1. Introduction
Magnetic resonance spectroscopy (MRS) is a powerful methodology that allows the direct detection of endogenous metabolites in the human body non-invasively in vivo. Although historically most often applied within a clinical context, there is increasing uptake (as there is with structural and functional MR imaging) of MRS to study the healthy brain. Although the principal inhibitory neurotransmitter in the human brain GABA, or γ-aminobutyric acid (H3N+CH2CH2CH2COO−), is only present in the human brain at millimolar levels, it is possible to measure GABA concentration with MRS, usually by tailoring the MRS experiment specifically to isolate GABA signals from the spectrum. This review will summarize the MRS methods proposed for detecting GABA, and address their various applications to date.
Although the majority of cortical neurons are glutamatergic, approximately one sixth of cortical neurons are GABAergic inter-neurons [1]. There is wide interest within the clinical and basic neuroscience community in studying the role of inhibitory processes in normal brain function and the pathophysiology of disease. Both GABA receptors and transporters have been well characterized, and associated genes have been implicated by genetic studies of schizophrenia and bipolar disorder. Two main sub-types of GABA receptor, GABAA and GABAB, mediate the synaptic effects of GABA, and synaptic GABA is rapidly removed by high-affinity GABA transporters (GAT). GABA is produced from glutamate by glutamic acid decarboxylase (GAD) within GABAergic neurons and is metabolised to succinic acid semialdehyde by GABA transaminase (GABA-T) and thence to succinate, primarily within astrocytic mitochondria [2].
Among a wide variety of methods for studying GABA and GABAergic processes, the only technique that allows the direct, non-invasive detection of endogenous GABA in vivo in the brain is magnetic resonance spectroscopy (MRS). The purpose of this review is to introduce MRS methods that detect GABA in vivo, and to summarize the existing published literature applying these methods.
2. Methodological summary
2.1. In vivo 1H magnetic resonance spectroscopy
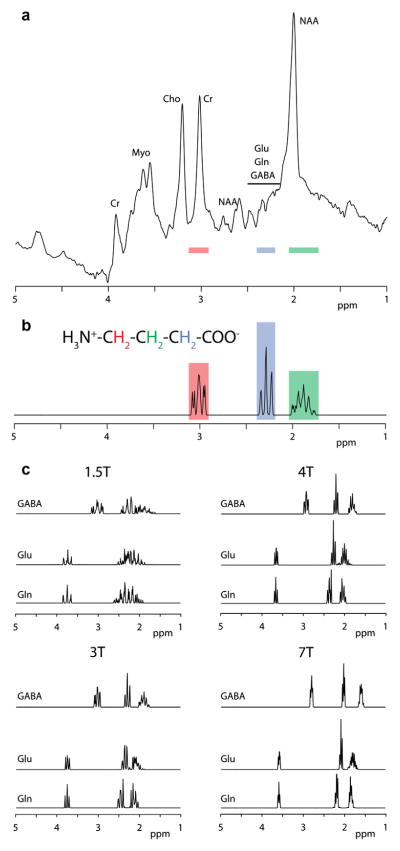
Magnetic resonance spectroscopy (MRS) is the in vivo application of nuclear magnetic resonance (NMR) spectroscopy, and is implemented on magnetic resonance imaging (MRI) scanners. 1H MRS detects radiofrequency signals that arise from hydrogen nuclear spins within tissue metabolites, and these signals have chemically specific frequencies, determined by the chemical environment of the hydrogen spins. Thus, MRS signals are separated in the MR spectrum along chemical lines, in what is known as the chemical shift dimension. Fig. 1a shows the MR spectrum acquired from an occipital region of the human brain, showing signals associated with a number of metabolites, most prominent among them being N-acetyl aspartate (NAA), creatine- (Cr) and choline-containing compounds (Cho). The spectrum is a plot of signal intensity (roughly proportional to metabolite concentration) against chemical shift. Chemical shift is reported in field-independent units, ppm (or parts per million of the proton frequency), so that NAA, for example, always gives a signal at 2.0 ppm even though signals are acquired at close to 64 MHz in a 1.5T scanner and 128 MHz in a 3T scanner.
Fig. 1.
MR spectra of GABA. (a) 1H MR spectrum of the human brain acquired at 3T, showing peaks corresponding to N-acetyl aspartate (NAA), creatine-containing compounds (Cr), choline-containing compounds (Cho), Myoinositol (Myo), glutamate (Glu), glutamine (Gln) and GABA. Coloured bars correspond to the simulated peak locations in (b). (b) Simulated MR spectrum of GABA at 3T, showing the assignments to the GABA CH2 spins. (c) Simulated MR spectra of GABA, Glu and Gln at a range of field strengths.
In order to perform useful MRS experiments, it is necessary to localize the MR signal acquired to a particular region in the body, either by exciting signals only within a specific volume (or voxel), known as single-voxel MRS or by performing a hybrid MRS and imaging experiment, known as magnetic resonance spectroscopic imaging (MRSI, also referred to as chemical shift imaging, CSI). Single-voxel spectra are usually acquired using either the Point-RE-Solved Spectroscopy (PRESS) [3] or Stimulated Echo Acquisition Mode (STEAM) [4] methods.
Since it is well-known which metabolites are present in the brain, the MR spectrum from a given region can be analysed as a linear combination of basis spectra, using tools such as the LCModel [5], in order to measure metabolite concentrations. The success of this kind of analysis is affected heavily by the quality of data – the signal-to-noise ratio (SNR) of signals increases linearly with the measurement volume and the square root of acquisition time, and the width of signals (and thus their resolution from adjacent signals) depends on the homogeneity of the magnetic field within the measurement volume. Therefore, a typical single-voxel measurement might be made from a volume of 8 cm3 in about 5 min, after a preparation step to optimize the field homogeneity known as shimming.
The spectrum in Fig. 1a exhibits a fundamental problem of in vivo 1H MRS, especially as applied to GABA – the dispersion of different signals along the chemical shift axis is limited in comparison with the linewidth of signals (even with good shimming). Therefore signals from different metabolites often overlap, and signals from more abundant metabolites often obscure those from less abundant metabolites such GABA.
2.2. Coupling in MRS
One common feature of the MR spectrum is the appearance of multiplets, signals associated with a single hydrogen environment that are split into a number of sub-peaks (see for example Fig. 1b). This phenomenon arises because of spin–spin coupling, or J-coupling, whereby the field experienced by a spin is affected by adjacent spins within the molecule. The splittings due to coupling result in signals that have lower peak intensity (in terms of signal height) and a broader footprint along the chemical shift axis, both of which make coupled species such as GABA and glutamate harder to detect and quantify.
2.3. MRS of GABA
GABA is present in the human brain at a concentration of about 1 mM, which is an order of magnitude lower than some of the more concentrated metabolites and ~40,000 times lower than water. The chemical structure and MR spectrum of GABA is shown in Fig. 1b; the three different multiplets correspond to the three methylene (CH2) groups in the molecule. Comparison with the spectrum in Fig. 1a reveals that these signals are overlapped by more intense signals arising from the more abundant metabolites NAA at 2 ppm, Cr at 3 ppm and glutamate (Glu) and glutamine (Gln) at 2.3 ppm (Total Glu + Gln is often referred to as Glx). Fig. 1c compares the MR spectra of GABA, Glu and Gln at a range of field strengths, further demonstrating that at field strengths of 3T and below, differentiation between these compounds becomes difficult.
Faced with the limited dispersion of signals along the chemical shift axis, there are three possible approaches to reducing signal overlap in order to resolve GABA signals. Firstly, it is possible to reduce the information content in the spectrum by applying one of a series of spectral editing methods. Alternatively, overlap between signals can be alleviated by spreading signals out into a second frequency dimension in two-dimensional MRS methods. The third approach is to move to higher field strength, since the relative width of multiplets (in ppm) scales inversely with field strength (as seen in Fig. 1c). Each of these approaches will be considered in turn.
2.4. Edited detection of GABA
Although the coupling between the GABA spins results in wider signals with lower intensity, hindering detection, it can also be used to advantage to separate GABA signals from the rest of the spectrum. The GABA signal at 3.0 ppm is coupled to the signal at 1.9 ppm, whereas the majority of the signals at 3.0 ppm are not coupled to signals at 1.9 ppm; therefore it is possible to separate the signals on this basis. A frequency-selective pulse which only directly affects signals close to 1.9 ppm can be added to the MRS experiment. This will also have an indirect effect (mediated by the coupling) on GABA signals at 3.0 ppm, because these GABA signals at 3.0 ppm are coupled to the GABA signals at 1.9 ppm. Such a pulse will have no effect on other signals at 3 ppm, because they are not coupled to spins close to 1.9 ppm. If two experiments are performed, with and without this frequency-selective pulse (referred to as the editing pulse), the difference will give a spectrum that only contains those signals that are affected by the pulse (the edited spectrum). Thus, the edited spectrum contains both those signals close to 1.9 ppm that appear trivially due to the direct effect of the pulse, as well as those signals remote from 1.9 ppm that only appear due to couplings between spins. This editing approach, referred to as J-difference editing, is used by both the first proposed method for specifically detecting GABA signals [6], and the method most widely applied currently, the MEGA-PRESS method [7]. Edited detection of GABA has been validated by correlation with chromatographic measurements of GABA [8].
One disadvantage of such J-difference methods is their reliance upon subtraction to remove the strong overlapping signals from the spectrum. Any instability in the experiment, whether caused by instrumental factors or subject movement, will result in subtraction artefacts that can obscure the intended edited GABA signals. For this reason, several experimental editing methods have been proposed which rely upon exciting multiple quantum coherences, which are conceptually preferable to difference methods since they suppress overlying signals within a single scan and do not rely upon subtraction, although often at a cost. Difference methods are usually acquired in an interleaved fashion and post-processing phase- and frequency-corrections are applied [9], both of which steps mitigate the impact of instabilities during the measurement.
2.5. Two-dimensional MRS
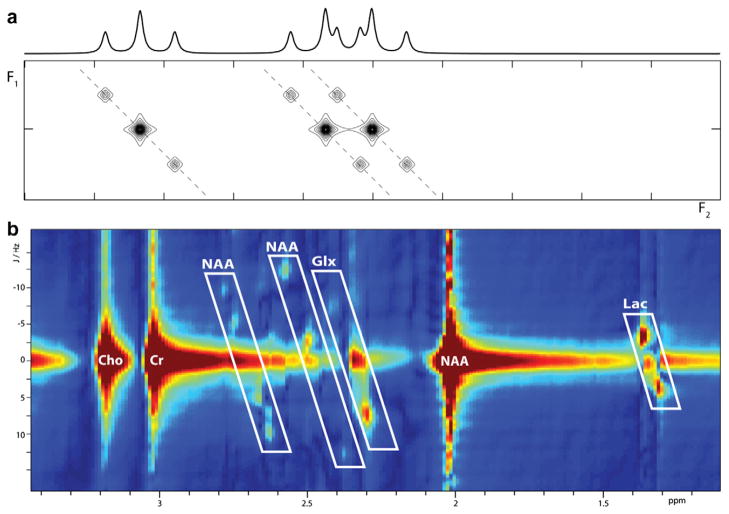
As the name suggests, rather than plotting signals along a single frequency axis, two dimensional MR spectra plot signals against two frequency axes, as seen in Fig. 2. Experimentally, this is achieved by acquiring a series of experiments that differ by a single incremented parameter, such as the duration of a delay or spin echo, referred to as t1. In J-resolved methods [10], the directly acquired dimension F2 contains both chemical shift and coupling information (as in the one-dimensional spectrum) and the indirectly acquired dimension F1 only contains the coupling information. Thus as seen in Fig. 2a, a multiplet, in this case a triplet, will be slanted diagonally into the second dimension rather than appearing as a series of adjacent peaks in a one-dimensional spectrum. This may allow resolution of overlapping multiplets with different chemical shifts, as seen on the right in Fig. 2a.
Fig. 2.
Comparison of multiplets in 1D spectrum and 2DJ spectrum at 3T showing (a) an isolated triplet, and separation of two overlapping triplets and (b) J-PRESS spectrum acquired at 3T.
Other two-dimensional approaches give an indirect dimension that contains both coupling and chemical shift information, such as COSY-based methods [11] in which off-diagonal cross peaks are caused by couplings between spins, or the frequency of double-quantum coherences excited during the experiment. In general, the evolution that occurs during t1 can be manipulated to determine the information content of the second dimension.
One problem with two-dimensional methods is the richness of the information acquired. In contrast to edited one-dimensional spectra, two-dimensional spectra typically contain peaks from all observable metabolites (albeit with reduced overlap due to expansion into a second dimension). If GABA is the principal metabolite of interest, it is common to compress the information back down to a one-dimensional spectrum prior to analysis and either extract the F2 = 7.5 Hz row e.g. [12] or the F2 = 0 Hz row (mathematically equivalent to TE-averaging [13], as described in Section 3.2.2) to quantify GABA signal. More computationally intensive approaches extend the LCModel linear fitting approach to fitting the full two-dimensional spectrum; for example ProFit [14] fits a 2D J-spectrum with a set of 2D metabolite basis spectra.
2.6. High Field MRS
In vivo human scanners at field strengths greater than 3T have been used in a small number of sites with considerable hardware expertise for a considerable time. However more recently a growing number of sites have installed 7T scanners produced by the three major manufacturers, and such magnets are likely to be more widely used to quantify GABA in the future. Methodological considerations at high field are extensively covered in reference [15]. As seen in Fig. 1, one advantage of high field (in addition to the primary motivation of increased SNR) is the ‘tightening’ of the multiplets from the metabolites, which occurs because chemical shift differences between peaks remain at a constant number of ppm moving from one field to another, while splittings due to couplings maintain a constant value in hertz but have a reduced value in ppm. A further advantage is the increasing frequency selectivity of edited experiments carried out at high field [16]. An editing pulse with bandwidth of 90 Hz applied at 7T will excite a narrower range of the spectrum (90 Hz ~ 0.3 ppm) than at 3T (90 Hz ~ 0.7 ppm) resulting in far less contamination of the spectrum with unwanted co-editing macromolecular contributions [17].
3. Literature review
Articles discussed in this review are classified into the following categories: methods; basic neuroscience applications; pharmacological applications; and clinical applications.
3.1. Literature review methods
Primary search terms applied to PubMed on April 14 2010 were ‘GABA MRS’ (196 results), ‘GABA editing’ (60 results). Additional searches were made based on known high profile authors (such as ‘GABA Rothman’ – 90 results) and methods (such as ‘GABA MEGA-PRESS’ – 12 results). These search results were filtered to remove: all references that do not use MRS; all studies of non-human subjects; all studies not carried out in vivo; all studies of organs other than the brain. The scope of this review was also restricted to only cover ‘second generation’ methods as applied to the detection of GABA. The classification ‘second generation’ is intended to exclude those methods that simply localize signals (whether in single voxel or MRSI experiments), and to include those methods that employ additional steps to isolate GABA signals, either in the form of spectral editing or additional dimensionality. Under this slightly arbitrary distinction, TE-averaged experiments (as described in Section 3.2.2) are included, but parameter-optimized STEAM experiments are not. The reason for this distinction is the somewhat limited ability of fitting to successfully resolve GABA from NAA, Cr and Glx at 1.5T and 3T and the huge range of SNR and spectral quality of data in the literature for which GABA output from fitting tools is quoted.
3.2. Methods
Table 1 provides an overview of methods proposed for measuring GABA. These will be considered in terms of the three categories above, with additional consideration for MRSI of GABA.
Table 1.
| Reference | Method | Field (T) | Volume (ml) | Expt. (min) | Region 1 | Region 2+ | Application | Finding | Effect % | nc |
|---|---|---|---|---|---|---|---|---|---|---|
| [6] | JE | 2.1 | 16 | OCC | Vigabatrin | GABA↑ | 238% | 12 | ||
| [7] | MP, MS | 4.7 | 27 | 6 | OCC | 4 | ||||
| [18] | DQF-MRSI | 2.1 | 27 | 35 | MRSI | |||||
| [10] | 2DJ | 1.5 | 12 | 42 | OCC | 36 | ||||
| [16] | MP | 7 | 27 | 19 | OCC | 14 | ||||
| [19] | DQF | 1.5 | 27 | 17 | OCC | 15 | ||||
| [20] | DQF | 3 | 35 | 9 | FRO-PAR | 10 | ||||
| [12] | 2DJ-MRSI | 4 | 8 | 48 | MRSI | 6 | ||||
| [21] | DQF | 3 | 43 | 19 | FRO-PAR | 11 | ||||
| [22] | DQF | 3 | 23 | 20 | PAR-OCC | ACC | 6 | |||
| [23] | HHE | 3 | 35 | 34 | FRO-PAR | 11 | ||||
| [24] | MP | 4 | 5.3 | 38 | SN | OCC | SN > OCC | 11 | ||
| [25] | 2DJ | 3 | 16 | 17 | ParGM | 27 | ||||
| [26] | DQF-MRSI | 3 | 19 | 17–34 | MRSI | 13 | ||||
| [27] | MP | 3 | 27 | 7 | OCC | |||||
| [9] | MP | 3 | 27 | 11 | ACC | 20 | ||||
| [28] | MP | 4 | 18 | 10 | PAR-OCC | 3 | ||||
| [29] | MP | 3 | 27 | 16 | PAR | 5 | ||||
| [30] | JE | 4 | 17 | None | 4 | |||||
| [13] | TEA | 3 | 12 | 9 | ACC | 6 | ||||
| [31] | MRSI | 7 | 1 | 26 | MRSI | |||||
| [32] | 2DJ | 1.5 | 27 | 35 | OCC | 1 |
Study designs and findings are summarized: ‘SN > OCC … 11’ should be interpreted as ‘GABA concentration in substantia nigra is greater than that in an occipital region in 11 participants’; ‘GABA↑ … 238% … 12’ should be interpreted as ‘GABA concentration is elevated by an average of 238% in 12 subjects’.
Abbreviations include: Methods (JE, J-difference editing; MP, MEGA-PRESS; MS, MEGA-STEAM; MRSI, magnetic resonance spectroscopic imaging; DQF, Double-quantum filter; HHE, Hartman-Hahn editing; 2DJ, 2D J-resolved spectroscopy; TEA, echo time averaging); Region (OCC, Occipital; FRO, frontal; PAR, parietal; SN, sustantia nigra; ACC, anterior cingulate).
Number of subjects in the study.
3.2.1. Edited methods
The pioneering work of Rothman et al. [6] first made possible the unambiguous detection of GABA in vivo. This sequence uses ISIS localization (which relies upon spatially selective pre-inversion) followed by a single spin echo, using a DANTE pulse train to modulate coupling evolution. Rothman’s group has applied this method extensively at 2.1T, for example to studies of epilepsy and depression and their associated therapies as outlined below, but these studies have largely been restricted to measuring GABA in the occipital lobe due to the experimental constraints of surface coil detection.
J-edited PRESS-based methods, such as MEGA-PRESS (MP in Table 1), have been applied in a number of different brain regions – occipital, parietal and frontal regions (including the anterior cingulate). MEGA-PRESS combines PRESS localization with two frequency-selective editing pulses (which can also act to suppress water signal), and is relatively simple to implement as a development of the PRESS single-voxel experiment. It is probably fair to say that edited methods in general, and the MEGA-PRESS method specifically, are currently the most widely used methods to quantify GABA at 3T.
As mentioned previously, double-quantum filtered (DQF) methods are less susceptible to movement artefacts than J-difference methods, but they can suffer from signal losses due to the selection of a given coherence and may require pulse phase calibrations. An additional consideration contributing to the uptake of J-difference methods is that editing is ‘non-destructive’ in the sense that either the ‘editing pulse off’ spectrum or the ‘sum’ spectrum can be analysed to quantify uncoupled metabolites.
3.2.2. Two-dimensional methods
Both 2D J-resolved MRS e.g. [10] and COSY-based methods e.g. [33] have been applied in vivo for the quantification of GABA. While Schulte et al. have implemented a two-dimensional fitting algorithm for 2D J-resolved spectra [14,25], Jensen et al. have analysed the J = 7.5 ppm row only, which is presumably a more feasible approach when combined with MRSI [12]. It is worth noting that one of the most exciting recent GABA MRS findings in the neuroscience field [34] applied the Schulte method.
Mullins et al. have shown that an implementation of TE-averaged PRESS (in essence a 2D experiment in which the second frequency dimension is discarded) performed less reproducibly than PRESS at TEs of 30 or 40 ms for the quantification of GABA [13]. Banakar et al. have applied two-dimensional localized COSY spectroscopy to study GABA in HIV-positive children [33].
3.2.3. High-field MRS
The MEGA-PRESS method [7] was originally implemented at 4.7T (although it has been very widely applied at 3T e.g. [29]), and has subsequently been applied at 7T [16]. Although high-field methods are not yet widely applied for GABA detection, there is promise for the quantification of GABA from single voxel methods, such as STEAM [15,35], and MRSI methods [31].
3.2.4. MRSI of GABA
Several approaches have been made to the spectroscopic imaging of GABA: DQF-MRSI [26]; J-edited MRSI [18]; and 2DJ-MRSI [12]. Although an MRSI method for the spatially resolved quantification of GABA over a whole slice would certainly be valuable, these methods struggle to reconcile low GABA SNR with imaging voxel size and experiment duration. Due to large voxel sizes and experimental difficulties, these methods are rightly described as MRSI from a methodological perspective, but meaningful images of GABA concentration remain elusive.
3.3. Neuroscience applications
Table 2 shows methodological details and summarized results of these studies. Within this category, there are three clear groups of studies: those looking at normal temporal changes in GABA; those involving non-pharmacological intervention to modulate GABA; and those that seek to correlate individual measurements of baseline GABA concentration with other measures.
Table 2.
| Reference | Method | Field (T) | Volume (ml) | Expt. (min) | Region 1 | Region 2+ | Application | Finding | Effect % | n |
|---|---|---|---|---|---|---|---|---|---|---|
| [36] | JE | 2.1 | 14 | 20 | OCC | PDD + menstrual cycle | GABA↓ | 30 | 23 | |
| [37] | 2D-J 0 | 1.5 | 13 | 6 | SM | OCC | Ischemic Nerve Block | GABA↓ | 41 | 12 |
| [38] | JE | 2.1 | 14 | 14 | OCC | MDD + ECT | GABA↑ | 76 | 8 | |
| [39] | JE | 2.1 | 14 | 20 | OCC | Menstrual cycle + smoking | GABA↓c | 31 | 36 | |
| [40] | JE | 2.1 | 20 | 1.3 | OCC | Puerperal women | GABA↓ | 26 | 33 | |
| [41] | MP | 3 | 8 | 25 | SM | SM | Motor Learning | GABA↓ | 20 | 36 |
| [42] | MP | 3 | 18 | 6 | OCC-PAR | Typtophan depletion | ns | 12 | ||
| [34] | 2DJ | 3 | 18 | 16 | ACC | SM | Negative BOLD | GABA ∝ BOLD | 25 | |
| [43] | 2DJ-MRSI | 4 | 8 | 48 | MRSI | Yoga | GABA↑ | 23 | 19 | |
| [44] | PRESS | 3 | 8.8 | 4 | ACC | Pain (Heat) | GABA↑ | 15 | 13 | |
| [45] | MP | 3 | 8 | 17 | SM | TMS | GABA↑ | 13 | 16 | |
| [46] | MP | 3 | 8 | 15 | SM | Direct Current Stimulation | GABA↓ | 10 | 11 | |
| [47] | MP | 3 | 27 | 15 | OCC | Orientation Discrimination | GABA ∝ OD | 15 | ||
| [48] | MP | 3 | 27 | 16 | OCC | BOLD and MEG gamma | GABA ∝γ ∝ BOLD | 12 | ||
| [49] | MP | 3 | 27 | 6 | FRO | PAR-OCC | Extroversion Trait | GABA ∝ Extro. | 41 | |
| [50] | MP | 3 | 27 | 10 | OCC | SM | Time-Of-Day | OCC > SM | 8 | |
| [51] | MP | 3 | 26 | 6.5 | OCC | Schizophrenia + OSS | GABA ∝ OSS | 10 | 26 | |
| [52] | MP | 3 | 27 | 15 | FEF | OCC | Eye movement control | GABA ∝ EMD | 26 |
Study designs and findings are summarized: the first row should be interpreted as ‘GABA concentration in an occipital region is reduced (by an average of 30%) in premenstrual dysphoric disorder in 23 participants (patients + controls)’.
Abbreviations include: Methods (JE, J-difference editing; MP, MEGA-PRESS; MRSI, magnetic resonance spectroscopic imaging; 2DJ 0, 2D J-resolved spectroscopy, F1 = 0 Hz); Region (OCC, occipital; SM, sensorimotor; PAR, parietal; SN, sustantia nigra; ACC, anterior cingulate; FEF, frontal eye field); Application (PDD, premenstrual dysphoric disorder; MDD, major depressive disorder; ECT, electrocortical therapy; BOLD, blood oxygen level dependent functional MRI; TME, transcranial magnetic stimulation; MEG, magnetoencephalography); Finding (G, GABA; OD, orientation discrimination; γ, MEG gamma frequency; Extro., extroversion trait; OSS, orientation-specific surround suppression; EMD, eye movement distractibility).
GABA is reduced in the luteal phase by 31%.
3.3.1. Temporal studies
It has been reported that current methods do not reveal any variation of GABA with time-of-day (over the range 7.30 AM–7.30 PM), which is important for study design [50]. Epperson et al. have shown that GABA changes with menstrual cycle phase [36,39], with GABA being reduced in the luteal phase relative to the follicular phase in healthy non-smokers but not in smokers or women with premenstrual dysphoric disorder. They have also shown that GABA is reduced post-partum (although less so in depressed subjects) relative to follicular phase healthy controls [40]. Due to menstrual phase variations, some studies of individual differences in GABA choose to exclude female participants.
3.3.2. Interventional studies
Stagg et al. have shown that theta burst transcranial magnetic stimulation causes a rise in GABA concentration and transcranial direct current stimulation (both anodal and cathodal) causes a reduction in GABA [45,46]. Sanacora et al. have shown that electro-convulsive therapy causes a rise in GABA in depressed subjects [38].
In spite of the established role of GABA in cortical plasticity, there is limited MRS literature showing changes in GABA after behavioural interventions. GABA concentration in sensorimotor cortex has been shown to drop over a period of 50 min during and after a motor learning paradigm [41], and by over 40% during an ischemic nerve block [37]. Painful heat stimuli cause an increase in GABA in the ACC [44]. Yoga (but not reading) causes a global rise in GABA [43]. Cognitive behavioural therapy in depressed patients resulted in a sizeable but non-significant reduction in occipital GABA [53]. Dietary manipulation (tryptophan depletion in healthy subjects [53] and ketogenic diet in epilepsy patients [54]) showed no significant effects.
3.3.3. Correlational studies
Given the importance of functional neuroimaging within cognitive neuroscience, the finding that BOLD fMRI signal changes correlate with individual differences in GABA concentration among healthy individuals [34] is likely to provoke much further work. It has been shown that larger negative BOLD changes occur in healthy individuals with high GABA [34], whereas task-related positive BOLD changes are larger in those subjects with lower GABA [48]. No relationship between negative BOLD and GABA exists in depressed subjects [55]. Magnetoencephalography has been used to observe individually characteristic sustained gamma oscillations in the visual cortex, the frequency of which correlates with GABA concentration and visually stimulated positive BOLD signal change [48].
Orientation discrimination has long been linked with GABAergic function in visual cortex [56]. It has recently been shown that individual differences in performance at an orientation discrimination task among healthy individuals correlate with GABA concentration in visual cortex [47]. It has also been shown that orientation-specific surround suppression of contrast discrimination also correlates with GABA concentration among a mixed cohort of schizophrenic patients and healthy controls [51]. It has also recently been shown that differences in frontal GABA concentration among healthy subjects may correlate with the NEO-FFI extro-version score, although results did not reach significance after multiple comparisons correction [49]. Additionally, it has been shown that eye movement distractibility correlates negatively with GABA concentration in frontal eye field (FEF), the frontal region associated with eye movement planning, but not GABA concentration in occipital cortex [52].
3.4. Pharmacological applications
In this section, studies of drug action in both healthy controls and patient groups are considered. In some clinical groups, recruitment of treatment-naïve subjects is practically difficult, whereas ethical difficulties may hinder some pharmacological studies in healthy controls. For both these reasons, the overlap between this section and Section 3.5 (Clinical Applications) is substantial.
3.4.1. Epilepsy medications
GABA is produced from glutamate by glutamic acid decarboxylase (GAD) and is metabolised to succinic acid semialdehyde by GABA transaminase (GABA-T) and thence to succinate. Vigabatrin is an irreversible inhibitor of GABA-T, and therefore of GABA breakdown. It has been widely been shown to raise the level of GABA in occipital regions in healthy and epileptic subjects e.g. [6,58,67] and see Table 3, although it can be assumed that these changes are not restricted to occipital regions. Rises in GABA are linked to seizure control in patients [60,69]. GABA measurements rise linearly with increasing vigabatrin dose up to 60 mg/kg per day [60] and then show no further increase.
Table 3.
| Reference | Method | Field (T) | Volume (ml) | Expt. (min) | Region 1 | Region 2+ | Application | Finding | Effect % | n |
|---|---|---|---|---|---|---|---|---|---|---|
| [57] | JE | 2.1 | OCC | Vigabatrin | GABA↑ | 150 | ||||
| [58] | JE | 2.1 | OCC | Epilepsy + vigabatrin | GABA↑ | 190 | 10 | |||
| [59] | JE | 2.1 | 14 | OCC | Epilepsy + gabapentin | GABA↑ | 28 | |||
| [60] | JE | 2.1 | 14 | 7 | OCC | Epilepsy + vigabatrin | GABA↑ | 150 | 26 | |
| [61] | JE | 2.1 | OCC | Epilepsy + vigabatrin | GABA↑ | 100 | 18 | |||
| [62] | JE | 2.1 | 14 | OCC | Epilepsy + vigabatrin | GABA↑ | 40 | |||
| [63] | JE | 2.1 | OCC | Epilepsy + vigabatrin | GABA↑ | 11 | ||||
| [64] | JE | 2.1 | 14 | OCC | Epilepsy + topiramate | GABA↑ | 56 | 21 | ||
| [65] | JE | 2.1 | 14 | OCC | Epilepsy + valproate et al. | 24 | ||||
| [66] | JE | 2.1 | 14 | OCC | Epilepsy + vigabatrin | GABA↑ | 6 | |||
| [67] | JE | 1.5 | 8 | 23 | OCC | Vigabatrin | GABA↑ | 40 | 5 | |
| [68] | JE | 2.1 | 14 | OCC | Epilepsy + gabapentin | GABA↑ | 6 | |||
| [69] | JE | 2.1 | 14 | OCC | Epilepsy seizure control | GABA↓ | 26 | |||
| [70] | JE | 2.1 | 14 | OCC | Epilepsy + topiramate | GABA↑ | 100 | 15 | ||
| [71] | JE | 2.1 | 14 | 14 | OCC | MDD + SSRI | GABA↑ | 34 | 11 | |
| [72] | DQF | 3 | 27 | 13 | OCC | SSRI citalopram | GABA↑ | 35 | 10 | |
| [73] | JE | 2.1 | 14 | 21 | OCC | Benzodiazepine | GABA↓ | 24 | 19 | |
| [74] | 2DJ | 1.5 | 19 | 20 | OCC | Cocaine-dep | GABA↓ | 32 | 55 | |
| [75] | 2DJ | 1.5 | 19 | 20 | PF | Cocaine-dep + venlafaxine Cocaine-dep + pramipexole |
ns GABA↑ |
17 | 34 | |
| [76] | JE | 2.1 | 20 | 1.3 | OCC | Ethanol Recovery + Smoking | GABA↓ | 25 | 20 | |
| [77] | JE | 2.1 | 14 | OCC | Epilepsy + topiramate/gabapentin | ns | 20 | |||
| [78] | 2DJ-MRSI | 4 | 8 | 48 | MRSI | SAD + levetiracetam | Th SAD ↓ | 58 | 20 | |
| [79] | MP | 3 | 27 | 13 | ACC | BG | Lithium | ns | 8 | |
| [80] | MEGA | 4 | 12 | 13 | ACC | Thalamus | Zolpidem | ACC = Th↓ | 25 | 19 |
| [81] | MP | 3 | 27 | 13 | ACC | BG | Schizophrenia | drug effects | 21 | 67 |
| [82] | MP | 3 | 22.5 | 6 | OCC | Epilepsy + levetiracetam | GABA↑ | 19 | 16 |
Study designs and findings are summarized: the first row should be interpreted as ‘GABA concentration in an occipital region is elevated (by 150%) by administration of vigabatrin’. ‘ACC = Th↓’ should be interpreted as ‘GABA concentration in ACC is unchanged, while thalamic GABA is reduced’.
Abbreviations include: Methods (JE, J-difference editing; MP, MEGA-PRESS; MRSI, magnetic resonance spectroscopic imaging; 2DJ, 2D J-resolved spectroscopy); Region (OCC, occipital; PF, prefrontal; ACC, anterior cingulate; BG, basal ganglia); Application (SSRI, selective seratonin reuptake inhibitor; MDD, major depressive disorder; SAD, social anxiety disorder); Finding (ns, non-significant finding; Th, Thalamus).
Gabapentin was designed as a GABA analogue (it is a γ-amino carboxylic acid), but its mechanism of action is not known, although it may upregulate GABA synthesis by GAD. Petroff et al. have shown that gabapentin increases GABA concentration in epilepsy patients [59], while a retrospective analysis of patients with refractory complex seizures shows no link between seizure control and gabapentin dose [77].
Topiramate acts as a GABAA receptor agonist, and also inhibits glutamatergic neurotransmission as an AMPA/kainate receptor inhibitor. It has been shown to cause large increases in GABA concentration in epilepsy patients (see Table 3).
Valproate is an effective treatment for complex partial seizures, but does not cause a rise in GABA in occipital cortex and there is no observed difference in GABA levels between patients taking valproate, carbamazepine and phenytoin [65] or lamotrigine [69].
Levetiracetam caused a significant increase in occipital GABA among five of sixteen epilepsy patients in whom it caused partial seizure reduction [82] and has also been shown to a non-significant increase in GABA in the thalamus of subjects with social anxiety disorder [78].
3.4.2. Other pharmacological agents
Administration of the benzodiazepine clonazepam causes a 24% reduction in occipital cortex GABA in healthy controls, but not in subjects with panic disorder (PD) [73]; these subjects had significantly lower GABA at baseline than healthy controls. The non-benzodiazopine sedative zolpidem caused a similar 25% reduction in thalamic GABA concentration, but not in ACC [80]. It is interesting to note that inter-regional differences in drug response can be observed, particularly in light of the overwhelming bias of studies towards measurement in occipital cortex (see Section 3.6.1 for further comment). Non-significant increases in GABA have been observed in healthy controls after lithium adminstration [79].
In separate studies, the selective serotonin reuptake inhibitors (SSRI) citalopram [72] and fluoxetine [71] have been shown to increase occipital cortex GABA by about a third in healthy volunteers and depressed subjects respectively. [71]. The SSRI venlafaxine caused a non-significant increase in GABA in prefrontal regions in cocaine-dependent subjects, whereas pramipexole administration gave a significant increase in the same study [75]. These results are in the context of a >30% reduction in GABA among cocaine-dependent subjects [74].
It is interesting to contrast pharmaceutical agents that directly alter the concentration of GABA to GABA-receptor agonists and antagonists. Vigabatrin, which inhibits breakdown of GABA, is clearly expected to increase GABA concentration; it is less clear what impact GABA receptor pharmaceuticals would be expected to have, and any impact on GABA concentration is presumably secondary to their primary action. In this context it is therefore perhaps surprising the wide diversity of drugs that have been shown to alter GABA concentration.
3.5. Clinical applications
Table 4 shows the methodological details and summarized results of a range of published clinical studies.
Table 4.
| Reference | Method | Field (T) | Volume (ml) | Expt. (min) | Region 1 | Region 2+ | Application | Finding | Effect % | n |
|---|---|---|---|---|---|---|---|---|---|---|
| [83] | JE | 2.1 | OCC | Epilepsy seizure control | GABA↓ | 13 | ||||
| [84] | JE | 2.1 | 14 | 14 | OCC | MDD | GABA↓; | 52 | 32 | |
| [85] | JE | 2.1 | 14 | OCC | Panic Disorder | GABA↓ | 22 | 28 | ||
| [36] | JE | 2.1 | 14 | 20 | OCC | PDD + menstrual cycle | GABA↓ | 30 | 23 | |
| [38] | JE | 2.1 | 14 | 14 | OCC | MDD + ECT | GABA↑ | 76 | 8 | |
| [86] | DQF | 1.5 | 35 | 17 | l FRO | r FRO | IGE | ns | 38 | |
| [87] | DQF | 1.5 | 35 | 17 | OCC | IGE | GABA↑ | 13 | 30 | |
| [54] | 2D-DQF | 1.5 | 343 | 20 | Brain | Ketogenic diet | ns | 7 | ||
| [73] | JE | 2.1 | 14 | 21 | OCC | Panic Disorder | GABA↓ | 24 | 19 | |
| [88] | JE | 2.1 | 14 | 20 | OCC | MDD | GABA↓ | 14 | 71 | |
| [89] | JE | 3 | 18,30 | 27 | VM-PFC | DD-PFC | Remitted MDD | ns | 31 | |
| [24] | MP | 4 | 5.3 | 38 | SN | OCC | SN > OCC | 11 | ||
| [53] | JE | 2.1 | 20 | 14 | OCC | MDD + CBT | ns | 15 | ||
| [90] | MP | 3 | 18 | 7 | OCC-PAR | Recovered MDD and BD | GABA↓ | 9 | 49 | |
| [91] | JE | 3 | 18,30 | 27 | VM-PFC | DD-PFC | MDD | GABA↓ | 11 | 40 |
| [92] | DQF | 1.5 | 35 | Malform’n of Cort’l Devel’t | ns | 19 | 30 | |||
| [33] | LCOSY | 1.5 | 27 | 26 | FWM | HIV + children | GABA↑ | 37 | 21 | |
| [93] | MP | 3 | 12 | 7 | FRO | OCC-PAR | rMDD | GABA↓ | 11 | 23 |
| [94] | 2DJ-MRSI | 4 | 7 or 112 | 20 | MRSI | Primary insomnia | GABA↓ | 30 | 32 | |
| [95] | JE | 3 | 18,30 | 27 | VM-PFC | DD-PFC | Panic Disorder | ns | 34 | |
| [96] | 2DJ-MRSI | 4 | 4.5 | 48 | MRSI | Bipolar Disorder | ns | 24 | ||
| [97] | JE | 3 | 18 | 13 | OCC | ACC | tr MDD | GABA↓ | 18 | 42 |
| [98] | DQF | 1.5 | 35 | 17 | TL | Epilepsy + resection | ns | 63 | ||
| [55] | 2DJ | 3 | 18 | 16 | ACC | MDD | ns | 43 | ||
| [81] | MP | 3 | 27 | 13 | ACC | BG | Schizophrenia | Drug effects | 21 | 67 |
Study designs and findings are summarized: ‘SN > OCC … 11’ should be interpreted as ‘GABA concentration in substantia nigra is greater than that in an occipital region in 11 participants’; ‘MDD … GABA↓ … 52% … 32’ should be read as ‘GABA concentration is reduced in subjects with major depressive disorder’.
Abbreviations: (JE, J-difference editing; DQF, double-quantum filtered; MP, MEGA-PRESS; MRSI, magnetic resonance spectroscopic imaging; 2DJ, 2D J-resolved spectroscopy); (OCC, occipital; l, left; r, right; FRO, frontal; VM-PFC, ventromedial prefrontal cortex; DD-PFC, dorsomedial dorsoanterolateral prefrontal cortex; SN, substantia nigra; PAR, parietal; FWM, frontal white matter; ACC, anterior cingulate; TL, temporal lobe; BG, basal ganglia); (SSRI, selective seratonin reuptake inhibitor; PDD, pre-menstrual dysphoric disorder; MDD, major depressive disorder; ECT, electroconvulsive therapy; IGE, idiopathic generalized epilepsy; CBT, cognitive behavioural therapy; BD, bipolar disorder; tr, treatment resistant; SAD, social anxiety disorder); (ns, non-significant finding).
3.5.1. Epilepsy
As the major inhibitory neurotransmitter, there is great interest in GABA with regards to epilepsy, both in terms of the pathophysiology of seizures and their treatment and prevention. As discussed in Section 3.4.1, a number of epilepsy medications have been shown to increase GABA, and measures of seizure control correlate with GABA concentration in patients with complex partial seizures [83].
A study of epilepsy patients with idiopathic generalized epilepsy (IGE) or occipital lobe epilepsy and being treated with a variety of medications (vigabatrin, topiramate, gabapentin, valproate, lamotrigine, carbamazepine, clobazam and acetazolamide, oxcarbazepine, phenytoin, and phenobarbitol) showed an elevation of GABA (versus healthy controls) in occipital cortex attributed to increased grey matter inclusion of the MRS volume [87]. Frontal lobe measurements of GABA in IGE [86] and temporal measurements in temporal lobe epilepsy before and after resection [98] showed no differences between patients and controls.
These studies demonstrate the difficulty of performing epilepsy studies: the pathology itself is very heterogeneous, and treatment options are diverse, with multiple therapies common. It is worth noting that treatment decisions are made on the basis of seizure control, so studies of patients treated with various medications represent a far from random allocation.
3.5.2. Major depressive disorder and bipolar disorder
Both depressed male and female subjects have been shown to have reduced GABA concentration in occipital cortex, with a larger difference in women than men [84]. A similar difference has been reported in dorsomedial/dorsal anteriolateral prefrontal cortex, although no significant difference was seen in ventromedial prefrontal cortex [91]. In addition to replicating the reduction in subjects with major depressive disorder (MDD), significant differences have been demonstrated between depression groups classified as atypical, melancholic and no-subtype, with the melancholic group having the greatest reduction [88]. Two studies have looked at GABA in remitted MDD subjects: measurements in ACC and occipital cortex show a significant reduction in GABA versus healthy controls [93], while measurements in prefrontal cortex show no change [89]. It has also been shown that treatment-resistant (TR) depressed patients have lower GABA in ACC and occipital cortex than non-TR depressed subjects and healthy controls [97].
Recovered bipolar disorder (BD) patients have been shown to have lower occipital GABA than controls [90], although MRSI-based methods show no significant difference between symptomatic BD patients and healthy controls [96].
3.5.3. Additional clinical studies
Tayoshi et al. did not find a significant difference in GABA concentration between schizoprenia subjects and healthy controls in the ACC and basal ganglia [81]. However, Yoon et al. have shown a significant reduction in occipital GABA in schizophrenia patients, and correlation of GABA concentration with performance at a psychophysical task as mentioned above [51].
In panic disorder, significant reduction in GABA has been seen in occipital cortex [85], but not in two regions of prefrontal cortex [95]. No significant GABA differences between patients and controls were seen in either social anxiety disorder [78] or primary insomnia [94]. GABA has been observed to be high in the substantia nigra of subjects with Parkinson’s disease [24], and in frontal white matter of children with HIV [33].
3.6. Discussion
3.6.1. Regions studied
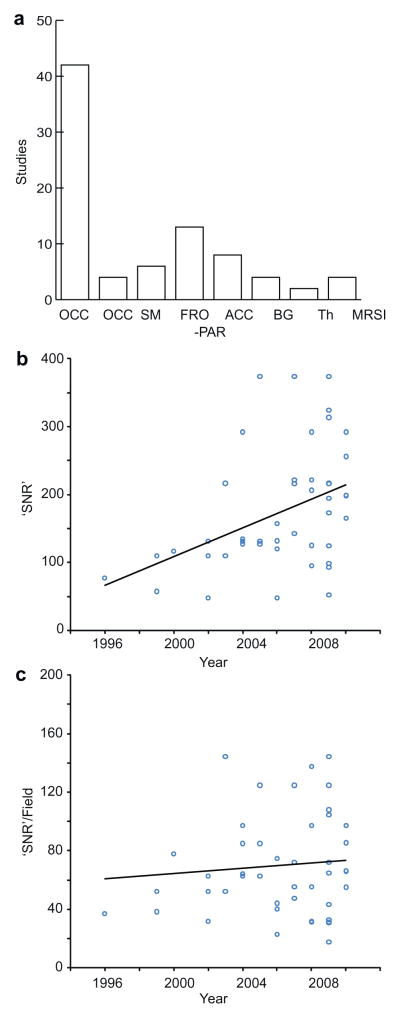
There has been a large bias towards studying occipital cortex, as seen in Fig 3a. This is largely due to experimental limitations – measurements that use a surface receive-coil are most conveniently carried out in this location, and those that use a volume coil often have best SNR in this location due to proximity to the coil elements. However, additional regions are being studied with increasing frequency.
Fig. 3.
Literature review. (a) Histogram of brain regions studied (OCC occipital; PAR parietal; SM sensorimotor; FRO frontal; ACC anterior cingulate; BG basal ganglia; Th Thalamus). (b) Plot of ‘SNR’ metric (volume × √(experiment time)) against year of publication. (c) Plot of ‘SNR’ metric divided by field strength against year of publication.
It is becoming increasingly apparent that neither pathological changes in GABA concentration, nor individual GABA differences between healthy controls [50,52] are necessarily global across the brain. Both MRSI methods and studies of multiple regions will be important in investigating this further.
3.6.2. Experiment duration, volume and SNR
In the design of MRS studies in general, and especially those studying the low concentration metabolites, such as GABA, there is a tension between needing to minimize experiment time to increase subject comfort (and thereby data quality) and control costs, to minimize study volume in order to increase anatomical specificity, and to maximize SNR and measurement precision. It is possible to generate a very crude estimate of the SNR of measurements (as tabulated) from the product of the field strength (in T), the measurement volume (in ml) and the square root of the measurement time (in minutes). This approach ignores any SNR differences between methodologies.
Calculating this metric for all non-methodological studies for which all three parameters are known, the mean value (215) is equivalent to a 7-min measurement of a 27 ml volume at 3T. Fig. 3b shows how this metric has changed with year of publication. There is a significant upward trend that is removed after controlling for field strength, as seen in Fig. 3c which plots (volume × √(experiment time)) against year of publication. This suggests that, in general, higher field strength has largely been used to increase SNR rather than to reduce measurement volumes or times.
From a methodological standpoint, it is extremely important to be able to see both the SNR and the quality of spectra in judging the merits of a study. Unfortunately, it is relatively common for MRS studies of GABA to show no example data (either typical or bestcase spectra), particularly in journals of psychiatry, and it is not clear whether this arises from the preferences of authors, reviewers or editors.
3.6.3. Analysis methods
Traditionally, MRS spectra have often been quantified by manual integration of metabolite peaks. This approach is time consuming and has the potential for user error and bias to impact results. Therefore, it is generally accepted that automated analysis is preferable, using tools such as LCModel for 1D spectra or ProFit for 2D spectra. Several groups have applied the LCModel to quantify J-edited spectra, but great care must be taken to optimize control parameters for appropriate fitting. One potential pitfall is to assign almost the entire edited signal at 3 ppm to the macromolecular baseline e.g. Fig. 2 in [81].
Quantification of MRS signals is usually done in a relative fashion. In the studies described, quantification relative to creatine, NAA and water have all been used. Each method has its advantages: the creatine signal has a chemical shift of 3.0 ppm and therefore numerator and denominator measurements come from identical volumes with no chemical shift displacement artifact; NAA can be quantified directly from the edited difference spectrum, in which it appears as a strong negative signal at 2 ppm; and water quantification has excellent signal-to-noise and brings GABA measurements into line with most other MRS measurements. It has been shown that quantification relative to Cr is slightly more reproducible than relative to water, possibly as this method does not require an additional scan [99].
4. Conclusion
MRS has become established as a robust and powerful tool for the investigation of the inhibitory neurotransmitter GABA. This is a significant success, given the significant methodological hurdles involved: low concentration; coupled spin systems; and overlapping metabolite peaks to name a few. MRS has traditionally been applied within a clinical context, but is increasingly being used to study the healthy brain, contemporaneous with an increase in the number of MRI scanners being installed in non-clinical departments for functional MRI studies. From a cognitive neuroscience perspective, a particularly interesting recent field, which is anticipated to flourish, is the application of MRS to study individual differences in GABA concentration as they relate to inhibition-dependent cognitive processes.
Acknowledgments
We would like to acknowledge useful discussions with Peter Barker and John Evans.
Nomenclature
- 2DJ
two-dimensional J-resolved spectroscopy (in tables only)
- ACC
anterior cingulate cortex
- BG
basal ganglia (in tables only)
- BOLD
blood-oxygen level dependent functional MRI
- Cho
choline
- COSY
correlation spectroscopy
- Cr
creatine
- DANTE
delay alternating with nutation for tailored excitation
- DQF
double-quantum filtered
- ECT
electrocortical therapy
- FEF
frontal eye field (in tables only)
- FRO
frontal (in tables only)
- GABA
γ-amino butyric acid
- GABA-T
GABA transaminase
- GAD
Glutamic acid decarboxylase
- GAT
GABA transporters
- Gln
glutamine
- Glu
glutamate
- Glx
glutamate + glutamine
- HHE
Hartmann–Hahn editing (in tables only)
- IGE
idiopathic generalized epilepsy
- JE
J-difference editing (in tables only)
- MDD
major depressive disorder
- MEG
magnetoencephalography
- MEGA-PRESS
J-difference edited PRESS
- MP
MEGA-PRESS (in tables only)
- MR
magnetic resonance
- MRS
magnetic resonance spectroscopy
- MRSI
magnetic resonance spectroscopic imaging
- NAA
N-acetyl aspartate
- NMR
nuclear magnetic resonance
- ns
non-significant (in tables only)
- OCC
occipital (in tables only)
- PAR
parietal (in tables only)
- PDD
premenstrual dysphoric disorder (in tables only)
- PF
prefrontal (in tables only)
- PRESS
Point-RESolved Spectroscopy
- SAD
social anxiety disorder
- SM
sensorimotor (in tables only)
- SN
substantia nigra
- SNR
signal-to-noise ratio
- SSRI
selective serotonin reuptake inhibitor
- STEAM
Stimulated-Echo Acquisition Mode
- TE
echo time
- Th
thalamus (in tables only)
- TMS
transcranial magnetic stimulation
- TR
treatment-resistant
Footnotes
This work was supported in part by NIH P41RR015241.
References
- 1.Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang L, Cloak CC, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry. 2003;64(Suppl 3):7–14. [PubMed] [Google Scholar]
- 3.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 4.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9:79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- 5.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 6.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Bielicki G, Chassain C, Renou JP, Farges MC, Vasson MP, Eschalier A, Durif F. Brain GABA editing by localized in vivo (1)H magnetic resonance spectroscopy. NMR Biomed. 2004;17:60–68. doi: 10.1002/nbm.863. [DOI] [PubMed] [Google Scholar]
- 9.Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging. 2007;25:1032–1038. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke Y, Cohen BM, Bang JY, Yang M, Renshaw PF. Assessment of GABA concentration in human brain using two-dimensional proton magnetic resonance spectroscopy. Psychiatry Res. 2000;100:169–178. doi: 10.1016/s0925-4927(00)00075-5. [DOI] [PubMed] [Google Scholar]
- 11.Ryner LN, Sorenson JA, Thomas MA. 3D localized 2D NMR spectroscopy on an MRI scanner. J Magn Reson B. 1995;107:126–137. doi: 10.1006/jmrb.1995.1068. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JE, Frederick BD, Wang L, Brown J, Renshaw PF. Two-dimensional, J-resolved spectroscopic imaging of GABA at 4 Tesla in the human brain. Magn Reson Med. 2005;54:783–788. doi: 10.1002/mrm.20644. [DOI] [PubMed] [Google Scholar]
- 13.Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med. 2008;60:964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- 14.Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- 15.Tkac I, Gruetter R. Methodology of 1H NMR spectroscopy of the human brain at very high magnetic fields. Appl Magn Reson. 2005;29:139–157. doi: 10.1007/BF03166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47:1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 17.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45:517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Shungu DC, Rothman DL. In vivo chemical shift imaging of gamma-aminobutyric acid in the human brain. Magn Reson Med. 1999;41:35–42. doi: 10.1002/(sici)1522-2594(199901)41:1<35::aid-mrm7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.McLean MA, Busza AL, Wald LL, Simister RJ, Barker GJ, Williams SR. In vivo GABA+ measurement at 1.5T using a PRESS-localized double quantum filter. Magn Reson Med. 2002;48:233–241. doi: 10.1002/mrm.10208. [DOI] [PubMed] [Google Scholar]
- 20.Choi IY, Lee SP, Merkle H, Shen J. Single-shot two-echo technique for simultaneous measurement of GABA and creatine in the human brain in vivo. Magn Reson Med. 2004;51:1115–1121. doi: 10.1002/mrm.20082. [DOI] [PubMed] [Google Scholar]
- 21.Choi IY, Lee SP, Shen J. In vivo single-shot three-dimensionally localized multiple quantum spectroscopy of GABA in the human brain with improved spectral selectivity. J Magn Reson. 2005;172:9–16. doi: 10.1016/j.jmr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Choi IY, Lee SP, Shen J. Selective homonuclear Hartmann-Hahn transfer method for in vivo spectral editing in the human brain. Magn Reson Med. 2005;53:503–510. doi: 10.1002/mrm.20381. [DOI] [PubMed] [Google Scholar]
- 23.Choi C, Coupland NJ, Hanstock CC, Ogilvie CJ, Higgins AC, Gheorghiu D, Allen PS. Brain gamma-aminobutyric acid measurement by proton double-quantum filtering with selective J rewinding. Magn Reson Med. 2005;54:272–279. doi: 10.1002/mrm.20563. [DOI] [PubMed] [Google Scholar]
- 24.Oz G, Terpstra M, Tkac I, Aia P, Lowary J, Tuite PJ, Gruetter R. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med. 2006;55:296–301. doi: 10.1002/mrm.20761. [DOI] [PubMed] [Google Scholar]
- 25.Schulte RF, Lange T, Beck J, Meier D, Boesiger P. Improved two-dimensional J-resolved spectroscopy. NMR Biomed. 2006;19:264–270. doi: 10.1002/nbm.1027. [DOI] [PubMed] [Google Scholar]
- 26.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya PK, Lowe MJ, Phillips MD. Spectral quality control in motion-corrupted single-voxel J-difference editing scans: an interleaved navigator approach. Magn Reson Med. 2007;58:808–812. doi: 10.1002/mrm.21337. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser LG, Young K, Matson GB. Elimination of spatial interference in PRESS-localized editing spectroscopy. Magn Reson Med. 2007;58:813–818. doi: 10.1002/mrm.21407. [DOI] [PubMed] [Google Scholar]
- 29.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 31.Henning A, Fuchs A, Murdoch JB, Boesiger P. Slice-selective FID acquisition, localized by outer volume suppression (FIDLOVS) for (1)H-MRSI of the human brain at 7 T with minimal signal loss. NMR Biomed. 2009;22:683–696. doi: 10.1002/nbm.1366. [DOI] [PubMed] [Google Scholar]
- 32.Lymer K, Haga K, Marshall I, Sailasuta N, Wardlaw J. Reproducibility of GABA measurements using 2D J-resolved magnetic resonance spectroscopy. Magn Reson Imaging. 2007;25:634–640. doi: 10.1016/j.mri.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Banakar S, Thomas MA, Deveikis A, Watzl JQ, Hayes J, Keller MA. Two-dimensional 1H MR spectroscopy of the brain in human immunodeficiency virus (HIV)-infected children. J Magn Reson Imaging. 2008;27:710–717. doi: 10.1002/jmri.21251. [DOI] [PubMed] [Google Scholar]
- 34.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 35.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 37.Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol. 2002;52:755–761. doi: 10.1002/ana.10372. [DOI] [PubMed] [Google Scholar]
- 38.Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 39.Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, Rothman DL, Mason GF. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 41.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 42.Selvaraj S, Wylezinska M, Evans J, Jezzard P, Matthews PM, Cowen PJ. Tryptophan depletion does not lower brain GABA levels in healthy volunteers. Psychopharmacology (Berl) 2006;187:131–132. doi: 10.1007/s00213-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 43.Streeter CC, Jensen JE, Perlmutter RM, Cabral HJ, Tian H, Terhune DB, Ciraulo DA, Renshaw PF. Yoga Asana sessions increase brain GABA levels: a pilot study. J Altern Complement Med. 2007;13:419–426. doi: 10.1089/acm.2007.6338. [DOI] [PubMed] [Google Scholar]
- 44.Kupers R, Danielsen ER, Kehlet H, Christensen R, Thomsen C. Painful tonic heat stimulation induces GABA accumulation in the prefrontal cortex in man. Pain. 2009;142:89–93. doi: 10.1016/j.pain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goto N, Yoshimura R, Moriya J, Kakeda S, Hayashi K, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Oonari N, Korogi Y, Nakamura J. Critical examination of a correlation between brain gamma-aminobutyric acid (GABA) concentrations and a personality trait of extroversion in healthy volunteers as measured by a 3 Tesla proton magnetic resonance spectroscopy study. Psychiatry Res. 2010;182:53–57. doi: 10.1016/j.pscychresns.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 51.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 53.Sanacora G, Fenton LR, Fasula MK, Rothman DL, Levin Y, Krystal JH, Mason GF. Cortical gamma-aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry. 2006;59:284–286. doi: 10.1016/j.biopsych.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy–exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- 55.Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 56.Sillito AM. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat’s striate cortex. J Physiol. 1975;250:287–304. doi: 10.1113/jphysiol.1975.sp011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattson RH, Petroff O, Rothman D, Behar K. Vigabatrin: effects on human brain GABA levels by nuclear magnetic resonance spectroscopy. Epilepsia. 1994;35(Suppl 5):S29–32. doi: 10.1111/j.1528-1157.1994.tb05963.x. [DOI] [PubMed] [Google Scholar]
- 58.Petroff OA, Rothman DL, Behar KL, Mattson RH. Initial observations on effect of vigabatrin on in vivo 1H spectroscopic measurements of gamma-aminobutyric acid, glutamate, and glutamine in human brain. Epilepsia. 1995;36:457–464. doi: 10.1111/j.1528-1157.1995.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 59.Petroff OA, Rothman DL, Behar KL, Lamoureux D, Mattson RH. The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Ann Neurol. 1996;39:95–99. doi: 10.1002/ana.410390114. [DOI] [PubMed] [Google Scholar]
- 60.Petroff OA, Behar KL, Mattson RH, Rothman DL. Human brain gamma-aminobutyric acid levels and seizure control following initiation of vigabatrin therapy. J Neurochem. 1996;67:2399–2404. doi: 10.1046/j.1471-4159.1996.67062399.x. [DOI] [PubMed] [Google Scholar]
- 61.Petroff OA, Rothman DL, Behar KL, Mattson RH. Human brain GABA levels rise after initiation of vigabatrin therapy but fail to rise further with increasing dose. Neurology. 1996;46:1459–1463. doi: 10.1212/wnl.46.5.1459. [DOI] [PubMed] [Google Scholar]
- 62.Petroff OA, Rothman DL, Behar KL, Collins TL, Mattson RH. Human brain GABA levels rise rapidly after initiation of vigabatrin therapy. Neurology. 1996;47:1567–1571. doi: 10.1212/wnl.47.6.1567. [DOI] [PubMed] [Google Scholar]
- 63.Petroff OA, Mattson RH, Behar KL, Hyder F, Rothman DL. Vigabatrin increases human brain homocarnosine and improves seizure control. Ann Neurol. 1998;44:948–952. doi: 10.1002/ana.410440614. [DOI] [PubMed] [Google Scholar]
- 64.Petroff OA, Hyder F, Mattson RH, Rothman DL. Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy. Neurology. 1999;52:473–478. doi: 10.1212/wnl.52.3.473. [DOI] [PubMed] [Google Scholar]
- 65.Petroff OA, Rothman DL, Behar KL, Hyder F, Mattson RH. Effects of valproate and other antiepileptic drugs on brain glutamate, glutamine, and GABA in patients with refractory complex partial seizures. Seizure. 1999;8:120–127. doi: 10.1053/seiz.1999.0267. [DOI] [PubMed] [Google Scholar]
- 66.Petroff OA, Hyder F, Collins T, Mattson RH, Rothman DL. Acute effects of vigabatrin on brain GABA and homocarnosine in patients with complex partial seizures. Epilepsia. 1999;40:958–964. doi: 10.1111/j.1528-1157.1999.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 67.Weber OM, Verhagen A, Duc CO, Meier D, Leenders KL, Boesiger P. Effects of vigabatrin intake on brain GABA activity as monitored by spectrally edited magnetic resonance spectroscopy and positron emission tomography. Magn Reson Imaging. 1999;17:417–425. doi: 10.1016/s0730-725x(98)00184-2. [DOI] [PubMed] [Google Scholar]
- 68.Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41:675–680. doi: 10.1111/j.1528-1157.2000.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 69.Petroff OA, Hyder F, Rothman DL, Mattson RH. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology. 2001;56:709–715. doi: 10.1212/wnl.56.6.709. [DOI] [PubMed] [Google Scholar]
- 70.Petroff OA, Hyder F, Rothman DL, Mattson RH. Topiramate rapidly raises brain GABA in epilepsy patients. Epilepsia. 2001;42:543–548. doi: 10.1046/j.1528-1157.2001.18800.x. [DOI] [PubMed] [Google Scholar]
- 71.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 72.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 73.Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Behar KL, Krystal JH. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161:2186–2193. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- 74.Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Streeter CC, Hennen J, Ke Y, Jensen JE, Sarid-Segal O, Nassar LE, Knapp C, Meyer AA, Kwak T, Renshaw PF, Ciraulo DA. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berl) 2005;182:516–526. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- 76.Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Petroff OA, Hyder F, Rothman DL, Mattson RH. Brain homocarnosine and seizure control of patients taking gabapentin or topiramate. Epilepsia. 2006;47:495–498. doi: 10.1111/j.1528-1167.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 78.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:739–743. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 79.Shibuya-Tayoshi S, Tayoshi S, Sumitani S, Ueno S, Harada M, Ohmori T. Lithium effects on brain glutamatergic and GABAergic systems of healthy volunteers as measured by proton magnetic resonance spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:249–256. doi: 10.1016/j.pnpbp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 80.Licata SC, Jensen JE, Penetar DM, Prescot AP, Lukas SE, Renshaw PF. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 T. Psychopharmacology (Berl) 2009;203:819–829. doi: 10.1007/s00213-008-1431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Ueno S, Harada M, Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Thorsten DM, Thilo H, Wolfgang B, Angelika M, Andreas S, Uwe B, Arnd D, Hermann S. Alterations of intracerebral gamma-aminobutyric acid (GABA) levels by titration with levetiracetam in patients with focal epilepsies. Epilepsia. doi: 10.1111/j.1528-1167.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 83.Petroff OA, Rothman DL, Behar KL, Mattson RH. Low brain GABA level is associated with poor seizure control. Ann Neurol. 1996;40:908–911. doi: 10.1002/ana.410400613. [DOI] [PubMed] [Google Scholar]
- 84.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 85.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, Charney DS, Krystal JH. Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 86.Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MRS reveals frontal lobe metabolite abnormalities in idiopathic generalized epilepsy. Neurology. 2003;61:897–902. doi: 10.1212/01.wnl.0000086903.69738.dc. [DOI] [PubMed] [Google Scholar]
- 87.Simister RJ, McLean MA, Barker GJ, Duncan JS. A proton magnetic resonance spectroscopy study of metabolites in the occipital lobes in epilepsy. Epilepsia. 2003;44:550–558. doi: 10.1046/j.1528-1157.2003.19102.x. [DOI] [PubMed] [Google Scholar]
- 88.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 89.Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, Drevets WC, Charney DS. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 91.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 92.Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton magnetic resonance spectroscopy of malformations of cortical development causing epilepsy. Epilepsy Res. 2007;74:107–115. doi: 10.1016/j.eplepsyres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, MMP, JCP Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 94.Winkelman JW, Buxton OM, Jensen JE, Benson KL, O’Connor SP, Wang W, Renshaw PF. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS) Sleep. 2008;31:1499–1506. doi: 10.1093/sleep/31.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hasler G, van der Veen JW, Geraci M, Shen J, Pine D, Drevets WC. Prefrontal cortical gamma-aminobutyric Acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2009;65:273–275. doi: 10.1016/j.biopsych.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, Renshaw PF, Pollack MH. Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:427–434. doi: 10.1016/j.pnpbp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 97.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MR spectroscopy of metabolite concentrations in temporal lobe epilepsy and effect of temporal lobe resection. Epilepsy Res. 2009;83:168–176. doi: 10.1016/j.eplepsyres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T. In vivo quantification of intracerebral GABA by single-voxel 1H-MRS-How reproducible are the results? Eur J Radiol. 2009;73:526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]