Abstract
Pitch can be conveyed to cochlear implant (CI) listeners via both place of excitation and temporal cues. The transmission of place cues may be hampered by several factors including limitations on the insertion depth and number of implanted electrodes, and the broad current spread produced by monopolar stimulation. The following series of experiments investigate several methods to partially overcome these limitations. Experiment 1 compares two recently published techniques that aim to activate more apical fibers than produced by monopolar or bipolar stimulation of the most apical contacts. The first technique (phantom stimulation) manipulates the current spread by simultaneously stimulating two electrodes with opposite-polarity pulses of different amplitudes. The second technique manipulates the neural spread of excitation by using asymmetric pulses and exploiting the polarity-sensitive properties of auditory nerve fibers. The two techniques yielded similar results and were shown to produce lower place pitch percepts than stimulation of monopolar and bipolar symmetric pulses. Furthermore, combining these two techniques may be advantageous in a clinical setting. Experiment 2 proposes a novel method to create place pitches intermediate to those produced by physical electrodes by using charge-balanced asymmetric pulses in bipolar mode with different degrees of asymmetry.
I. INTRODUCTION
A. Background
Contemporary cochlear implants (CIs) mimic the frequency-to-place mapping performed by the normal cochlea by passing sounds through a bank of bandpass filters and directing the outputs of these filters to several electrodes implanted inside the cochlea. “Place pitch” commonly refers to the perceived difference produced by presenting the same stimulus to different intracochlear electrodes; CI listeners are usually able to scale their electrodes from “low pitch” to “high pitch” when stimulating progressively more basal contacts. However, the range of place pitches that a CI user can experience may be limited by several factors, including (1) the limited insertion depth of the electrode array, (2) the finite number of implanted electrodes, and (3) the presumably broad current spread produced by monopolar stimulation. Here, we present the results of two experiments designed to partially overcome these limitations.
B. Phantom stimulation and asymmetric pulses
The electrode array of most available CIs is usually not inserted all the way to the apex of the cochlea. Consequently, auditory nerve fibers that would normally code the low-frequency part of the spectrum may not be activated, even by the most apical contacts. This hypothesis is supported by experiments with patients having residual low-frequency hearing contralateral to the implanted ear (Baumann and Nobbe, 2006; Vermeire et al., 2008; McDermott et al., 2009). We recently performed a pitch matching study with CI subjects having completely normal contralateral hearing and no prior listening experience with their device (Carlyon et al., 2010). Subjects were asked to compare the pitch elicited by monopolar stimulation of individual electrodes of their CI to the pitch of acoustic pulse trains filtered in different frequency regions and presented to their contralateral ear. It was found that the pitch of the most apical electrode corresponded to acoustic center frequencies ranging from 952 to 966 Hz for the three subjects for whom reliable estimates could be obtained. Because dropping acoustic frequency information below this cut-off may impair speech perception (Faulkner et al., 2003; Başkent and Shannon, 2005), speech processors usually redirect this part of the spectrum to the most apical electrodes of the array, thereby leading to a mismatch between the acoustic frequency and the place of stimulation in the cochlea. While patients can get used to this mismatch over time, it is unclear whether complete adaptation may be obtained (Rosen et al., 1999; Fu et al., 2002).
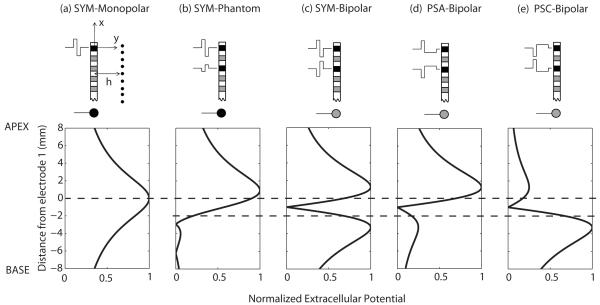
Two techniques have been proposed that may reduce this mismatch, and, possibly, increase the number of functional CI channels (Saoji and Litvak 2010; Macherey et al., 2011). Both techniques involve manipulating the waveform delivered to the electrodes to recruit more apical fibers than is normally achieved by existing CI processing strategies. To predict the effects of such waveform manipulations, let us consider a very simple geometrical model where the electrodes are assumed to be point current sources arranged along an axis x and where neural elements are located at a distance y=h from the electrode array (Fig. 1a, top panel). We assume a homogeneous medium with constant resistivity ρ. Fig. 1a (bottom panel) shows the extracellular potential, distributed along the x axis, produced by the anodic phase of a monopolar symmetric biphasic pulse. This configuration is currently implemented in the vast majority of commercial CIs and shows a broad distribution centered on the stimulating electrode. Note that the second, cathodic phase of the pulse produces a voltage distribution that is the exact opposite to that produced by the anodic phase (negative values). As a first approximation, let us assume (1) that only positive current injection produces neural excitation, which is at least approximately true for the loudness levels used here (Macherey et al., 2006, 2008, 2010; Undurraga et al., 2010), (2) that neural excitation at a given place is proportional to the extracellular potential at this same place, and (3) that the place pitch corresponds to the center of gravity of the voltage distribution. In accordance with the first assumption, the plots in Fig. 1 and elsewhere show only positive voltage deflections arising from the two phases of each pulse. The contribution of each active electrode i to the total voltage at point (x,h). is given by (cf. Rattay et al., 1989) where x and xi are measured relative to the location of electrode 1, and Ii is the current delivered by electrode i. Contributions from the different active electrodes are summed to produce the final distribution.
Figure 1.
Top: Schematic illustration of different stimulation techniques. The black electrode contacts are the active contacts. The gray contacts are not active. The ball electrode at the bottom represents the extracochlear ground. Neurons are located at a distance h from the electrode array. Bottom: Predicted voltage distributions along the x axis for (a) symmetric monopolar, (b) symmetric phantom, (c) symmetric bipolar, (d) pseudomonophasic anodic-first bipolar, and (e) pseudomonophasic cathodic-first bipolar. Only positive voltage deflections are shown. For illustration purposes, neighboring electrodes are assumed to be separated by 1 mm., which corresponds approximately to the separation found in the HiRes 90k implant, h is assumed to be 3 mm and the fraction of current returning to electrode E3 in condition (b) is 0.75. The voltage plots are normalized re the maximum. The dashed lines indicate the positions of the active intracochlear electrode(s) E1 and E3.
The first technique, referred to as phantom electrode stimulation, was initially proposed by Wilson et al. (1992) and recently tested by Saoji and Litvak (2010). The idea, illustrated in Fig. 1b, is to present a symmetric biphasic pulse on the most apical electrode (called the “primary” electrode) and, at the same time, to present a lower-amplitude, opposite-polarity pulse on a neighbouring electrode (called the “compensating” electrode). The remainder of the current presented via the primary electrode is returned via an extra-cochlear electrode. The ratio of amplitude between the currents applied to the compensating and primary electrodes is referred to as σ. When σ is relatively small, the peak of the distribution is shifted apically compared to a purely monopolar pulse. As σ is further increased, the peak continues to shift apically but, at the same time, a secondary peak appears proximal to the compensating electrode (Fig. 1b). This secondary peak may produce excitation of fibers near the compensating electrode, thereby counteracting the effect of lowering the place pitch. When the two pulses have equal amplitude (i.e., σ=1; Fig. 1c), the stimulation is referred to as bipolar and shows a symmetric bimodal distribution. The pitch of the bipolar signal may in this case become higher than that of a monopolar signal because the center of gravity is located in-between the two electrodes of the bipolar channel. Consistent with this simple model, Saoji and Litvak (2010) found in half of their subjects that the perceived pitch first decreased with increases in σ and subsequently increased for higher values of σ. For their other subjects, no pitch reversal was observed. However, apart from one subject for whom pitch decreased up to σ=1, the other subjects could not be tested at high values of σ due to current source limitations. It is worth noting that the model predictions of Fig. 1 represent the extracellular voltage distribution and not neural excitation per se. It is likely that additional factors such as differences in neural survival or in the distance between the electrodes and the nerve fibers play an important role in perceptual experiments. For example, if the compensating electrode is in a “dead” region, it may not excite auditory nerve fibers in its vicinity, even for high values of σ, and so pitch will decrease monotonically with increasing σ.
The second technique stems from psychophysical and electrophysiological data showing that the positive phase of a pulse is responsible for exciting auditory neurons in CI patients, and from the fact that, with a “pseudomonophasic anodic-first” waveform (PSA, Fig. 1d), neural stimulation arises from the short, high-amplitude phase rather than from the “long low” phase of each pulse (Macherey et al., 2006, 2008, 2010; Undurraga et al., 2010). Still assuming that neural excitation is only produced by positive current injection, the use of PSA (Fig. 1d) should have the effect of reducing the excitation of fibers near the more basal electrode compared to symmetric pulses (Fig. 1c) because, for the same amount of charge, a long-duration pulse sounds softer and presumably produces less neural excitation than a short-duration pulse (Shannon, 1985; Macherey et al., 2006).
Consistent with this prediction, we have shown that the pitch is lower when the short-high amplitude anodic phase is applied to the more apical (PSA on Fig. 1d) than when it is applied to the more basal electrode (PSC on Fig. 1e; Macherey et al., 2011). More importantly, we have shown that the pitch of PSA is even lower than the pitches obtained with monopolar (Fig. 1a) or bipolar symmetric pulses (Fig. 1c) presented to the most apical channel of a CI. This result was observed at the apex of the cochlea and at a low pulse rate of 12 pps. The use of this very low pulse rate precluded the subjects from using possible temporal pitch cues and ensured that the results only reflected place-of-excitation cues (i.e., place-pitch cues).
In Section II, we compare and combine these two techniques (phantom stimulation and asymmetric pulses) in order to find the stimulus configuration that can produce the lowest place pitch.
C. Manipulation of amplitude and duration ratios
Place-pitch percepts intermediate to those produced by stimulation of physical electrodes can be created by simultaneous or sequential stimulation of neighbouring intracochlear electrodes (McDermott and McKay, 1994; Donaldson et al., 2005). Simultaneous activation of adjacent electrodes is referred to as current steering and is implemented in the commercially-available Fidelity 120 strategy of the Advanced Bionics device. Despite the ability to increase the number of place-pitch percepts beyond the number of physical electrodes (Donaldson et al., 2005), this strategy has only produced modest or no improvement in speech perception compared to more common (CIS-like) strategies (Buechner et al., 2008; Firszt et al., 2009; Donaldson et al., 2011). One possible reason for this is that the current steering technique involves the summation of current produced by the two electrodes, and does not improve spatial resolution per se. Several authors have proposed that better spatial resolution may be obtained via current focusing techniques where current flows from one intracochlear electrode to another or to several other intracochlear electrodes so as to restrict the spatial extent of stimulation (cf. Bonham and Litvak, 2008 for a review). Recently, Landsberger and Srinivasan (2009) and Srinivasan et al. (2010) have proposed a combination of current steering and current focusing that both increase the number of pitch percepts while sharpening the spatial pattern of stimulation.
In Section III, we investigate a novel method that we term “neural steering”, also designed to create place pitches intermediate to those produced by stimulation of the physical electrodes. The stimuli are asymmetric pulses presented in bipolar configuration (Fig. 1d, e) and may therefore be more spatially-selective than the monopolar stimuli required for current steering. The basic idea is to create a continuum of place pitch between the PSA and PSC stimuli presented in Fig. 1d and 1e, respectively. As previously noted, we have shown that PSA has a lower pitch than PSC (Macherey et al., 2011). That result was obtained at two different intracochlear sites and at two different pulse rates (12 and 1031 pps), with a ratio of four between the amplitudes of the short-high and long-low phases. Presumably, lower ratios will produce smaller pitch differences and one aim of experiment 2 was to determine the minimum ratio needed to produce a significant pitch difference. In addition, by systematically modifying the ratio of duration/amplitude of the two phases of the asymmetric bipolar pulse, we predicted that it should be possible to generate a continuum of pitches. Specifically, for PSA (Fig. 1d), the pitch should get higher as the ratio of duration between the long and the short phase decreases up to the point where the pulse is symmetric (Fig. 1c). If the leading polarity is then reversed and the ratio is increased, the pitch should get even higher (cf. PSC, Fig. 1e).
II. EXPERIMENT 1: Symmetric and asymmetric phantom stimulation
A. Rationale and methods
Figure 2 shows the predicted voltage distributions produced by positive current injections for symmetric (a) and asymmetric (b) phantom stimuli. Our hypothesis is that for a given σ, the use of asymmetric pulses will reduce the excitation of nerve fibers proximal to the compensating electrode compared to the symmetric case (compare the middle panels of Fig. 2a and 2b). We expect that for small values of σ, the opposite-polarity pulse presented on electrode 3 would only shift the peak of excitation to a more apical site without producing unwanted excitation near the compensating electrode for both symmetric and asymmetric pulses. The pitch of the symmetric and asymmetric phantom should therefore be the same at such “low” σ values. However, for higher values of σ, neural excitation near the compensating electrode should be more prominent for a symmetric than for an asymmetric pulse. The pitch of symmetric phantom should therefore be higher than that of asymmetric phantom at these “high” σ values.
Figure 2.
Schematic representation of the stimuli used in Experiment 1 together with predicted voltage distributions. (a) symmetric phantom condition. (b) asymmetric phantom condition. σ=0 corresponds to monopolar stimulation; σ=1 corresponds to bipolar “BP+1” stimulation.
1. Subjects and Experimental platform
Seven users (S1-S4, S6, S9 and S10) of the CII/HiRes 90k CI manufactured by Advanced Bionics took part. This research was approved by the Cambridge Local Research Ethics committee and informed consent was obtained from all subjects. Six of the subjects (S1-S4, S6 and S9) participated in a related study on pitch perception and their labels are consistent with those used previously (c.f. Macherey et al., 2011). Subjects were paid for participating. The tests were performed using the Apex 2 experimental software platform (Laneau et al., 2005), modified to incorporate the BEDCS software provided by Advanced Bionics.
2. Stimuli
Stimuli were trains of 400-ms charge-balanced symmetric or asymmetric pulses delivered at a rate of 12 pps (five pulses per stimulus) or at a rate of 1031 pps. The low rate of 12 pps was chosen because it is below the lower limit of melodic pitch measured in normal-hearing listeners (Krumbholz et al., 2000). It ensured that pitch comparisons would solely reflect place pitch cues (Carlyon et al., 2010). The experiment was also repeated at a rate of 1031 pps because this rate is more comparable to those used in contemporary CI strategies. The phase duration was 97 μs for the symmetric and for the first phase of the asymmetric pulses. The second phase of the asymmetric pulses was four times longer with its amplitude reduced by the same factor. There were 10 stimuli in total differing in their pulse shape (symmetric or asymmetric) and in the proportion of current σ returning to the compensating electrode. Examples of these stimuli are illustrated in Fig. 2. Five values of σ were tested ranging from 0 to 1 in steps of 0.25, thereby creating a continuum between purely monopolar (σ=0) and purely bipolar “BP+1” (σ=1) mode. The primary electrode was the most apical electrode (E1). The compensating electrode was electrode E3 (about 2.2 mm away from E1) and the ground electrode was the extracochlear case electrode. A separation of two electrodes between the primary and the compensating electrodes was chosen because it produced similar pitch shifts as a separation of one electrode in the study of Saoji and Litvak (2010). Furthermore, it has the advantage of maximizing the chances of reaching comfortably loud percepts in bipolar mode without exceeding the current limits imposed by safe charge density injection or by compliance (cf. Saoji and Litvak, 2010). In the present study, impedances were monitored at the beginning and at the end of each session and it was ensured that the voltage requirements were always below compliance of the current sources (approximately 7.5 Volts).
3. Procedure
Most comfortable levels (MCLs) were first measured for the 10 stimuli. The monopolar symmetric stimulus was then set to its MCL and was used as the reference to balance in loudness the other nine stimuli, leading to nine stimulus pairs to balance in total. The loudness balancing procedure consisted of presenting one stimulus fixed in level (the standard) followed by a second stimulus to be adjusted (the signal). After each presentation, the subject was asked to press one of six virtual buttons that either increased or decreased the level of the signal by different amounts. The two sounds were then played again with the new level of the signal and this procedure was repeated until the subjects judged the two sounds to be equally loud. Four adjustments per pair were made in the same way as in Macherey and Carlyon (2010). As an example, let us consider that a stimulus termed “X” had to be balanced to the monopolar symmetric pulse train. In the first two adjustments, monopolar symmetric was the standard and X was the signal to be adjusted. In the two remaining adjustments, X was the standard (set at the mean balanced level found in the first two adjustments) and monopolar symmetric was the signal. The level differences in dB obtained in these four adjustments between the two stimuli were then averaged and the balanced MCL for stimulus X was inferred from this mean level difference.
The ten stimuli were then compared in pitch in a two-interval forced choice task using the optimally-efficient mid-point comparison procedure that has been described in detail elsewhere (Long et al. 2005; Macherey and Carlyon, 2010). The procedure consists of making pitch comparisons between pairs of sounds. The choice of sounds to be presented on a given trial is driven by the results of previous trials in such a way that the whole set of stimuli can be pitch-ranked in a minimum of comparisons. On each trial, subjects were presented with pairs of stimuli separated by a gap of approximately 500 ms and were asked to specify which stimulus had the higher pitch. No feedback was provided and the procedure was repeated 20 times, yielding for each of the 10 stimuli a mean pitch rank and a standard error of the mean.
B. RESULTS
1. Loudness balancing
The results of the loudness-balancing procedure were analysed in a three-way repeated-measures ANOVA with treatment factors “pulse rate”, “pulse shape” and “σ”. The levels at 1031 pps were on average 3.1 dB lower than those at 12 pps (F[1,6]=13.8, p=0.01). There was also a significant effect of σ (F[4,24]=244.6, p<0.001), reflecting the fact that more current is needed when σ increases (moving from monopolar to bipolar). Interestingly, there was also a significant effect of pulse shape (F[1,6]=9.3, p<0.023). On average, the levels for the asymmetric stimuli needed to be 0.4 dB lower than those for the symmetric stimuli to elicit the same loudness sensation. This last observation is consistent with previous results showing that asymmetric pulses are more efficient in stimulating nerve fibers than symmetric pulses both in monopolar and in bipolar mode (van Wieringen et al., 2005; Macherey et al., 2006). Finally, there was no significant effect of the different interaction factors (p>0.05).
2. Pitch judgments
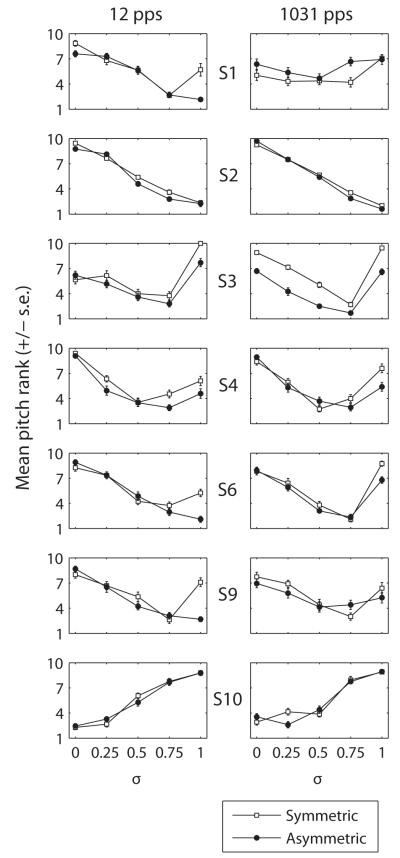
Figure 3 shows, for each subject, the mean pitch rank and the standard error as a function of σ for both symmetric (open squares) and asymmetric (filled circles) pulses. The left and right columns show results at 12 and 1031 pps, respectively. Six of the subjects (S1-S4, S6 and S9) showed a similar trend: pitch decreased with increases in σ up to some value, above which it sometimes increased. Surprisingly, S10 showed the exact opposite trend. He perceived the pitch to increase with increases in σ for both pulse shapes. Because no feedback was given, we further checked that this subject was not confusing the labels “low pitch” and “high pitch”. We asked him to compare the pitches of electrode E1 and E3, E7 and E9, and E14 and E16 all presented in monopolar mode, also without giving him feedback. His judgments were consistent with expected cochlear tonotopy for E7 vs. E9 and for E14 vs. E16 (E9 was judged higher in pitch than E7 and E16 was judged higher in pitch than E14). However, he consistently judged E3 as lower in pitch than E1. This suggests that S10 was not confusing “low” and “high” but that his peculiar results may reflect a pitch reversal at the apical end of his electrode array. The reason for this reversal is unclear; a CT scan revealed no kinks or other reversals in the electrode array. Note that similar pitch reversals have been observed elsewhere (Gani et al., 2007) and may reflect cross-turn stimulation. Given this pitch reversal at the apex, S10 was excluded from the statistical analysis.
Figure 3.
Individual subjects’ results for Experiment 1 at 12 pps (left) and at 1031 pps (right). Each panel shows the mean pitch rank (and standard error) as a function of σ for symmetric (open squares) and asymmetric (filled circles) stimuli.
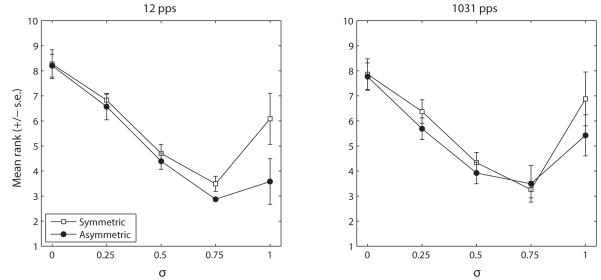
Fig. 4 shows the ranks averaged across all subjects (except S10) at 12 pps (a) and 1031 pps (b). The pitch ranks were analysed in a three-way repeated-measures ANOVA with σ, pulse shape, and stimulation rate as treatment factors. The Huyn-Feldt correction was used. Overall, asymmetric stimuli produced a significantly lower pitch than symmetric stimuli (F[1,5]=7.6, p=0.04). More importantly and consistent with our initial hypothesis, there was a highly significant interaction between σ and pulse shape (F[4,20]=7.0, p=0.001), reflecting the fact that the non-monotonic trend of the pitch rank vs. σ function was more prominent for symmetric than for asymmetric phantom pulses. Finally, the effect of σ was also significant (F[4,20]=12.2, p=0.003) but neither the factor “rate” nor any of the other interactions were significant (p>0.05). Subsequent pairwise comparisons showed that symmetric and asymmetric phantom data only significantly differed from each other at σ=1 (p=0.01 at 12 pps; p=0.02 at 1031 pps). As one aim of this experiment was to compare the decrease in pitch obtained with asymmetric pulses and with symmetric phantom pulses, data obtained at σ=1 for asymmetric stimulation and at the σ producing the lowest pitch for symmetric phantom stimulation (σ=0.75) were additionally compared in a t-test but showed no significant differences.
Figure 4.
Summary data of Experiment 1 at 12 pps (left) and at 1031 pps (right). The data of S10 were not included in the average for reasons given in Section II.B.2.
3. Individual pitch results
(1) 12 pps
For subjects S1-S4, S6 and S9, the results of the symmetric pulse condition were consistent with the findings of Saoji and Litvak (2010). Five of these six subjects showed non-monotonic functions of pitch vs. σ, likely due to a significant influence of the compensating electrode on neural excitation for σ higher than 0.5 or 0.75. One subject (S2) showed a monotonic decrease in pitch over the whole range of σ. For the asymmetric pulse condition, however, only two subjects showed a non-monotonic pattern. Furthermore, the value of σ at which pitch started to increase again for these two subjects was either the same or higher for asymmetric pulses than for symmetric pulses. For the other four subjects, pitch continued to go down or remained the same up to the highest value of σ=1.
Although there was no significant difference overall in pitch rank between symmetric and asymmetric phantom at the σ giving the lowest pitch (0.75), the pitch reversal never occurred for σ below 0.75 for asymmetric pulses whereas it could occur at a σ of 0.5 for symmetric phantom (cf. Saoji and Litvak, 2010). If one does not know a priori the functions relating pitch vs. σ (which would be the case in a clinical environment) and wants to reach the lowest pitch, asymmetric phantom may therefore be a better choice than symmetric phantom. In this case, our data suggest that an optimal σ would be 0.75.
(2) 1031 pps
Although there was no significant effect of stimulation rate, the data obtained at 1031 pps noticeably differed from those obtained at 12 pps for S1 and S6. First, the ranks obtained with S1 were highly variable and could vary substantially from one repetition to another. This is reflected by the fact that the ranks are all scattered around the middle of the range. The subject herself reported that the task was very difficult. Second, S6 showed a non-monotonic pattern for asymmetric phantom at 1031 pps whereas the function was monotonically decreasing at 12 pps. As discussed in Section IV, these discrepancies may have been due to the influence of temporal pitch cues.
III. EXPERIMENT 2: MANIPULATION OF DURATION AND AMPLITUDE RATIOS
A. Rationale and methods
Figure 5 illustrates several asymmetric pulse shapes presented in bipolar mode together with the predicted voltage distributions. The shapes differ in the ratio of duration between the second and the first phase and in the leading polarity relative to the most apical electrode. As previously mentioned, we hypothesize that the pitch elicited by such stimuli should progressively increase from the left panel to the right panel.
Figure 5.
Schematic representation of the stimuli used in Experiment 2a and 2b together with predicted voltage distributions.
1. Subjects
Five subjects (S2-S4, S6 and S11) took part. Four (S2-S4, S6) had already participated in Experiment 1.
2. Experiment 2a: Pitch Ranking
As a proof of concept, we first asked the 5 subjects to pitch rank eight 400-ms pulse trains differing in the ratio of duration between the long and the short phase and in their leading polarity relative to the most apical electrode (Fig. 5). Four ratios (8, 4, 2 and 1, i.e., symmetric) and two leading polarities (anodic or cathodic) were tested. The duration of the first phase was always 97 μs and the pulses were presented at a rate of 1031 pps. The stimuli were presented in bipolar mode on electrodes E1 and E3. They were first loudness balanced at a comfortably loud level using the same loudness-balancing procedure as in Expt 1. The reference sound for loudness-balancing was the symmetric anodic-first bipolar stimulus (A1, Fig. 5c). The eight stimuli were then pitch ranked, using between ten and twenty repetitions of the mid-point comparison procedure. We will denote each of these stimuli as a letter followed by a number. The letter (A or C) represents the leading polarity relative to the apical electrode and the number (from 1 to 8) represents the ratio of duration between the second and the first phase of the pulses.
3. Experiment 2b: Psychometric functions
In Experiment 2b, we used the method of constant stimuli and measured two psychometric functions in order to determine the smallest difference in duration ratio that produced a noticeable change in pitch. This measure has the advantage (compared to the midpoint comparison procedure used in Expt. 2a) of being comparable to traditional measures of discriminability. The stimuli included the same ones as those used in Expt. 2a plus two additional ratios of 1.3 and 2.7, also balanced in loudness to the same reference. The two psychometric functions were measured simultaneously. The standards were A8 and C8. They were each compared to 11 different stimuli in a two-alternative forced-choice task. This led to a total of 22 different trials presented in random order. These trials were presented in blocks of 110 (5 repetitions per trial) and each block was repeated 8 times (resulting in 40 trials per pair in total). Subjects were asked to indicate on each trial which sound was higher in pitch. The two psychometric functions were mixed to avoid presenting the subjects with the same standard on every trial. No feedback was provided. This measure was performed at two different rates (12 and 1031 pps) for all subjects except S11. S11 only performed the 1031-pps condition because she did not feel comfortable with the 12-pps sounds (their “pulsatile” sound quality was unusual and disturbing to her). For S3, the psychometric functions at 12 pps were collected in two separate sessions (on different days). At the beginning of the second session, the loudness of the stimuli was re-checked and it was found that the levels used in the first session sounded too loud. They were therefore decreased by a factor of 0.9 without performing the loudness-balancing again. For this particular subject at this particular rate, 5 blocks per session were performed, leading to a total of 50 trials per pair. Before collecting the pitch data in the second session, the stimuli with the new levels were played sequentially and the subject found them to have the same loudness. Importantly, given that his pitch results were very similar for the two sets of levels, they will be combined in section III.B.2.
B. Results
1. Experiment 2a: Pitch Ranking
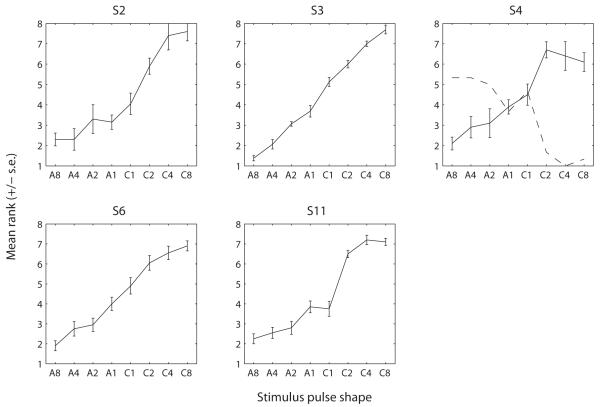
The solid lines in Fig. 6 show the mean pitch rank as a function of pulse shape for each subject. The pulse shapes on the x-axis were ordered according to our hypothesis: A8 (the first point) should give the lowest pitch. As the ratio of duration decreases, the pitch should get higher because there will be relatively more and more excitation near electrode E3. When the leading polarity becomes negative (C1, Fig. 5d), the excitation should then be less and less pronounced near electrode 1 as the ratio of duration is further increased. The results from all five subjects are consistent with this hypothesis. The function was overall monotonic over the range of ratios that we tested. There was also a substantial inter-subject variability. For example, subject S3 shows small standard errors and seemed to be able to discriminate all stimuli whereas subject S4 had large standard errors. It is interesting to note that C1 was higher in pitch than A1 for all subjects except S11 despite the predicted voltage distributions being identical. This could be due to nerve fibers being more effectively driven by the anodic phase when it is presented first than when it is presented second. Indeed, when it is presented first, it does not have to overcome the effect of a previous opposite-polarity phase. For each duration ratio, we performed paired-sample t-tests (using the Bonferroni correction) to determine the smallest ratio at which a change in polarity produced a significant difference in pitch rank. For S3, the ranks of A1 and C1 were significantly different (p=0.024). For all other subjects, a ratio of 2 was sufficient to produce a pitch difference between the two polarities (p<0.05).
Figure 6.
Results of Experiment 2a. Each panel shows the results for one subject. As for Figure 3, the function shows the mean pitch rank (and standard error) as a function of the pulse shape. The dashed line in panel S4 shows the results of the same task repeated in a subsequent session (discussed in the Section III.B.2).
2. Experiment 2b: Psychometric functions
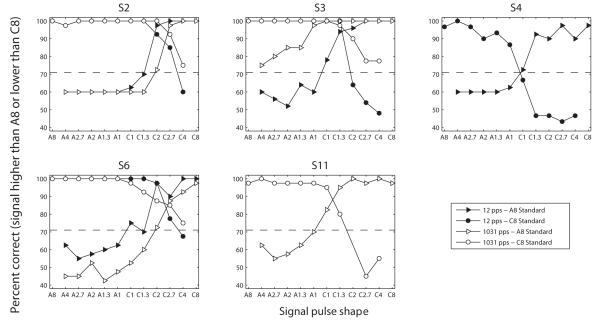
Fig. 7 illustrates the psychometric functions measured in Experiment 2b at 12 pps (filled symbols) and at 1031 pps (open symbols). The functions connecting triangles show the percentage of times the signals were judged higher in pitch than the A8 standard while the functions connecting circles show the percentage of times the signals were judged lower in pitch than the C8 standard. The dashed line shows the ratio at which the functions cross the 71% point; this corresponds to the value to which two-down, one-up adaptive procedures, which are common in auditory psychophysics, converge. For all subjects and both rates, the two psychometric functions intercept at or above this dashed line. This means that there was at least one intermediate pitch that the subjects could perceive between the two endpoint conditions A8 and C8. Thus the number of discrete pitch steps that can be produced with a single BP+1 bipolar channel is 3 or more, i.e. the same or more than the number of actual physical electrodes located in-between and including the electrodes of the bipolar pair.
Figure 7.
Results of Experiment 2b showing the psychometric functions for each subject. Due to a mistake in the experiment file, the comparison between C8 and C2 was not performed with S11 and the corresponding data point is missing in the figure.
The data of subject S4 are not shown for a rate of 1031 pps for the following reason. When we started running the task at 1031 pps, we observed that his judgments were opposite to what we expected: A8 was judged higher in pitch than the other stimuli and C8 was judged lower in pitch. Given that these results were inconsistent with the results obtained in Exp 2a with this same subject, we retested him on the exact same task as performed in Exp 2a and obtained ranks illustrated by the dashed line on Fig. 6 (cf. panel S4). This rank function shows the exact opposite trend as the one collected in the first session. Similarly as for subject S10 in Expt. 1, we wondered if this change could have resulted from the subject confusing “low pitch” and “high pitch” labels. So we asked him to discriminate monopolar pulse trains presented on E1 and E3. He always rated E3 as higher in pitch than E1, consistent with expected cochlear tonotopy, meaning that he was probably not confusing “high” and “low”. Therefore, his reversed judgments from one session to the next seem to reflect that these asymmetric stimuli have an ambiguous pitch. The possible reasons for this ambiguity will be discussed in Section IV. For now, it is worth noting that the psychometric functions obtained at 12 pps were consistent with the results of Exp. 2a and with the results obtained with the other subjects.
There was no consistent effect of pulse rate across the three subjects who performed both conditions. Only S3 seemed to be particularly better at 1031 pps (where A8 and A4 and C8 and C4 were already discriminable) than at 12 pps.
IV. DISCUSSION
A. Validity of the model’s assumptions
In the present study, we have used the distribution of the extracellular potential as a means of predicting the place of excitation along the cochlea. Although this simple model captures qualitatively many of our observations, it also has several limitations. First, we did not model neural excitation per se. Extracellular stimulation (whether it is anodic or cathodic) will produce regions of depolarization and of hyperpolarization along each nerve fiber (Rattay, 1989). These patterns of polarization depend on many factors including the geometry of the neuron, the presence of peripheral processes, their degree of myelination and their distance from the electrode (e.g., Rattay et al., 2001). There is at present no simple computational model that can account for the finding that anodic stimulation is more effective than cathodic stimulation at comfortably loud levels (as we have observed in CI listeners) so it was chosen here to only make simple geometrical considerations based on the current spread.
Second, we have only considered the instantaneous extracellular potential. However, the trans-membrane potential of the neurons needs time to be depolarized and to fire an action potential. If neurons behaved as perfect integrators of charge, the long, low-amplitude phase of asymmetric pulses would produce the same amount of neural excitation as the short, high-amplitude phase and we would expect the pattern of excitation in response to PSA or to PSC in Fig. 1 to be the same as that of a symmetric bipolar pulse (i.e., bimodal as shown in Fig. 1c). However, there is strong evidence that this is not the case and that long phases need more charge than short phases to reach detection threshold or to produce the same loudness percept (at least for the range of phase durations used here; e.g., Shannon, 1985; Moon et al., 1993, Zeng et al., 1998; McKay and McDermott, 1999; Macherey et al., 2006). So we still expect the short, high-amplitude phase to be more effective than the long, low-amplitude phase but it may well be that the voltage plots shown in this paper exaggerate the actual difference in neural excitation produced by the short-high and long-low phases.
Third, we have assumed that only positive current injection will produce neural excitation. However, Undurraga et al. (2011) have recently shown that the electrically-evoked auditory brainstem response to a cathodic phase of a biphasic pulse is, although smaller than that produced by the anodic phase, still present. Therefore, there is likely to be a small effect of cathodic currents that we have not considered here.
B. Differences between 12 and 1031 pps
While the results collected at 12 and at 1031 pps were overall similar in both experiments, there were also two noticeable differences.
Subject S1 did not manage to rank the different phantom stimuli at 1031 pps (showing great variability) while she could do so at 12 pps.
Subject S4 reversed his pitch judgments from one session to the next in Expt. 2a. This strongly suggests a pitch ambiguity associated with bipolar asymmetric pulses presented at 1031 pps. However, no such ambiguity was found at 12 pps (when collecting data in Expt. 2b).
It is worth noting that these two subjects already showed very different place pitch discrimination results at 12 and at 1031 pps in a previous experiment (c.f. Fig. 5 in Macherey et al., 2011). We also showed in this previous paper that the upper limit of temporal pitch could vary as a function of stimulation site and of pulse shape. In particular, we found that stimulating the apex of the cochlea with bipolar asymmetric pulses produced a consistently higher upper limit than stimulating other sites or with other pulse shapes. If the temporal pitch cues mediated by different pulse shapes at 1031 pps are different, they may influence the subjects’ place pitch judgments. More specifically, if stimulating more and more apically increases the temporal pitch cue that is being conveyed, subjects may have to choose to listen to one of two available cues (place pitch going down and temporal pitch going up). This may result in confusions as shown by S1 or to ambiguous judgments from one session to another as shown by S4. This may also explain why S6 showed a pitch reversal in Expt. 1 at 1031 pps but not at 12 pps for asymmetric phantom stimulation. Indeed, the possibility of conflicting temporal and spatial cues at higher pulse rates was why we chose to include the 12-pps rate in this and previous studies (Carlyon et al., 2010; Macherey et al., 2011). One could further test whether this ambiguity could account for pitch reversals of an individual subject by using a multidimensional scaling paradigm, as we and others have done in the past to test the independence of place and temporal pitch cues (Tong et al., 1983; Kong and Carlyon, 2010; Macherey et al., 2011). Nevertheless, given the small number of subjects tested here, it is at present unclear how often such “pitch ambiguities” may arise and what impact they would have on speech perception if these pulse shapes were implemented in a clinical processor.
C. Clinical implications
1. Stimulating more apically
Stimulating more apically may be interesting for three reasons. First, it would functionally extend the range of nerve fibers that can be recruited by the implant. This may result in more independence between the low-frequency channels of the CI; for example, if all except one channel is stimulated conventionally, and the most apical channel is stimulated in such a way as to produce more apical excitation, then this should result in a greater separation between the excitation patterns produced by the apical and other electrodes. However, this conjecture remains to be tested by, e.g., measuring forward-masked excitation patterns. In terms of speech perception, this may improve the neural representation of low-frequency phonetic cues such as nasality. Second, in patients with residual low-frequency hearing in the implanted ear, it may reduce the electrode insertion depth needed to stimulate a given range of the cochlea, thereby potentially reducing the amount of damage caused to that residual hearing. Third, it may also help to convey temporal pitch cues up to higher rates than can be achieved at other stimulation sites (cf. Macherey et al., 2011). We are currently carrying out experiments to investigate whether these additional cues can improve patients’ performance on music and speech perception tasks. The results presented in Section II also show that combining asymmetric pulses and phantom electrode may allow one to use higher σ values while still being in the decreasing pitch arm of the function relating pitch and σ.
Despite these possible advantages, the size of the pitch shift is likely to be modest. In Macherey et al. (2011), we asked two CI subjects with normal contralateral hearing to match the pitch of acoustic pulse trains presented to their normal-hearing ear to the pitch of electrical pulse trains. We found the pitch of PSA (σ =1) presented on (E1, E3) to be 10 and 20%, respectively, lower than the pitch of a monopolar pulse train presented on E1. This would be approximately equivalent to having an additional electrode inserted 1 mm. deeper in the cochlea. Saoji and Litvak (2010) found that their symmetric phantom stimuli produced a pitch shift that was similar in size. The benefits of such a small shift on speech recognition are unclear. The effects of spectral mismatch have been investigated in normal-hearing subjects listening to vocoded speech (Dorman et al., 1997; Fu and Shannon, 1999). The spectral mismatch conditions were obtained by shifting the center frequencies of the synthesis filters relative to those of the analysis filters. Fu and Shannon (1999) found that vowel recognition remained high for spectral shifts less than 3 mm. but significantly dropped for larger shifts. It is therefore possible that the pulse shape manipulations proposed in the present paper would benefit patients with shallow insertion depths. Another way of recruiting more apical fibers is to insert the electrode array deeper in the cochlea. However, Finley et al. (2008) suggested that deep insertions may cause mechanical trauma near the apex. In particular, they found that insertion depth was significantly related to the number of electrodes located in the scala vestibuli which had been shown previously to correlate negatively with word recognition scores (Skinner et al., 2007).
2. Manipulating amplitude and duration ratios with asymmetric pulses
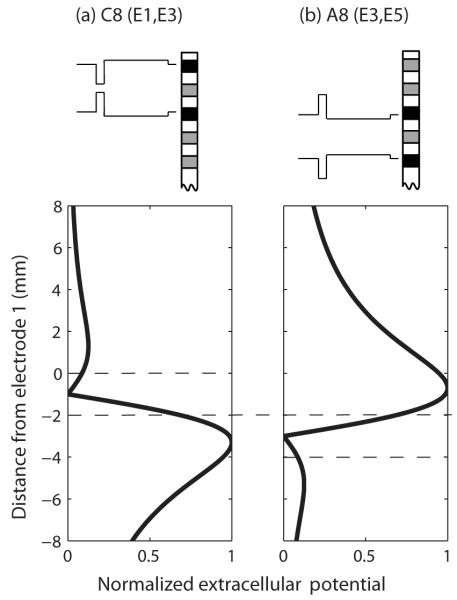
The results presented in Section III describe a novel way of producing place-pitch percepts intermediate to those produced by the physical electrodes of the implant. The potential advantages of such a technique compared to the current steering technique are that only a single current source is needed and that stimulation may be more spatially selective because it is bipolar. There are, however, also two important obstacles to be overcome. First, it remains to be determined whether this technique can be used to create a continuum of place pitches across the electrode array. In the simple situation shown in Fig. 5, every stimulus produces two peaks in the voltage distribution, on the same two electrodes (E1 and E3). The stimuli differ only in the relative amplitude of these two peaks. In contrast, Fig. 8 shows the predicted voltage distributions produced by C8 presented on electrodes E1 and E3 (Fig. 8a) and by A8 presented on electrodes E3 and E5 (Fig. 8b). In this situation, which stimulus has the higher pitch will likely depend on additional factors such as the relative amounts of neural survival near each of these electrodes. Our preliminary attempts to address this issue have so far produced inconclusive results. Second, the duration of the pulses used in experiment 2 were very long; we used a short phase of 97 μs which produced pulse durations ranging from about 200 to about 900 μs. If channels are stimulated non-simultaneously, this would impose substantial limitations on the pulse rates that could be used, at least if the asymmetric pulses were applied to all channels. Potential solutions include applying asymmetric pulses only to a subset of channels, or incorporating these pulse shapes into “sparse” signal processing schemes that require low overall pulse rates (e.g., the peak-derived timing “PDT” strategy, Van Hoesel and Tyler, 2003).
Figure 8.
Predicted voltage distributions produced by two asymmetric pulses presented on different bipolar channels.
SUMMARY.
Both phantom stimulation and the use of asymmetric pulses can produce lower place pitch percepts than can be achieved by symmetric monopolar or bipolar stimulation of the most apical channel.
Increasing σ causes place pitch to vary non-monotonically for symmetric pulse shapes. In contrast, most subjects show a monotonic decrease in pitch with increases in σ for the asymmetric pulse shape used here.
A continuous variation in place pitch can be obtained by variations in asymmetric pulse shape in bipolar mode.
In bipolar mode, place pitch can be varied significantly by inverting the polarity of asymmetric pulse shapes having a ratio of 2, or even less, between the duration of the long and short phases.
ACKNOWLEDGMENTS
This research was supported by grant #080216 from the Wellcome Trust. We thank Randy Kalkman and Johan Frijns of the Leiden University Medical Centre for analysing the CT scan of subject S10. The idea of combining asymmetric pulses and phantom electrode stimulation was suggested to us by Leo Litvak.
REFERENCES
- Başkent D, Shannon RV. Interactions between cochlear implant electrode insertion depth and frequency-place mapping. J. Acoust. Soc. Am. 2005;117:1405–1416. doi: 10.1121/1.1856273. [DOI] [PubMed] [Google Scholar]
- Baumann U, Nobbe A. The cochlear implant electrode-pitch function. Hear. Res. 2006;213:34–42. doi: 10.1016/j.heares.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Bonham BH, Litvak LM. Current focusing and steering: modeling, physiology, and psychophysics. Hear. Res. 2008;242:141–153. doi: 10.1016/j.heares.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner A, Brendel M, Krüeger B, Frohne-Büchner C, Nogueira W, Edler B, Lenarz T. Current steering and results from novel speech coding strategies. Otol. Neurotol. 2008;29:203–207. doi: 10.1097/mao.0b013e318163746. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Macherey O, Frijns JH, Axon PR, Kalkman RK, Boyle P, Baguley DM, Briggs J, Deeks JM, Briaire JJ, Barreau X, Dauman R. Pitch comparisons between electrical stimulation of a cochlear implant and acoustic stimuli presented to a normal-hearing contralateral ear. J. Assoc. Res. Otolaryngol. 2010;11:625–640. doi: 10.1007/s10162-010-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single-versus dual-electrode stimuli by cochlear implant users. J. Acoust. Soc. Am. 2005;118:623–626. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Dawson PK, Borden LZ. Within-subjects comparison of the HiRes and Fidelity 120 speech processing strategies: speech perception and its relation to place-pitch sensitivity. Ear. Hear. 2011;32:238–250. doi: 10.1097/AUD.0b013e3181fb8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman MF, Loizou PC, Rainey D. Simulating the effect of cochlear-implant electrode insertion depth on speech inderstanding. J. Acoust. Soc. Am. 1997;102:2993–2996. doi: 10.1121/1.420354. [DOI] [PubMed] [Google Scholar]
- Faulkner A, Rosen S, Stanton D. Simulations of tonotopically mapped speech processors for cochlear implant electrodes varying in insertion depth. J. Acoust. Soc. Am. 2003;113:1073–1080. doi: 10.1121/1.1536928. [DOI] [PubMed] [Google Scholar]
- Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, Skinner MW. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol. Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Reeder RM, Skinner MW. Speech recognition in cochlear implant recipients: comparison of standard HiRes and HiRes 120 sound processing. Otol. Neurotol. 2009;30:146–152. doi: 10.1097/MAO.0b013e3181924ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV. Recognition of spectrally degraded and frequency-shifted vowels in acoustic and electric hearing. J. Acoust. Soc. Am. 1999;105:1889–1900. doi: 10.1121/1.426725. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Galvin JJ., 3rd Perceptual learning following changes in the frequency-to-electrode asignment with the Nucleus-22 cochlear implant. J. Acoust. Soc. Am. 2002;112:1664–1674. doi: 10.1121/1.1502901. [DOI] [PubMed] [Google Scholar]
- Gani M, Valentini G, Sigrist A, Kós MI, Boëx C. Implications of deep electrode insertion on cochlear implant fitting. J. Assoc. Res. Otolaryngol. 2007;8:69–83. doi: 10.1007/s10162-006-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Carlyon RP. Temporal pitch perception at high rates in cochlear implants. J. Acoust. Soc. Am. 2010;127:3114–3123. doi: 10.1121/1.3372713. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Patterson RD, Pressnitzer D. The lower limit of pitch as determined by rate discrimination. J. Acoust. Soc. Am. 2000;108:1170–1180. doi: 10.1121/1.1287843. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Srinivasan AG. Virtual channel discrimination is improved by current focusing in cochlear implant recipients. Hear. Res. 2009;254:34–41. doi: 10.1016/j.heares.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneau J, Boets B, Moonen M, van Wieringen A, Wouters J. A flexible auditory research platform using acoustic or electric stimuli for adults and young children. J. Neurosci. Methods. 2005;142:131–136. doi: 10.1016/j.jneumeth.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Long CJ, Nimmo-Smith I, Baguley DM, O’Driscoll M, Ramsden R, Otto SR, Axon PR, Carlyon RP. Optimizing the clinical fit of auditory brain stem implants. Ear. Hear. 2005;26:251–262. doi: 10.1097/00003446-200506000-00002. [DOI] [PubMed] [Google Scholar]
- Macherey O, van Wieringen A, Carlyon RP, Deeks JM, Wouters J. Asymmetric pulses in cochlear implants: effects of pulse shape, polarity, and rate. J. Assoc. Res. Otolaryngol. 2006;7:253–266. doi: 10.1007/s10162-006-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP, van Wieringen A, Deeks JM, Wouters J. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J. Assoc. Res. Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP. Temporal pitch percepts elicited by dual-channel stimulation of a cochlear implant. J. Acoust. Soc. Am. 2010;127:339–349. doi: 10.1121/1.3269042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O, Deeks JM, Carlyon RP. Extending the limits of place and temporal pitch perception in cochlear implant users. J. Assoc. Res. Otolaryngol. 2011;12:233–251. doi: 10.1007/s10162-010-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott HJ, McKay CM. Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea. J. Acoust. Soc. Am. 1994;96:155–162. doi: 10.1121/1.410475. [DOI] [PubMed] [Google Scholar]
- McDermott H, Sucher C, Simpson A. Electro-acoustic stimulation. Acoustic and electric pitch comparisons. Audiol. Neurootol. 2009;14:2–7. doi: 10.1159/000206489. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ. The perceptual effects of current pulse duration in electrical stimulation of the auditory nerve. J. Acoust. Soc. Am. 1999;106:998–1009. doi: 10.1121/1.428052. [DOI] [PubMed] [Google Scholar]
- Moon AK, Zwolan TA, Pfingst BE. Effects of phase duration on detection of electrical stimulation of the human cochlea. Hear. Res. 1993;67:166–178. doi: 10.1016/0378-5955(93)90244-u. [DOI] [PubMed] [Google Scholar]
- Rattay F. Analysis of models for extracellular fiber stimulation. IEEE Trans. Biomed. Eng. 1989;36:676–682. doi: 10.1109/10.32099. [DOI] [PubMed] [Google Scholar]
- Rattay F, Leao RN, Felix H. A model of the electrically excited human cochlear neuron. I. Contributions of neural substructures on the generation and propagation of spikes. Hear. Res. 2001;153:43–63. doi: 10.1016/s0378-5955(00)00256-2. [DOI] [PubMed] [Google Scholar]
- Rosen S, Faulkner A, Wilkinson L. Adaptation by normal listeners to upward spectral shifts of speech: Implications for cochlear implants. J. Acoust. Soc. Am. 1999;106:3629–3636. doi: 10.1121/1.428215. [DOI] [PubMed] [Google Scholar]
- Saoji AA, Litvak LM. Use of “phantom electrode” technique to extend the range of pitches available through a cochlear implant. Ear. Hear. 2010;31:693–701. doi: 10.1097/AUD.0b013e3181e1d15e. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear. Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan AG, Landsberger DM, Shannon RV. Current focusing sharpens local peaks of excitation in cochlear implant stimulation. Hear. Res. 2010;270:89–100. doi: 10.1016/j.heares.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MW, Holden TA, Whiting BR, Voie AH, Brundsen B, Neely JG, Saxon EA, Hullar TE, Finley CC. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann. Otol. Rhinol. Laryngol. Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- Tong YC, Blamey PJ, Dowell RC, Clark GM. Psychophysical studies evaluating the feasibility of a speech processing strategy for a multiple-channel cochlear implant. J. Acoust. Soc. Am. 1983;74:73–80. doi: 10.1121/1.389620. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, van Wieringen A, Carlyon RP, Macherey O, Wouters J. Polarity effects on neural responses of the electrically stimulated auditory nerve at different cochlear sites. Hear. Res. 2010;269:146–161. doi: 10.1016/j.heares.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, Carlyon RP, Wouters J, van Wieringen A. Electrically evoked auditory brainstem responses to different pulse shapes. Conference on implantable auditory prostheses; Pacific Grove, CA. 2011. [Google Scholar]
- van Hoesel RJ, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J. Acoust. Soc. Am. 2003;113:1617–1630. doi: 10.1121/1.1539520. [DOI] [PubMed] [Google Scholar]
- van Wieringen A, Carlyon RP, Laneau J, Wouters J. Effects of waveform shape on human sensitivity to electrical stimulation of the inner ear. Hear. Res. 2005;200:73–86. doi: 10.1016/j.heares.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Nobbe A, Schleich P, Nopp P, Voormolen MH, Van de Heyning PH. Neural tonotopy in cochlear implants: an evaluation in unilateral cochlear implant patients with unilateral deafness and tinnitus. Hear. Res. 2008;245:98–106. doi: 10.1016/j.heares.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Lawson DT, Zerbi M, Finley CC. Speech processors for auditory prostheses: Virtual channel interleaved sampling (VCIS) processors—Initial studies with subject SR2, First Quarterly Progress Report, NIH project N01-DC-2-2401. Neural Prosthesis Program, National Institutes of Health; Bethesda, MD: 1992. [Google Scholar]
- Zeng FG, Galvin JJ, 3rd, Zhang C. Encoding loudness by electric stimulation of the auditory nerve. Neuroreport. 1998;9:1845–1848. doi: 10.1097/00001756-199806010-00033. [DOI] [PubMed] [Google Scholar]