.91 (0.85–0.97). This model demonstrated a strong association between serum β-HCH level and PD status and an effect for sex and age. The goodness of fit of the model was tested using the Hosmer-Lemeshow test (χ2 = 4.66; P = .79), and the fit was very good. For the PD vs AD analysis, the OR estimates (95% CIs) for the 3 variables were as follows: β-HCH, 5.20 (1.29–20.98); sex, 2.35 (0.50–9.66); and age, 0.77 (0.66–0.90). This model demonstrated a strong association between serum β-HCH level and PD status and an effect for sex and age. The goodness of fit of the model was tested using the Hosmer-Lemeshow test (χ2 = 2.02; P = .98), and the fit was excellent.
COMMENT
This study demonstrates that an elevated serum level of the organochlorine pesticide β-HCH is associated with a diagnosis of PD. This finding is of particular interest given the recent publication of a study24 from the Faroe Islands, which reported a small but significant association between serum β-HCH and PD. The Faroe Islands population is highly exposed to organochlorine pesticides and other persistent organic pollutants based on their diet,25 and they exhibit a prevalence of PD approximately twice as high as expected.26 A higher rate of PD also has been found among the Inuit in Greenland,27 who are likewise exposed to high levels of organochlorine pesticides through a diet similar to that in the Faroe Islands. The data from the Faroe Islands study24 reported mean serum β-HCH concentrations of 0.04 and 0.05 µg/g of lipid for controls and patients with PD, respectively. Based on the report from that study of a mean lipid concentration of 10 g/L, the mean values for controls and that for the patients with PD were 0.5 and 0.6 ng/mL, respectively. These values are similar to the median values found in patients with PD in the present study (0.36 ng/mL). However, our calculated OR for PD (vs control) risk based on β-HCH levels is much higher than that reported in the Faroe Islands study (4.39 vs 1.44). The similar levels of serum β-HCH in controls and patients with PD in the Faroe Islands are likely a significant contributor to the differences in the calculated risk factors. This possibility is further supported by the finding that the highest quartile of β-HCH levels in the present study contained only patients with PD. Taken together, these data suggest that high levels of β-HCH may be an important risk factor for PD.
In the United States, β-HCH levels have been detected in varying percentages of the population for the past few decades.28–30 The results of the 1979 National Human Adipose Tissue Survey estimated that almost 92% of the population had detectable levels of β-HCH,30 a finding that suggests that exposure to β-HCH was wide-spread in the United States. For individuals who were 25 to 44 years of age during this time frame, which would be most representative of samples analyzed in the present study, there was a median serum value of 11.7 ng/mL.30 This value is much higher than that found in the present study and may be indicative of the fact that the samples from the Second National Health and Nutrition Examination Survey (NHANES II) were taken during the time that HCH use was just starting to be restricted and high environmental levels of the chemical were still present. More recent data from the NHANES (1999–2000) revealed that the mean serum level of β-HCH in the US population 20 years and older was 0.07 ng/g.31 With the assumption of a weight of 1.02 g/mL of serum, this would result in a mean value of 0.058 ng/mL, which is similar to the mean (SD) value found in control patients in the present study (0.05 [0.02] ng/mL) (Figure 2).
We also found β-HCH in some controls, although generally at much lower levels than the median level found in patients with PD (0.36 ng/mL). This result suggests that there are other factors that may interact with β-HCH exposure to alter the risk of PD. For example, caffeine consumption and cigarette smoking have been associated with a decreased risk of PD.32 For patients with PD, it is possible that genetic polymorphisms in enzymes that metabolize β-HCH may contribute to their high levels of β-HCH. A few studies33–35 have found that genetic polymorphisms in drug-metabolizing enzymes interact with pesticide exposure to cause an increased risk of PD. The present data that compares serum levels in the same patients 5 years apart provides some support for the gene polymorphism contention. Because we found no difference in β-HCH levels across time in the samples taken at the 2 time points (June 10, 2002, and December 31, 2007) and the half-life of β-HCH is approximately 7 to 8 years, these data could suggest that there is decreased clearance of β-HCH in these patients. Future studies with DNA samples and demographic data on smoking and caffeine consumption from the patients in the present study will provide a powerful mechanism for the determination of whether the elevated serum β-HCH levels are the result of genetic, polymorphisms in 1 or more enzymes involved in the metabolism of β-HCH or environmental factors.
In data from the NHANES II, increasing age, residence on a farm, and male sex conferred increased risks of exposure to β-HCH,36 all of which have also been associated with a higher risk of PD. In the present study, sex (male > female) and age (lower levels with age) were only mildly associated with β-HCH levels and PD. Ascherio and coworkers6 found no interaction with pesticide exposure and sex as it pertained to risk of PD, but Petersen and coworkers24 found that women had higher mean levels of β-HCH than did men for controls (0.05 vs 0.04 µg/g) and patients with PD (0.08 vs 0.04 µg/g). Future studies with larger sample sizes are needed to rigorously explore the relationship of β-HCH concentrations with demographic variables.
We also found that levels of p,p′-DDE are significantly higher in patients with AD vs patients with PD and controls and that 4,4′-DDD is detected in 35% of patients with AD vs 5% or fewer in the controls and patients with PD. Although there have been many fewer studies published that explore the relationship between pesticide exposure and risk of AD, Baldi and coworkers37 found an adjusted risk ratio of 2.39 in men occupationally exposed to pesticides. Fleming and coworkers9 found that p,p′-DDT was more likely to be found in AD brain tissue than in the brain tissue of those with PD or of control samples. Because the present data and those from the study by Fleming et al involve small numbers of patients with AD (20 in the present study), further studies with larger sample sizes are warranted to determine whether exposure to certain pesticides increases the risk of AD.
Although this study indicates that elevated serum β-HCH levels are associated with a diagnosis of PD, there are some limitations. First, these analyses were restricted to patients seen at the UTSWMC, which could suggest that the results may simply be due to the sampling of a highly exposed patient population and that those results may not generalize to other areas. However, the ubiquitous global contamination pattern of β-HCH38 and the findings from the National Human Adipose Tissue Survey and the NHANES II suggest that it is unlikely that there is something unique about this patient population. The recent study from the Faroe Islands21 that reports a significant association between β-HCH and PD further suggests that the present results generalize to other populations, although this remains to be established in future studies. Also, some patients with PD (12 of 50) have nondetectable levels of β-HCH, which suggests that exposure to β-HCH may contribute to PD only in a subset of cases, a hypothesis that is consistent with the multifactorial nature of PD. Second, we cannot definitively state that β-HCH causes PD; β-HCH is a persistent ingredient in technical-grade lindane (γ-HCH),12 but both isomers of HCH have been demonstrated to cause oxidative stress and to reduce brain concentrations of dopamine in animals.13,16 Finally, our measures were taken from peripheral blood and may not accurately reflect the concentration of the pesticide in the brain. Because of its longer half-life,12 it is possible that β-HCH is a marker for exposure to other HCH components, such as γ-HCH, which causes dopaminergic toxicity. Indeed, elevated levels of γ-HCH have been found in postmortem substantia nigra samples from patients with PD in I small study.11 The finding that β-HCH levels accumulate to a greater extent in the blood than in the brain compared with those of γ-HCH12 provide further support for this possibility. However, no studies to date have been conducted with matched brain and blood samples from controls and patients with PD. Future studies involving such matched samples would aid in the clarification of this relationship.
In summary, these findings support epidemiology studies that associate exposure to pesticides with increased risk of PD, and we identified a specific pesticide that is linked with the diagnosis of PD. It is possible that elevated levels of β-HCH may be a useful clinical measure to identify people who may have an increased risk of PD, particularly when combined with information about genetic polymorphisms in genes that metabolize organochlorine pesticides. This is particularly important because by the time patients with PD are diagnosed, neurodegeneration has often progressed to a point where neuroprotective strategies are largely ineffective. Thus, determination of those who may be at risk for PD based on pesticide exposure and genetic information may allow for early detection and more efficacious treatments aimed at slowing or preventing further dopaminergic neurodegeneration.
Acknowledgments
Funding/Support: This study was supported in part by grant P30ES005022 from the National Institute of Environmental Health Sciences, grant P30-AG012300 from the National Institute on Aging, the Dallas Area Parkinsonism Society, Rowe & Co Inc, the Dallas Foundation, and the Michael J. Fox Foundation for Parkinson’s Research. Opinions, interpretations, conclusions, and recommendations are those of the authors.
Footnotes
Author Contributions: Drs Richardson and German had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Richardson, Diaz-Arrastia, and German. Acquisition of data: Richardson, Buckley, Winnik, O’Suilleabhain, Diaz-Arrastia, and German. Analysis and interpretation of data: Richardson, Shalat, Buckley, Reisch, and German. Drafting of the manuscript: Richardson, Shalat, Buckley, Winnik, German, and O’Suilleabhain. Critical revision of the manuscript for important intellectual content: Richardson, Shalat, O’Suilleabhain, Diaz-Arrastia, Reisch, and German. Statistical analysis: Shalat, Reisch, and German. Obtained funding: Richardson, Diaz-Arrastia, and German. Administrative, technical, and material support: Richardson, Buckley, Winnik, and O’Suilleabhain. Study supervision: Richardson.
Financial Disclosure: None reported.
REFERENCES
- 1.Tanner CM, Ottman R, Goldman SM, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281(4):341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 2.Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63(2):305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- 3.Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to rural environmental factors: a population based case-control study. Can J Neurol Sci. 1991;18(3):279–286. doi: 10.1017/s0317167100031826. [DOI] [PubMed] [Google Scholar]
- 4.Le Couteur DG, McLean AJ, Taylor MC, Woodham BL, Board PG. Pesticides and Parkinson's disease. Biomed Pharmacother. 1999;53(3):122–130. doi: 10.1016/S0753-3322(99)80077-8. [DOI] [PubMed] [Google Scholar]
- 5.Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21(4):435–440. [PubMed] [Google Scholar]
- 6.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60(2):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 7.Kamel F, Tanner CM, Umbach DM, et al. Pesticide exposure and self-reported Parkinson's disease in the Agricultural Health Study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 8.Hancock DB, Martin ER, Mayhew GM, et al. Pesticide exposure and risk of Parkinson's disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36(1):100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol. 1998;150(2):339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- 11.Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59(4):229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Toxic Substances and Disease Registry. Toxicological profile for hexachlorocyclohexane. [Accessed August 12, 2008]; http://www.atsdr.cdc.gov/toxprofiles/tp43.html. [PubMed]
- 13.Ortiz Martinez A, Martinez-Conde E. The neurotoxic effects of lindane at acute and subchronic dosages. Ecotoxicol Environ Saf. 1995;30(2):101–105. doi: 10.1006/eesa.1995.1011. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Exp Neurol. 1998;150(2):263–271. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- 15.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson's disease pathogenesis. Neurotoxicology. 2005;26(4):701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava A, Shivanandappa T. Hexachlorocyclohexane differentially alters the antioxidant status of the brain regions in rat. Toxicology. 2005;214(1–2):123–130. doi: 10.1016/j.tox.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Hatcher JM, Richardson JR, Guillot TS, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204(2):619–630. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 19.O'Suilleabhain PE, Sung V, Hernandez C, et al. Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations. Arch Neurol. 2004;61(6):865–868. doi: 10.1001/archneur.61.6.865. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Struciński P, Ludwicki JK, Góralczyk K, et al. Organochlorine pesticides residues in human breast adipose tissue in Poland. Cent Eur J Public Health. 2000;8(suppl):25–26. [PubMed] [Google Scholar]
- 22.Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113(7):853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar JH. Biostatistical Analyses. 4th ed. Upper Saddle River, NJ: Prentice Hall Publishing; 1999. pp. 563–564. [Google Scholar]
- 24.Petersen MS, Hailing J, Bech S, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology. 2008;29(4):584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Deutch B, Hansen JC. AMAP Greenland and the Faroe Islands 1997–2001. Copenhagen, Denmark: Danish Environmental Protection Agency; 2003. [Google Scholar]
- 26.Wermuth L, Joensen P, Bunger N, Jeune B. High prevalence of Parkinson's disease in the Faroe Islands. Neurology. 1997;49(2):426–432. doi: 10.1212/wnl.49.2.426. [DOI] [PubMed] [Google Scholar]
- 27.Wermuth L, Pakkenberg H, Jeune B. High age-adjusted prevalence of Parkinson's disease among Inuit in Greenland. Neurology. 2002;58(9):1422–1425. doi: 10.1212/wnl.58.9.1422. [DOI] [PubMed] [Google Scholar]
- 28.Murphy RS, Kutz FW, Strassman SC. Selected pesticide residues or metabolites in blood and urine specimens from a general population survey. Environ Health Perspect. 1983;48:81–86. doi: 10.1289/ehp.834881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy R, Harvey C. Residues and metabolites of selected persistent halogenated hydrocarbons in blood specimens from a general population survey. Environ Health Perspect. 1985;60:115–120. doi: 10.1289/ehp.8560115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutz FW, Strassman SC. Trends of organochlorine pesticide residues in human tissue. In: Han MAQ, Stanton RH, editors. Toxicology of Halogenated Hydrocarbons: Health and Ecological Effects. New York, NY: Pergamon Press; 1981. pp. 38–49. [Google Scholar]
- 31.Needham LL, Barr DB, Caudill SP, et al. Concentrations of environmental chemicals associated with neurodevelopmental effects in U.S. population. Neurotoxicology. 2005;26(4):531–545. doi: 10.1016/j.neuro.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 33.Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson’s disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352(9137):1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- 34.Elbaz A, Levecque C, Clavel J, et al. CYP2D6 polymorphism, pesticide exposure, and Parkinson's disease. Ann Neurol. 2004;55(3):430–434. doi: 10.1002/ana.20051. [DOI] [PubMed] [Google Scholar]
- 35.Fong CS, Wu RM, Shieh JC, et al. Pesticide exposure on southwestern Taiwanese with MnSOD and NQ01 polymorphisms is associated with increased risk of Parkinson's disease. Clin Chim dcfa. 2007;378(1–2):136–141. doi: 10.1016/j.cca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Stehr-Green PA. Demographic and seasonal influences on human serum pesticide residue levels. J Toxicol Environ Health. 1989;27(4):405–421. doi: 10.1080/15287398909531312. [DOI] [PubMed] [Google Scholar]
- 37.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157(5):409–414. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- 38.Li YF, Scholtz MT, Van Heyst BJ. Global gridded emission inventories of β-hexachlorocyclohexane. Environ Sci Technol. 2003;37(16):3493–3498. doi: 10.1021/es034157d. [DOI] [PubMed] [Google Scholar]