Abstract
Background
Compared to troponin alone, a dual-marker strategy with natriuretic peptides may improve acute coronary syndrome (ACS) diagnosis with a single blood draw and provide physiologic information regarding underlying heart disease. We evaluate the value of adding natriuretic peptides (myocyte stress markers) to troponins (myocardial injury markers) for diagnosing ACS in emergency department (ED) patients with chest pain.
Methods
In 328 patients (53 ± 12 years, 63% men) with an initially negative conventional troponin and nonischemic electrocardiogram who underwent 64-slice cardiac computed tomography (CT), we measured conventional troponin-T (cTnT), high-sensitivity troponin-T (hsTnT), N-terminal pro-B type natriuretic peptide (NT-proBNP), and mid-regional pro-atrial natriuretic peptide (MR-proANP). ACS was defined as myocardial infarction or unstable angina. CT was evaluated for coronary plaque, stenosis, and regional wall motion abnormality (RWMA).
Results
Patients with ACS (n=29, 9%) had higher concentrations of each biomarker compared to those without (all p <0.01). Adding natriuretic peptides, especially NT-proBNP, to both cTnT orhsTnT improved the C-statistics and net reclassification index for ACS, largely driven by correctly reclassifying events. Dual-negative marker results improved sensitivity (cTnT 38% to 83–86%, hsTnT 59% to 86–90%; all p <0.01) and negative predictive value (cTnT94% to 97–98%, hsTnT 96% to 97–98%) for ACS. Patients with dual-negative markers had the lowest percentage of CT coronary plaque, stenosis, and RWMA (all p-trend <0.001).
Conclusion
Among ED patients with low-intermediate likelihood of ACS, combining natriuretic peptides with either conventional or highly-sensitive troponin improved discriminatory capacity and allowed for better reclassification of ACS, findings supported by structural and functional CT results.
Keywords: natriuretic peptides, troponins, acute coronary syndrome, emergency department, computed tomography
INTRODUCTION
The current standard for the diagnosis of acute myocardial infarction (MI) includes the measurement of conventional troponin (cTn) levels due to the high specificity of this biomarker for detecting myocardial injury and necrosis, but these assays are limited by their low first-draw sensitivity, and the need for serial blood draws.1 Moreover, unstable angina (UAP) is part of the spectrum and represents a significant number of acute coronary syndrome (ACS) diagnoses that does not involve an elevation or the typical “rise and fall” pattern of cTn.1 Newer and more precise assays of myocardial injury and necrosis, high-sensitivity troponin (hsTn) assays, could replace cTn in the future because of their ability to detect minute quantities of myocardial injury, thus improving sensitivity for the diagnosis of acute MI.2–4 As we and others have shown,2–4 high-sensitivity troponin-T (hsTnT) has greater sensitivity than cTnT for ACS diagnosis in acute chest pain patients presenting to the emergency department (ED); however, its sensitivity remains moderate, particularly for the diagnosis of UAP.5
The natriuretic peptides, including N-terminal pro-B type natriuretic peptide (NT-proBNP) and the newer mid-regional pro-atrial natriuretic peptide (MR-proANP), are cardiac biomarkers secreted into the circulation via atrial and ventricular myocytes,6 and used for diagnosis or exclusion of heart failure (HF); both correlating well with cardiac structure and function.7–8 While natriuretic peptides are cardiac-specific, their role for diagnostic evaluation of patients with suspected ACS remains less well-understood, and results in this regard are mixed.9–10 Given the reduced first-draw sensitivity of troponin for ACS diagnosis, in theory, a clinical approach utilizing biomarkers with complementary physiologies (e.g. myocardial stress and necrosis) might be superior to a single biomarker strategy alone.
There are few data comparing the effectiveness of a multimarker strategy for ACS diagnosisin a single blood draw,9, 11–12 and data are lacking regarding the value of this approach using newer assays such as hsTnT and MR-proANP. Thus, we aim to determine the diagnostic value of a dual marker strategy, particularly dual-negative marker results, for ACS and UAP diagnosis by combining markers of myocardial injury (cTnT or hsTnT) with markers of myocyte stress (NT-proBNP or MR-proANP) in acute chest pain patients presenting to the ED. Additionally, we examine the dual marker results with imaging by evaluating its relationship with prevalence of cardiac computed tomography (CT) features of coronary plaque, stenosis, and regional wall motion abnormalities (RWMA).
METHODS
Study population
The “Rule Out Myocardial Infarction Using Computer Assisted Tomography” (ROMICAT) trial was a prospective study of 368 consecutive adult patients at low-to-intermediate likelihood of ACS who presented to the ED with acute chest pain, hadinitially negative cTnT concentrations and nonischemic electrocardiogram (ECG), and were awaiting hospital admission. The details of the study design have been previously reported.13 All eligible patients who consented underwent ECG-gated contrast-enhanced 64-slice CT and had blood biomarkers drawn at time of CT scan acquisition. We included 328 patients, who had circulating levels of all 4 biomarkers (cTnT, hsTnT, NT-proBNP, and MR-proANP), measured at time of CT scan for the ACS analysis. Since elevated troponin levels are part of the definition for MI,1 we performed a subgroup analysis in 320 patients for UAP without MI. Our institutional review board approved the study protocol and all patients provided written informed consent.
Clinical Endpoint
ACS was defined as either (1) a MI or (2) UAP as previously described.13 For our clinical endpoints of ACS and UAP, an outcome panel of two experienced physicians reviewed patient data forms containing prospectively collected information on the history and nature of chest pain, risk factors and medical history, as well as medical records including the serial cTn levels pertaining to the hospital admission. The outcome panel was blinded to the findings of all the biomarker results used in this analysis and the CT results with disagreement resolved by consensus with an additional cardiologist.
Biomarker Testing
Blood samples for biomarker testing were taken at the time of CT angiography, at a median of 4.2 hours from initial ED presentation. The samples were obtained from a peripheral vein and collected into ethylenediaminetetraacetic acid (EDTA) coated tubes and non-coated tubes. The blood was immediately centrifuged and the aliquoted plasma and serum were stored in microcentrifuge tubes at −80°C until assayed. Specimens were tested on the first freeze thaw cycle. All analyses were run in a blinded fashion. The inter-run coefficient of variation (CV) was ≤ 6.6% and intra-assay CV was ≤3.65% for all markers.
Levels of cTnT (Stat T, Roche Diagnostics, Penzberg, Germany) was measured using a 4th generation immunoassay on an Elecsys® 2010 platform. We used the diagnostic threshold for acute MI of 0.03 ng/mL for this analysis, which is the 99thpercentile for this assay.1 For hsTnT, we used a pre-commercial hsTnT method (Roche Diagnostics, Penzberg, Germany) on an Elecsys® 2010 platform. Given enhanced sensitivity, this assay is reported with units of pg/mL (rather than ng/mL for cTnT) and the 99th percentile for a normal reference population is reported to be 13 pg/mL, which was the cut-point used for this analysis.1
Serum NT-proBNP measurements were performed by an electrochemiluminescence sandwich immunoassay (ELECSYS 2010, Roche Diagnostics). Plasma MR-proANP concentrations were determined with immunoluminometric assays (BRAHMS AG), as described elsewhere.14 For NT-proBNP, the median concentration was 50 ng/L while the optimal cutpoint for ACS using Youden’s index was 44 ng/L. For MR-proANP, the median concentration was 58 pmol/L with an optimal cutpoint for ACS of 65 pmol/L. Since there are currently no established cutpoint for either NT-proBNP or MR-proANP for ACS diagnosis in non-heart failure cohort, we used the cutpointsof 50 ng/L for NT-proBNP and 60 pmol/L for MR-proANP for simplicity.
Cardiac CT Imaging
CT imaging was performed using a 64-slice CT scanner (Sensation 64; Siemens Medical Solutions, Forchheim, Germany), as previously described,13 and evaluated for the presence of coronary plaque and stenosis, which was defined as a luminal obstruction >50% diameter or if coronary stenosis could not be excluded (n=28) and deemed “inconclusive”. RWMA was assessed qualitatively based on the 17-segment model, as previously described.15 Patients where RWMA could not be assessed due to incomplete LV function study (n=11) were classified as having no RWMA.
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) or median with interquartile range [IQR] for continuous variables and as frequency and percentages for nominal variables. Differences between groups were assessed using Student’s t test or Wilcoxon rank sum test for continuous variables and Chi-square test or Fisher’s Exact test for categorical variables, as appropriate. For the diagnostic accuracy of the biomarkers for detecting ACS, we calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Comparisons between sensitivities of two tests and specificities of two tests were performed using McNemar’s test. Logistic regression was used to determine the C-statistic, or area under the receiver operating characteristic curve (AUC), for each biomarker to evaluate the discriminatory capacity of the individual dichotomous biomarker model for ACS. We compared the difference in C-statistics of nested models (model 1 contains first biomarker, model 2 contains the first and second biomarker) using the likelihood ratio testto determine the incremental value. Lastly, net reclassification improvement (NRI) was performed with biomarkers kept as dichotomous variables as described by Pencina et al.16 When comparing added value of one biomarker to another, NRI measures the correctness of reclassification of subjects based on their predicted probabilities of events using the new model. We performed similar analyses for the subgroup of patients without MI for the clinical outcome of UAP. A 2-tailed p-value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS (Version 9.2, North Carolina) and MedCalc (Version 11.6 for Windows, Belgium).
RESULTS
Patient Characteristics
Baseline patient characteristics of the overall cohort and as stratified by ACS are summarized in Table 1. There were 29 (8.8%) patients with ACS diagnosis, of which 8 (2.4%) were MI and 21 (6.4%) were UAP. As compared to patients without ACS, those with ACS were older, more likely to have hypertension, hyperlipidemia, and on aspirin and statin therapy. They also had higher prevalence of CT coronary plaque and extent, coronary stenosis, and RWMA despite similar preserved LV function, volumes, and mass.
Table 1.
Characteristics of the Overall Cohort and as Stratified by Presence of ACS.
| Overall (n = 328) | ACS (n=29) | No ACS (n=299) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 52.6 ± 11.6 | 61.7 ± 11.6 | 51.7 ± 11.3 | <0.0001 |
| Male | 206 (62.8%) | 23 (79.3%) | 183 (61.2%) | 0.07 |
| Caucasian | 281 (85.7%) | 27 (93.1%) | 254 (85.0%) | 0.40 |
| BMI (kg/m2) | 28.9 ± 5.7 | 28.9 ± 4.4 | 28.9 ± 5.8 | 0.63 |
|
| ||||
| Risk factors | ||||
| Diabetes | 37 (11.3%) | 4 (13.8%) | 33 (11.0%) | 0.55 |
| Hypertension | 129 (39.3%) | 18 (62.1%) | 111 (37.1%) | 0.02 |
| Hyperlipidemia | 121 (36.9%) | 16 (55.2%) | 105 (35.1%) | 0.04 |
| Smoking | 155 (47.3%) | 17 (58.6%) | 138 (46.2%) | 0.24 |
| Family history of CAD | 80 (24.4%) | 9 (31.0%) | 71 (23.8%) | 0.37 |
|
| ||||
| Vital signs at triage | ||||
| Systolic blood pressure (mmHg) | 139 ± 22 | 138 ± 21 | 139 ± 22 | 0.89 |
| Diastolic blood pressure (mmHg) | 81 ± 14 | 75 ± 14 | 81 ± 13 | 0.05 |
| Heart rate (bpm) | 77 ± 16 | 72 ± 18 | 78 ± 16 | 0.03 |
|
| ||||
| Medications on presentation | ||||
| Aspirin | 108 (32.9%) | 15 (51.8%) | 93 (31.1%) | 0.037 |
| Beta-blockers | 73 (22.3%) | 11 (37.9%) | 62 (20.7%) | 0.058 |
| Statins | 93 (28.4%) | 15 (51.8%) | 78 (26.1%) | 0.0082 |
| ACE-I | 50 (15.2%) | 6 (20.7%) | 44 (14.7%) | 0.42 |
|
| ||||
| Cardiac CT results | ||||
| Any coronary plaque | 164 (50.0%) | 29 (100%) | 135 (45.2%) | <0.0001 |
| Extent of plaque, median [IQR] | 0.5 [0, 3.5] | 7 [4, 10] | 0 [0, 2] | <0.0001 |
| Coronary stenosis | 61 (18.6%) | 22 (75.9%) | 39 (13.0%) | <0.0001 |
| RWMA | 40 (12.2%) | 21 (72.4%) | 19 (6.4%) | <0.0001 |
| LV EF (%) | 67.5 ± 9.7 | 66.8 ± 11.3 | 67.6 ± 9.6 | 0.86 |
| LV end-systolic volume (ml) | 39.5 ± 20.9 | 43.7 ± 27.5 | 39.1 ± 20.2 | 0.998 |
| LV end-diastolic volume (ml) | 118.1 ± 31.4 | 123.2 ± 35.6 | 117.7 ± 31.0 | 0.62 |
| LV mass (g/m2) | 149.6 ±42.3 | 153.5 ± 34.9 | 149.2 ± 43.0 | 0.51 |
|
| ||||
| Laboratory tests | ||||
| cTnT (ng/mL) | 0.005 ± 0.029 | 0.050 ± 0.085 | 0.0006 ±0.005 | <0.0001 |
| hsTnT (pg/mL) | 5.2 [2.5, 8.6] | 30.5 [8.6, 68.7] | 4.9 [2.4, 7.7] | <0.0001 |
| NT-proBNP (ng/L) | 50 [24, 108] | 100 [53, 238] | 45 [22, 98] | <0.0001 |
| MR-proANP (pmol/L) | 58 [41, 84] | 83 [55, 127] | 57 [41, 80] | 0.005 |
ACS denotes acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; ACE-I, angiotensin converting enzyme inhibitor; CT, computed tomography; IQR, interquartile range; RWMA, regional wall motion abnormality; LV, left ventricular; EF, ejection fraction.
Biomarker Results
All 4 of the biomarkers were elevated in patients with ACS compared to those without (Table 1, all p <0.01). Similar results were seen when patients with UAP were compared to those without (cTnT: 0.005 ng/mL vs. 0.0006 ng/mL, hsTnT: 12.3 pg/mL vs. 4.9 pg/mL, NT-proBNP: 133 ng/L vs. 45 ng/L, and MR-proANP: 84 pmol/L vs. 57 pmol/L; all p <0.005).
Diagnostic Accuracy of Troponins and Natriuretic Peptides for ACS and UAP
Diagnostic accuracies of the biomarkers individually and in combination as dual-negative results are summarized in Table 2. In keeping with the use of troponins as part of the definition of MI, the natriuretic peptides were less specific for the detection of ACS than troponin; however, natriuretic peptides were more sensitive than troponins. Thus, in comparing the single troponin marker to a dual-negative marker combination with natriuretic peptides, we found that a dual-negative marker strategy of troponins and natriuretic peptides improved sensitivity (cTnT 38% to 83–86%, hsTnT 59% to 86–90%; all p <0.005) and NPV (cTnT 94% to 97–98%, hsTnT 96% to 97–98%) for ACS. Similar results were seen for the detection of UAP (Appendix 1), as a dual-negative approach improved both sensitivity (cTnT 19% to 81%, hsTnT 48% to 86%; all p <0.005) and NPV (cTnT 95% to 98%, hsTnT 96% to 98%).
Table 2.
Diagnostic Accuracy of Individual Troponins and the Natriuretic Peptides and Dual-negative Combinations to Detect ACS.*Cutpoints: cTnT0.03 ng/mL, hsTnT, 13 pg/mL, NT-proBNP 50 ng/L, MR-proANP 60 pmol/L.
| Analyte, cut-point | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| cTnT | 11/29 | 295/299 | 11/15 | 295/313 |
| 38% (21–58) | 99% (97–100) | 73% (45–92) | 94% (91–97) | |
| hsTnT | 17/29 | 268/299 | 17/48 | 268/280 |
| 59% (39–76) | 90% (86–93) | 35% (22–51) | 96% (93–98) | |
| NT-proBNP | 24/29 | 160/299 | 24/163 | 160/165 |
| 83% (64–94) | 54% (48–59) | 15% (10–21) | 97% (93–99) | |
| MR-proANP | 20/29 | 162/299 | 20/157 | 162/171 |
| 69% (49–85) | 54% (48–60) | 13% (8–19) | 95% (90–98) | |
|
| ||||
| cTnT(−), NT-proBNP(−) | 25/29 | 160/299 | 25/164 | 160/164 |
| 86% (68–96) | 54% (48–59) | 15% (10–21) | 98% (94–99) | |
| hsTnT(−), NT-proBNP(−) | 26/29 | 154/299 | 26/171 | 154/157 |
| 90% (73–98) | 52% (46–57) | 15% (10–21) | 98% (95–100) | |
| cTnT(−), MR-proANP(−) | 24/29 | 162/299 | 24/161 | 162/167 |
| 83% (64–94) | 54% (48–60) | 15% (10–21) | 97% (93–99) | |
| hsTnT(−), MR-proANP(−) | 25/29 | 153/299 | 25/171 | 153/157 |
| 86% (68–96) | 51% (45–57) | 15% (10–21) | 97% (94–99) | |
Appendix 1.
Diagnostic Accuracy of Individual Troponins and the Natriuretic Peptides and Dual-negative Combinations to Detect Unstable Angina. Cutpoints: cTnT0.03 ng/mL, hsTnT, 13 pg/mL, NT-proBNP 50 ng/L, MR-proANP 60 pmol/L.
| Analyte, cut-point | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| cTnT | 4/21 | 295/299 | 4/8 | 295/312 |
| 19% (5–42) | 99% (97–100) | 50% (16–84) | 95% (91–97) | |
| hsTnT | 10/21 | 268/299 | 10/41 | 268/279 |
| 48% (26–70) | 90% (86–93) | 24% (12–40) | 96% (93–98) | |
| NT-proBNP | 17/21 | 160/299 | 17/156 | 160/164 |
| 81% (58–95) | 54% (48–59) | 11% (6–17) | 98% (94–99) | |
| MR-proANP | 16/21 | 162/299 | 16/153 | 162/167 |
| 76% (53–92) | 54% (48–60) | 10% (6–16) | 97% (93–99) | |
|
| ||||
| cTnT(−), NT-proBNP(−) | 17/21 | 160/299 | 17/156 | 160/164 |
| 81% (58–95) | 54% (48–59) | 11% (6–17) | 98% (94–99) | |
| hsTnT(−), NT-proBNP(−) | 18/21 | 154/299 | 18/163 | 154/157 |
| 86% (64–97) | 52% (46–57) | 11% (7–17) | 98% (95–100) | |
| cTnT(−), MR-proANP(−) | 17/21 | 162/299 | 17/154 | 162/166 |
| 81% (58–95) | 54% (48–60) | 11% (7–17) | 98% (64–99) | |
| hsTnT(−), MR-proANP(−) | 18/21 | 153/299 | 18/164 | 153/156 |
| 86% (94–97) | 51% (45–57) | 11% (7–17) | 98% (94–100) | |
Incremental Value of Adding Natriuretic Peptides to Troponins for ACS Diagnosis
Combining natriuretic peptides with both conventional and highly sensitive methods for troponin improved the C-statistics (AUC) and had significant values of NRI for ACS, largely driven by correct reclassification of events (Table 3). More specifically, between the two natriuretic peptides, NT-proBNP had best improvement in C-statistics and NRI when added to either troponins. Impressively, adding NT-proBNP to cTnT and hsTnT improved the AUC from 0.68 to 0.78 (p=0.005) and 0.74 to 0.80 (p=0.02) respectively, and correctly reclassified 66% of events (p=0.0004). Furthermore, in our subgroup analysis of patients without MI (Appendix 2), we found that both natriuretic peptides had similar incremental value for UAP diagnosis when used in a dual marker strategy with either troponins.
Table 3.
Incremental Value of Natriuretic Peptides (NP) beyond Troponins using C-statistics (AUC)and Net Reclassification Index (NRI) for ACS. Analytecutpoints as in Table 2.
| AUC (Troponin) | AUC (Troponin+NP) | Δ AUC(95% CI) | P | NRI(95% CI) | P | % Events Correctly Reclassified | P | % Nonevents Correctly Reclassified | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| cTnT+NT-proBNP | 0.68 | 0.78 | 0.10 (0.03–0.17) | 0.005 | 73% (43–102) | 0.0002 | 66% | 0.0004 | 7% | 0.23 |
| hsTnT+NT-proBNP | 0.74 | 0.80 | 0.06 (0.01–0.12) | 0.02 | 73% (43–102) | 0.0002 | 66% | 0.0004 | 7% | 0.23 |
| cTnT+MR-proANP | 0.68 | 0.77 | 0.08 (0.01–0.15) | 0.02 | 46% (11–82) | 0.02 | 38% | 0.04 | 8% | 0.15 |
| hsTnT+MR-proANP | 0.74 | 0.79 | 0.04 (−0.01–0.10) | 0.12 | 46% (11–82) | 0.02 | 38% | 0.04 | 8% | 0.15 |
Appendix 2.
Incremental Value of Natriuretic Peptides (NP) beyond Troponins using C-statistics (AUC) and Net Reclassification Index (NRI) for unstable angina (in patients without myocardial infarction). Analytecutpoints as in Table 2.
| AUC (Troponin) | AUC (Troponin+NP) | Δ AUC(95% CI) | P | NRI(95% CI) | P | % Events Correctly Reclassified | P | % Nonevents Correctly Reclassified | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| cTnT+NT-proBNP | 0.59 | 0.71 | 0.12 (0.03–0.21) | 0.007 | 69% (33–104) | 0.002 | 62% | 0.005 | 7% | 0.23 |
| hsTnT+NT-proBNP | 0.69 | 0.76 | 0.07 (0.002–0.14) | 0.04 | 69% (33–104) | 0.002 | 62% | 0.005 | 7% | 0.23 |
| cTnT+MR-proANP | 0.59 | 0.71 | 0.13 (0.04–0.21) | 0.006 | 61% (23–99) | 0.007 | 52% | 0.02 | 8% | 0.15 |
| hsTnT+MR-proANP | 0.69 | 0.76 | 0.07 (0.001–0.14) | 0.047 | 61% (23–99) | 0.007 | 52% | 0.02 | 8% | 0.15 |
Relation of Combined Natriuretic Peptides and Troponins to Cardiac CT features
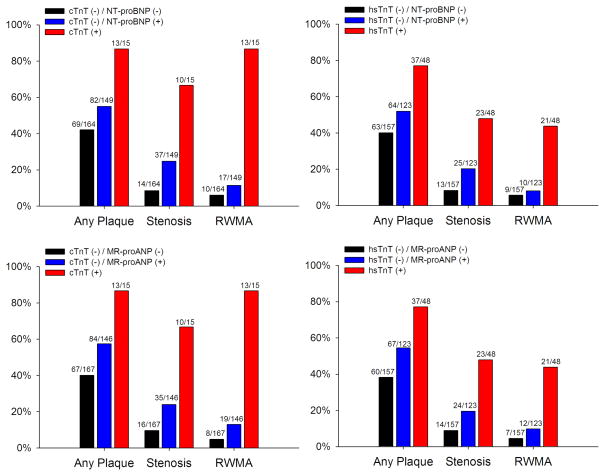
Coronary plaque was present in 164 (50.0%) patients, stenosis in 61 (18.6%), and RWMA in 40 (12.2%). All 4 of the biomarkers were elevated in patients with abnormal cardiac CT features as compared to those without (Appendix 3, all p <0.001). Patients with dual-negative markers had the lowest percentage of CT coronary plaque, stenosis, and RWMA (Figure 1, all p-trend <0.001)
Appendix 3.
Difference in Biomarkers Levels as Stratified by Cardiac CT Features. Abbreviations as in Table 1.
| Plaque (n=164) | No Plaque (n=164) | P value | |
|---|---|---|---|
| cTnT (ng/mL) | 0.009 ± 0.04 | 0.0005 ± 0.004 | 0.0001 |
| hsTnT (pg/mL) | 6.3 [3.6, 12.3] | 4.2 [2.1, 6.5] | <0.0001 |
| NT-proBNP (ng/L) | 59 [27, 156] | 42 [21, 75] | 0.0004 |
| MR-proANP (pmol/L) | 67 [45, 102] | 53 [38, 71] | <0.0001 |
|
| |||
| Stenosis (n=61) | No Stenosis (n=267) | ||
|
| |||
| cTnT (ng/mL) | 0.016 ± 0.048 | 0.002 ± 0.021 | <.0001 |
| hsTnT (pg/mL) | 8.9 [5.5, 30.5] | 4.6 [2.3, 7.4] | <.0001 |
| NT-proBNP (ng/L) | 100 [51, 238] | 42 [21, 81] | <.0001 |
| MR-proANP (pmol/L) | 75 [53, 114] | 54 [41, 78] | 0.0005 |
|
| |||
| RWMA (n=40) | No RWMA (n=288) | ||
|
| |||
| cTnT (ng/mL) | 0.038 ± 0.074 | 0.0004 ± 0.004 | <0.0001 |
| hsTnT (pg/mL) | 19.9 [6.6, 60.1] | 4.9 [2.4, 7.7] | <0.0001 |
| NT-proBNP (ng/L) | 97 [44, 331] | 47 [22, 94] | <0.0001 |
| MR-proANP (pmol/L) | 84 [57, 130] | 55 [41, 78] | <0.0001 |
Figure 1.
Distribution of Cardiac CT Structural and Functional Findings as Stratified by Dual-marker Approach of Troponin/Natriuretic Peptides.
DISCUSSION
In this low-to-intermediate risk cohort of acute chest pain patients in the ED with no previous history of CAD and initial negative cTn, we found that there was considerable incremental value for ACS and UAP diagnosis by using a dual marker strategy of combining established and emerging biomarkers of myocardial injury (either cTnT or hsTnT) with myocyte stress (NT-proBNP or MR-proANP) from a single blood draw after ED presentation (median 4.2 hours). Importantly, even in the context of superior sensitivity provided by hsTnT, the addition of a natriuretic peptide (particularly NT-proBNP) improved diagnostic accuracy, and increased NPV for excluding an ACS.
Cardiac troponins have become the biomarker of choice for the detection of myocardial injury and are central to the diagnosis of MI.1 The development of hsTn assays has allowed for the detection of much smaller concentrations of the biomarker, but the diagnostic and prognostic implications of this enhanced sensitivity are still under investigation,2–4 and the ramifications of reduced specificity of the biomarker for ACS are undefined. The additive diagnostic value of natriuretic peptides to hsTnTin this cohort is intriguing given the enhanced sensitivity of the latter biomarker. Given this, it is not surprising that there was value from adding NT-proBNP to cTnT as well. Because our analyses include both cTnT and hsTnT, our study is applicable independent of what type of troponin assay may be used in the future.
Given the reduced specificity of hsTn methods, adding a second biomarker may enhance diagnostic performance. The natriuretic peptides are a natural choice, given their tight association with structural heart disease, including ischemic heart disease. Indeed, while concentrations of natriuretic peptides have taken on special meaning relative to the presence and severity of heart failure,7, 17 their release is not limited to this diagnosis. Early in the course of myocardial ischemia, natriuretic peptide levels have been shown to rise, correlate with extent of myocardial damage, and have prognostic implications after MI.18,19 As the combination of adding NT-proBNP to cTNT has already provided complementary information for prognosis in those with ACS20 as well as further risk stratification even in patients with negative cTnT results,18 strategies employing multiple cardiac markers will likely improve the diagnostic abilityin ACS beyond single marker strategy alone,9 and may negate the need for serial blood sampling and prolonged hospitalization, particularly when both markers are low. It is of note and interest that while NT-proBNP and MR-proANP delivered relatively comparable diagnostic information, there were differences in their overall performance, perhaps favoring NT-proBNP. As these two natriuretic peptides are biologically distinct and released from different areas in the heart,6 there may be subtle differences in their clinical behavior. Notably, in this cohort, we previously showed that another candidate chest pain marker, copeptin, had no added value to hsTnT.21 Our findings extend to the understanding of which biomarkers can be used synergistically to optimally evaluate patients with acute chest pain.
When assessed individually, all 4 biomarkers levels were higher in patients with ACS and those with UAP compared to those without. Reflecting their role as a clinical mainstay for diagnosis of ACS, both troponins had excellent specificity (90–99%) for adjudicated ACS and UAP diagnoses, however sensitivity was low for cTnT (19–38%) and improved but still modest sensitivity (48–59%) was noted for hsTnT. Given its high specificity, a positive troponin value could not be overlooked clinically because of a negative natriuretic peptide result. Therefore, we incorporated natriuretic peptides with troponins in a dual-negative marker strategy, whereby only patients with negative values for both assays were considered negative. In a dual-negative marker approach with the natriuretic peptides, we found enhanced sensitivities to 81–90% with corresponding near perfect NPV of 97–98% for ACS and UAP detection, thus identifying a cohort at a very low risk of ACS. This is of particular importance in our study population of patients presenting to the ED, as this approach can give clinicians, particularly ED physicians, more confidence to manage patients with dual-negative result in a lesser aggressive manner.
As reflected in differential patterns of sensitivity and specificity, we found significantly improved C-statistics and NRI for ACS and UAP diagnosis when combining troponin with a natriuretic peptide, largely driven by correctly reclassifying events. As confirmed in our subgroup analysis of patients without MI, these ACS events that are reclassified are essentially UAP. All combinations of natriuretic peptides and troponins showed significant improvement in C-statistics over the relevant troponin assay alone for both ACS and UAP, in addition to improved NRI with impressive reclassification when natriuretic peptides were added to troponins. Specifically, adding NT-proBNP to either troponin correctly reclassified 62–66% of patients for ACS and UAP diagnosis, while adding MR-proANP to either troponins correctly reclassified 38–52% of patients. Moreover, NT-proBNP performed much better than MR-proANP for the diagnosis of ACS but both natriuretic peptides performed comparably well when used in combination with the troponins for the diagnosis of UAP. Notably, neither dual-marker strategies resulted in significant degrees of incorrect reclassification, an important point in this patient population with low rates of diagnoses likely to affect natriuretic peptide values.
Interestingly, in a higher-risk ED multicenter cohort of acute chest pain patients, NT-proBNP was incremental to cTnT9, 12 but did not reclassify patients beyond that of hsTnI.9 While our findings of the incremental of NT-proBNP to cTnT for ACS diagnosis were consistent despite different cutpoints for NT-proBNP (50 ng/L in this analysis, 100 ng/L in the IMAGINE study,9 and 402 ng/L in the study by Haaf et al.12), several reasons could explain our conflicting findings with high-sensitivity troponin. First, we used different assays for high-sensitivity troponin in the two studies (hsTnT [Roche] vshsTnI [Singulex]9). Probably most important is the use of category-free NRI instead of categorical NRI in this analysis. The use of the category-free NRI provides a more stable result when lack of optimal risk categories exist (such as in this ED cohort) and is less prone to changes of the pre-test categories.16 Lastly, this ROMICAT cohort includes low-intermediate risk patients for suspected ACS and more strict criteria including normal renal function and no prior history of CAD. The IMAGINE cohort consisted of patients with a generally higher-risk presentation, with a mixture of diagnoses typical of such a population, including heart failure, as well as impaired renal function.9 While adding NT-proBNP to hsTnI correctly reclassified ACS events by 15%, it incorrectly reclassified 32% of non-ACS cardiac events due to the majority (78%) being acutely decompensated heart failure patients. Of note, while the study by Haaf et al. included more heterogeneous patients such as those with known CAD, NT-proBNP was examined only with cTnT and not high-sensitivity troponin.12 Our study specifically targets the low risk patients with acute chest pain and excludes co-existing diagnosis that causes abnormal natriuretic peptide values (e.g. heart failure, pulmonary thromboembolism, impaired renal function). In aggregate, the multi-marker approach of troponin plus natriuretic peptide adds considerable diagnostic value for ACS.
We had the unique opportunity to describe the relationship between cardiac CT with respect to the dual marker strategy since both were performed at the same time and measured or interpreted in a blinded fashion, without the clinical history or each other results. Using anatomic imaging with cardiac CT to explain the etiology of our dual marker results, we found elevated biomarker levels for all 4 markers in relation to CT abnormalities(plaque, stenosis, and RWMA). Moreover, we observe a gradient effect with the lowest proportion of patients with CT coronary plaque, stenosis, or RWMA having dual-negative result, followed by those with negative troponin but positive natriuretic peptide, and the highest prevalence of CT abnormalities in those with positive troponin (either cTnT or hsTnT). Thus, above and beyond delivering important diagnostic value for ACS, the patterns of biomarker release in this context are supported by the underlying cardiac structural and functional findings.
Limitations
Our cohort is limited to symptomatic patients at low to intermediate risk of ACS and so is not applicable to medically complex patients or those with heart failure. Our study population also excluded patients with known CAD, making this different from an overall ED population. Nevertheless, our findings are appropriate in the large population of patients that present to the ED in whom troponin assays are of clinical importance. Also, the blood samples measured for cTnT and hsTnT were drawn at the time of the CT scan, which was a median of 4.2 hours after presentation. We could not use the first draw troponin blood sample on ED presentation since it was used to determine study eligibility.13 Since an earlier hsTnT draw of <3 hours from symptom onset have been reported to have less sensitivity as compared to that drawn at >3 hours,22 the initial reduced sensitivity from an earlier draw of troponin may lead to even more profound results when used in combination with the natriuretic peptides. Further prospective studies examining these biomarkers in combination at initial ED presentation would be of great utility for this ED cohort and help facilitate triage decision.
CONCLUSION
Among ED chest pain patients, a dual marker strategy of combining natriuretic peptides totroponins improved discriminatory capacity and allowed for better reclassification of ACS, largely driven by correctly identifying events. These findings are supported by structural and functional cardiac CT results.
Acknowledgments
Sources of Funding: This work was supported by the NIH R01 HL080053 and additional funds from the University of Ulm. Dr. Truong received support from NIH grant K23HL098370 and L30HL093896.
Footnotes
Disclosure: No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 2.Giannitsis E, Becker M, Kurz K, et al. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010;56:642–50. doi: 10.1373/clinchem.2009.134460. [DOI] [PubMed] [Google Scholar]
- 3.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 4.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 5.Januzzi JL, Jr, Bamberg F, Lee H, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation. 2010;121:1227–34. doi: 10.1161/CIRCULATIONAHA.109.893826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 7.Neuhold S, Huelsmann M, Strunk G, et al. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: prediction of death at different stages of the disease. J Am Coll Cardiol. 2008;52:266–72. doi: 10.1016/j.jacc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Truong QA, Siegel E, Karakas M, et al. Relation of natriuretic peptides and midregional proadrenomedullin to cardiac chamber volumes by computed tomography in patients without heart failure: from the ROMICAT Trial. Clin Chem. 2010;56:651–60. doi: 10.1373/clinchem.2009.138586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhardwaj A, Truong QA, Peacock WF, et al. A multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency department. Am Heart J. 2011;162:276–282. e1. doi: 10.1016/j.ahj.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Haaf P, Reichlin T, Corson N, et al. B-type natriuretic peptide in the early diagnosis and risk stratification of acute chest pain. Am J Med. 2011;124:444–52. doi: 10.1016/j.amjmed.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 11.McCann CJ, Glover BM, Menown IB, et al. Investigation of a multimarker approach to the initial assessment of patients with acute chest pain. Adv Ther. 2009;26:531–4. doi: 10.1007/s12325-009-0032-7. [DOI] [PubMed] [Google Scholar]
- 12.Haaf P, Balmelli C, Reichlin T, et al. N-terminal pro B-type natriuretic peptide in the early evaluation of suspected acute myocardial infarction. Am J Med. 2011;124:731–9. doi: 10.1016/j.amjmed.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgenthaler NG, Struck J, Thomas B, et al. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–6. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 15.Seneviratne SK, Truong QA, Bamberg F, et al. Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging. 2010;3:375–83. doi: 10.1161/CIRCIMAGING.109.892638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–9. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 18.Weber M, Bazzino O, Navarro Estrada JL, et al. N-terminal B-type natriuretic peptide assessment provides incremental prognostic information in patients with acute coronary syndromes and normal troponin T values upon admission. J Am Coll Cardiol. 2008;51:1188–95. doi: 10.1016/j.jacc.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 19.Eggers KM, Lagerqvist B, Venge P, et al. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2009;54:357–64. doi: 10.1016/j.jacc.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 20.Cameron SJ, Sokoll LJ, Laterza OF, et al. A multi-marker approach for the prediction of adverse events in patients with acute coronary syndromes. Clin Chim Acta. 2007;376:168–73. doi: 10.1016/j.cca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Karakas M, Januzzi JL, Jr, Meyer J, et al. Copeptin Does Not Add Diagnostic Information to High-Sensitivity Troponin T in Low- to Intermediate-Risk Patients with Acute Chest Pain: Results from the Rule Out Myocardial Infarction by Computed Tomography (ROMICAT) Study. Clin Chem. 2011;57:1137–45. doi: 10.1373/clinchem.2010.160192. [DOI] [PubMed] [Google Scholar]
- 22.Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58:1332–9. doi: 10.1016/j.jacc.2011.06.026. [DOI] [PubMed] [Google Scholar]