Abstract
Objective
To compare bone mass between overweight adolescents with and without cardiometabolic risk factors (CMR). Associations of bone mass with CMR and adiposity were also determined.
Study design
Overweight adolescents (aged 14–18 years) were classified in Healthy (n=55), 1CMR (n=46) or ≥2CMR (n=42). CMR were measured using standard methods and defined according to pediatric definitions of metabolic syndrome. Total body bone mass, fat mass and fat-free soft tissue mass (FFST) were measured by DXA. Visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAAT) were assessed using MRI.
Results
After controlling for age, sex, race, height and FFST, Healthy group had 5.4% and 6.3% greater bone mass than the 1CMR and ≥2CMR groups, respectively (both P<0.04). Multiple linear regression, adjusting for same covariates, revealed that VAT (β=−0.22), waist circumference (β= −0.23), homeostasis model assessment of insulin resistance (β= −0.23) and HDL-cholesterol (β=0.22) were associated with bone mass (all P<0.04). There was a trend towards a significant inverse association between bone mass and fasting glucose (P=0.056). No relations were found between bone mass and fat mass, SAAT, BP or triglycerides.
Conclusion
Being overweight with metabolic abnormalities, particularly insulin resistance, low HDL-cholesterol and visceral adiposity, may adversely influence adolescent bone mass.
Keywords: Adiposity, fat, visceral obesity, adolescent, childhood, bone, skeleton, metabolic syndrome, waist circumference
Peak bone mass, which is generally achieved by early adulthood, is an important determinant of adult risk for osteoporosis. Hence, any childhood disease or condition that reduces bone mineral accrual during the maturational period may lead to suboptimal peak bone mass and presumably a greater risk of fracture in later life.1 Recently, there has been a growing concern that childhood obesity may negatively affect bone development, as there is evidence linking childhood obesity to skeletal fractures.2 However, determining whether excess adiposity is either beneficial or detrimental to the growing skeleton has been challenging. Whereas some studies report greater bone mass in overweight children and adolescents compared with their healthy weight peers,3–5 others conclude that obesity is linked to lower bone mass or that extra weight from fat mass had no effect on bone mass.6–9
Discrepancies in the aforementioned childhood bone-fat investigations may be attributed, in part, to the methodological limitations when comparing bone mass between overweight and healthy weight children of the same age. At any given age, a wide variation exists among children in stature, body composition, rate of growth, and timing and tempo of biological maturation. Because overweight compared with healthy weight children of the same age are generally further advanced in maturation, their skeletal development is likewise more advanced, because of increased hormonal activity, than their healthy weight peers. In addition, the metabolic effects of obesity could have an impact on bone development. Currently, no studies have investigated the bone-fat relationship in overweight youth, while considering cardiometabolic risk factors (CMR).
The primary aim of this study was to compare total body bone mineral content (BMC) between overweight adolescents with no CMR (Healthy group), adolescents with only one CMR (1CMR group), and adolescents with two or more CMR (≥2CMR group). The secondary aim was to determine associations of total body BMC with CMR and robust measurements of total and central adiposity. We tested the hypotheses that: 1) total body BMC is lower in the overweight adolescents with CMR, and 2) total body BMC is negatively associated with CMR and central adiposity. Because age, sex, race, height, and muscle mass are known to be independent predictors of bone mass in children and adolescents,18 these variables were considered as potential confounders in our analyses.
METHODS
Participants in this cross-sectional investigation were 143 overweight adolescents that were recruited from high schools in Augusta, Georgia area to participate in an adiposity and cardiovascular fitness study. With approval from superintendents and school principals, flyers were distributed to all students in the high schools. Inclusion criteria for this study were the following: white or black/African-American race, aged 14–18 years, and overweight (BMI ≥ 85th percentile for age and sex). Adolescents were excluded if they were taking medications or had any medical conditions that could affect growth, maturation, physical activity, nutritional status, or metabolism. Informed consent and assent were obtained from all parents and adolescents, respectively. The protocol was approved by the Human Assurance Committee at the Medical College of Georgia (Institutional Review Board). All measurements were performed at the Georgia Prevention Institute at the Medical College of Georgia between 2001 and 2005.
Height, body weight, and waist circumference measurements were collected by a trained laboratory technician. Participants were measured in light indoor clothing after the removal of shoes. Height (cm) and body weight (kg) were assessed for calculation of sex- and age-specific BMI percentiles.19 Waist circumference (cm) was then obtained at the midpoint between the lowest rib and the iliac crest. Seated blood pressure was measured 5 times at 1 min intervals after a 10-minute rest using the Dinamap Pro 100 (Critikon Corporation, Tampa, FL), and the last 3 measures were averaged. Sexual maturation stage (or Tanner stage) was measured using a five-stage scale, ranging from I (prepubertal) to V (fully mature) as described by Tanner.20 Using a sex-specific questionnaire, participants reported their pubertal stage by comparing their own physical development to the five stages in standard sets of diagrams. A parent or research coordinator then reviewed the results with the children to make sure they understood the questionnaire. When an individual reported discordant stages of pubic hair and breast or genital development, the higher of the two stages was used. In addition, the females provided information about their menarcheal status.
Fasting blood samples were obtained from participants for assessment of glucose, insulin, triglycerides, total cholesterol, HDL-cholesterol, and LDL-cholesterol. Glucose was measured in 10 μL sera using an Ektachem DT system (Johnson and Johnson Clinical Diagnostics, Rochester, NY) with mean intra- and interassay CVs of 0.61% and 1.45%, respectively. Insulin was assayed in duplicate 100 μL with reagents obtained from Linco (St. Charles, MO) with mean intra- and interassay CVs of 5% and 5.6%, respectively. From the measures of glucose and insulin, the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated: fasting insulin (μU/mL) x fasting glucose (mg/dL)/405. Triglycerides (mg/dL), total cholesterol (mg/dL), and high-density lipoprotein cholesterol (HDL; mg/dL) were measured with the Ektachem DT II system. With this system, HDL-cholesterol is analyzed using a two-reagent system involving stabilization of low-density lipoprotein, very-low-density lipoprotein, and chylomicrons using cyclodextrin and dextrin sulfate, and subsequent enzymatic-colorimetric detection of HDL-cholesterol. Low-density lipoprotein cholesterol (LDL; mg/dL) was determined using the Friedewald formula.
Bone outcomes of the total body [BMC (g), bone area (cm2) and aBMD (g/cm2)] were measured using dual-energy X-ray absorptiometry (DXA; QDR-4500W, Hologic Waltham, MA). Because of the limitations of aBMD in children and adolescents, total body BMC has been proposed as the most appropriate outcome measure of bone mass status in youth.21 Therefore, total body BMC was chosen as our primary bone outcome measure for bone mass status. Total body composition was also determined by DXA for fat-free soft tissue mass (FFST, kg) and fat mass (kg). Anthropomorphic phantoms were scanned daily for quality assurance. In this laboratory, using a one-way random effects model, single measure intraclass correlation coefficients (ICC) were calculated in 219 adolescents, aged 13–18 y. Each participant was scanned twice within a 7-day period for BMC, bone area, aBMD, FFST mass, and fat mass (all R ≥ 0.97).
Visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAAT) were measured using a 1.5-T magnetic resonance imaging system (MRI, General Electric Medical Systems, Milwaukee, WI). Five transverse images were acquired from the lumbar region beginning at the inferior border of the fifth lumbar vertebra and proceeding toward the head; a 2-mm gap between images was used to prevent crosstalk. To calculate volumes for VAT and SAAT, the cross-sectional area (cm2) from each slice was multiplied by the slice width (1 cm) and then the individual volumes (cm3) were summed. The ICCs for repeat analyses of the same scans on separate days within a 7-day period were R ≥ 0.98 for both VAT and SAAT.
The amount of minutes per day spent in moderate and vigorous physical activities (PA) were assessed using MTI Actigraph monitors (model 7164; MTI Health Services, Fort Walton Beach, FL), uniaxial accelerometers that measure vertical acceleration and deceleration. With epoch length set at 1 minute and expressed as counts per minute, the accelerometers were to begin recording when the subject left our laboratory after the first day of testing. The subjects were instructed to 1) wear the monitor for a period of 7 days, 2) remove it for sleep, bathing, and any activity that may cause harm to either the monitor or another person (e.g., during contact sports), and 3) bring the monitor back to us 1 week later. Data from day 1 and day 7 were discarded because a full day of information was not available for those days. Movement counts were converted to average minutes per day spent in moderate [3–6 metabolic equivalents (METs)] and vigorous (>6 METs) PA by the software accompanying the device.
To assess mean daily intakes for energy (kcal), dietary calcium (mg/d), dietary vitamin D (μg), and the percentage of kcal/d from carbohydrates, protein, and fat, a trained registered dietitian conducted four to seven 24-h recalls (including 1 weekend day) using a multiple pass, computer-assisted interview approach [Nutrition Data System for Research (NDS-R), Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN]. Four, five, six, and seven days of dietary information were collected in 10%, 21%, 27%, and 42% of the adolescents, respectively, within four weeks of the blood collection. The first two recalls were performed in person at our institute with the use of food models, portion booklets, or serving containers to assist in estimating serving size, and the remaining interviews were conducted by telephone. Participants were not interviewed on days when they had been ill, or days that fell on a major holiday. To minimize the potential for under eating during the time frame for 24-h recalls, youths were blinded to the telephone recall schedule. A trained research assistant coded and analyzed dietary intake data using NDS-R software version 2006 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
For this analysis, the majority of CMR were defined according to the National Cholesterol Education Program Adult Treatment Panel III definition modified for age.22 These risk factors were defined accordingly: (1) waist circumference ≥90th percentile for age and sex;23 (2) HDL cholesterol ≤40 mg/dL; (3) triglycerides ≥110 mg/dL; (4) systolic or diastolic blood pressure ≥90th percentile for age, sex, and height;24 and (5) fasting glucose ≥100 mg/dL.25
Statistical Analyses
Data were checked for outliers and for normality using histograms and tests of skewness and kurtosis for normality. Insulin, HOMA-IR, and triglycerides were log-transformed so that each of these variables followed an approximate normal distribution. Because the results in the log-transformed models and untransformed models were similar, we report the untransformed data in Table I for clarity. We used analysis of covariance (ANCOVA) to compare descriptive characteristics between the three overweight groups, adjusting for age and sex. Differences in proportions were tested by the chi-square test of goodness of fit.
Table 1.
Descriptive characteristics of the participants
| Optimal rangea | Overweight + Healthy |
Overweight + 1 CMR |
Overweight + ≥2 CMR |
P-valueb | |
|---|---|---|---|---|---|
| (n = 55) | (n = 46) | (n = 42) | |||
| Sex (M/F)c | 12/43 | 29/17 | 28/14 | 0.06 | |
| Race (W/B)c | 20/35 | 18/28 | 20/22 | 0.52 | |
| Age (y)d | 16.2 ± 1.2 | 15.8 ± 1.1 | 16.0 ± 1.2 | 0.35 | |
| Tanner stage (1–5)d | 4.5 ± 0.6 | 4.4 ± 0.6 | 4.4 ± 0.7 | 0.50 | |
| Height (cm) | 163.9 ± 8.5 | 170.8 ± 8.7 e | 172.1 ± 8.9 e | <0.01 | |
| Weight (kg) | 73.2 ± 8.7 | 85.5 ± 11.7 e | 95.3 ± 16.6 e, f | <0.01 | |
| BMI percentile | 5th – 85th percentile (19) | 91.4 ± 3.9 | 94.8 ± 4.2 e | 96.6 ± 3.5 e, f | <0.01 |
| Waist circumference (cm) | <90th percentile (23) (age and sex) | 79.8 ± 5.7 | 87.9 ± 8.5 e | 94.5 ± 10.5 e, f | <0.01 |
| FFST mass (kg) | 46.2 ± 9.6 | 54.2 ± 9.7 e | 57.4 ± 10.1 e | <0.01 | |
| Fat mass (kg) | 23.6 ± 7.5 | 27.5 ± 9.2 e | 33.7 ± 11.9 e, f | <0.01 | |
| Total body BMC (g) | 2456 ± 466 | 2473 ± 418 | 2532 ± 407 | 0.37 | |
| Total body bone area (cm2) | 2112 ± 226 | 2155 ± 204 | 2205 ± 217 | 0.07 | |
| Total body aBMD (g/cm2) | 1.15 ± 0.10 | 1.14 ± 0.10 | 1.15 ± 0.10 | 0.89 | |
| VAT (cm3) | 121.6 ± 55.6 | 149.5 ± 55.8 e | 187.5 ± 85.4 e, f | <0.01 | |
| SAAT (cm3) | 1399 ± 605 | 1591 ± 739 | 2139 ± 923 e, f | <0.01 | |
| Systolic BP (mm Hg) | <90th percentile (24) (age, sex, and height) | 112 ± 10 | 115 ± 10 e | 117 ± 11 e | <0.01 |
| Diastolic BP (mm Hg) | <90th percentile (24) (age, sex, and height) | 59 ± 7 | 60 ± 7 | 61 ± 6 | 0.52 |
| Biochemical parameters | |||||
| Fasting glucose (mg/dL) | 70 – 100 mg/dL (25) | 88.9 ± 5.1 | 90.5 ± 6.8 | 94.2 ± 7.0 e, f | <0.01 |
| Fasting insulin (μU/mL) | ≤20 μU/mL (45) | 17.9 ± 8.0 | 23.3 ± 11.1 e | 28.7 ± 10.5 e, f | <0.01 |
| HOMA-IR | <4.4 (46) | 3.9 ± 1.8 | 5.2 ± 2.6 e | 6.7 ± 2.4 e, f | <0.01 |
| Triglycerides (mg/dL) | <110 mg/dL (22) | 56.7 ± 21.6 | 65.0 ± 23.5 | 122.3 ± 73.1 e, f | <0.01 |
| Total cholesterol (mg/dL) | <150 mg/dL (22) | 150.4 ± 24.5 | 147.7 ± 31.7 | 152.8 ± 28.1 | 0.68 |
| HDL-cholesterol (mg/dL) | >40 mg/dL (22) | 51.5 ± 8.9 | 41.1 ± 8.6 e | 36.6 ± 6.8 e, f | <0.01 |
| LDL-cholesterol (mg/dL) | <110 mg/dL (22) | 92.1 ± 23.6 | 100.0 ± 29.9 | 103.9 ± 30.0 e | 0.03 |
| PA and dietary parameters | |||||
| Moderate PA (min/d) | 41.3 ± 21.0 | 45.1 ± 26.6 | 40.3 ± 23.1 | 0.65 | |
| Vigorous PA (min/d) | 5.9 ± 6.0 | 3.8 ± 4.6 | 2.9 ± 3.2 | 0.07 | |
| Mod + Vig PA (min/d) | ≥60 min/d (47) | 47.1 ± 25.6 | 48.9 ± 29.4 | 43.2 ± 24.8 | 0.64 |
| Energy intake (kcal/d) | 1800 – 2200 kcal/d (48) | 1769 ± 746 | 1782 ± 607 | 1913 ± 605 | 0.47 |
| Carbohydrate (%) | 45 – 65% (48) | 52.9 ± 6.5 | 53.2 ± 6.9 | 52.8 ± 6.5 | 0.73 |
| Fat (%) | 25 – 35% (48) | 33.2 ± 5.7 | 33.1 ± 4.4 | 34.3 ± 4.3 | 0.58 |
| Protein (%) | 10 – 30% (48) | 14.8 ± 3.1 | 14.6 ± 3.2 | 13.8 ± 2.5 | 0.14 |
| Calcium intake (mg/d) | ≥1300 mg/d (49) | 731 ± 358 | 744 ± 382 | 697 ± 292 | 0.26 |
| Vitamin D intake (μg/d) | ≥5 μg/d (49) | 2.8 ± 2.3 | 3.8 ± 2.9 | 3.5 ± 1.9 | 0.55 |
Values are means ± SD. BMI, body mass index; FFST, fat-free soft tissue; BMC, bone mineral content, aBMD, areal bone mineral density; VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue; BP, blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and PA, physical activity.
Recommended optimal range for adolescents; reference in parentheses.
Tests of significance between groups were conducted by using ANCOVA (controlling for sex and age) with Least Square Difference adjustment for multiple comparisons.
Tests of significance between groups were conducted by using chi-square test of goodness of fit.
Tests of significance between groups were conducted by using ANOVA.
Significantly different from Overweight + Healthy group, P < 0.05.
Significantly different from Overweight + 1 CMR group, P < 0.05.
For comparison of the bone variables, an F test was performed to test the assumption of homogeneity of regression slopes for the interaction between the independent variables (i.e., Healthy, 1CMR, and CMR groups) and the covariates (age, sex, race, height and FFST mass). Because there was no interaction, ANCOVA was used to compare the bone variables between the Healthy, 1CMR, and ≥2CMR groups after adjusting for sex, race, height, and FFST mass. The Least Square Difference method was used to adjust for multiple comparisons and statistical differences are shown between each pair (Healthy vs. 1CMR, Healthy vs. ≥2CMR, and 1CMR vs. ≥2CMR). In the total sample, stepwise linear regression analysis was performed to identify independent correlates of total body BMC using the stepwise procedure. The variables with P < 0.05 in the hierarchical multiple regression model (using the covariates of age, sex, race, height, and FFST mass) were then entered in the stepwise multiple regression model. All statistical analyses were performed using SPSS version 18.02 for Mac OS X (PASW Statistics, Chicago, IL). A P-value < 0.05 was considered statistically significant for all analyses.
RESULTS
The sample was composed of 143 white and black adolescents, aged 14–18 years, with an approximately equal distribution of females (52%) and males (48%), (χ2 = 0.18, P = 0.68). Although there were a greater percentage of blacks (59%) than whites (41%), the racial distribution was not significantly different between females and males (χ2 = 1.87, P = 0.17). The majority of participants (91%) reported to be in pubertal stages IV and V; however, 10 subjects reported to be in pubertal stage III and three in stage II. All females reported having started menstruation. Of the 143 overweight adolescents, 62% were identified with at least one cardiometabolic risk factor, and the number of participants with one, two, three and four cardiometabolic risk factors was 46, 30, 11, and 1, respectively. The cardiometabolic risk factor distributions among the participants were the following: 24% (n = 35) with waist circumference ≥90th percentile for age and sex; 8% (n = 11) and 0% with systolic and diastolic blood pressure ≥90th percentile for age, sex, and height, respectively; 10% (n = 15) with fasting glucose ≥100 mg/dL; 15% (n = 22) with triglycerides ≥110 mg/dL; and 42% (n = 60) with HDL-cholesterol ≤40 mg/dL.
The descriptive characteristics of the three overweight groups (i.e., Healthy, 1CMR, and ≥2CMR groups) are shown in Table I. Height, weight, BMI percentile, FFST mass, fat mass, waist circumference, VAT, systolic BP, fasting insulin, and HOMA-IR were significantly lower and HDL-cholesterol were significantly higher in the Healthy group compared with the 1CMR and ≥2CMR groups (all P < 0.05, Table I). The CMR group vs. ≥2CMR group had significantly lower body weight, BMI percentile, fat mass, waist circumference, VAT, SAAT, fasting glucose, HOMA-IR, and triglycerides and significantly higher HDL-cholesterol (all P < 0.05). Age, Tanner stage, total body BMC, total body bone area, total body aBMD, diastolic BP, total cholesterol, moderate and vigorous PA, and the dietary variables were not statistically different between Healthy, 1CMR, and ≥2CMR groups (all P > 0.05).
Bone Comparisons between Groups
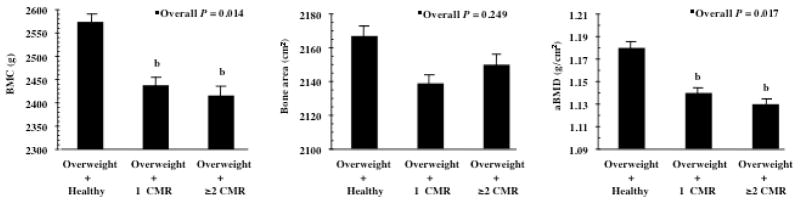
Group-specific means for each bone variable based on analysis of covariance that controls for age, sex, race, height and FFST mass are shown in Figure 1. After controlling for covariates, total body BMC was 5.4% higher in the Healthy vs. 1CMR (P = 0.018) and 6.3% greater in the Healthy vs. ≥2CMR (P = 0.007). Total body aBMD was 4.3% greater in the Healthy vs. 1CMR (P = 0.036) and 5.2% higher in the Healthy vs. ≥2CMR (P = 0.006). There were no significant differences in total body bone area between groups.
FIGURE 1.
Mean (± SE total body bone mineral content (BMC), bone area, and mineral density (aBMD) in overweight adolescents with no cardiometabolic risk factors (CMR) (Overweight adolescents + Healthy, n=55), overweight adolescents with only one CMR (Overweight + 1 CMR, n=46), and overweight adolescents with two or more CMR (Overweight + ≥2 CMR, n=42). ■Overall P-value on the basis of ANCOVA, adjusted for age, sex, race, height and fat-free soft tissue mass. b P <0.05, significantly different from Overweight + Healthy group (Least square difference adjustment for multiple comparisons.
Associations of Total BMC
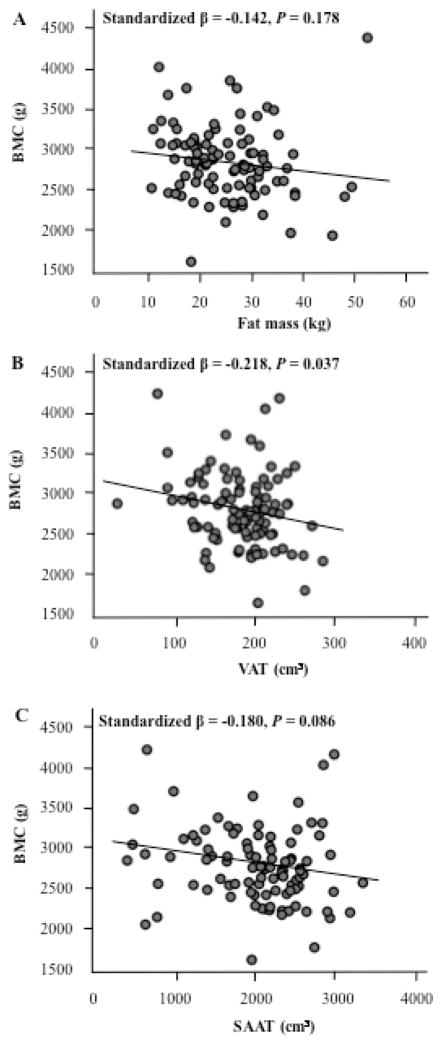
Multiple linear regression, adjusting for age, sex, race, height and FFST mass, revealed that VAT was negatively associated with total body BMC, and it could explain 4.8% of the variation in total body BMC (Figure 2, B, P = 0.037). The other adiposity variables, fat mass and SAAT, were not significantly associated with total body BMC (Figure 2, A and C). Multiple linear regression analyses were also conducted to investigate whether total body BMC was associated with CMR, PA, and dietary variables among all subjects, after adjustment for age, sex, race, height and FFST mass. Waist circumference (standardized β = −0.23), fasting insulin (standardized β = −0.23), and HOMA-IR (standardized β = −0.26) were negatively associated with total body BMC (all P < 0.02). Similarly, there was a trend towards a significant, negative association between total body BMC and fasting glucose (standardized β = −0.21, P = 0.056) and total cholesterol (standardized β = −0.11, P = 0.067). Conversely, HDL-cholesterol (standardized β = 0.22) and energy intake (standardized β = 0.20) were positively associated with total body BMC (both P < 0.05). There were no significant associations between total body BMC and systolic BP, diastolic BP, triglycerides, LDL-cholesterol, moderate PA, vigorous PA, moderate + vigorous PA, dietary calcium intake, or dietary vitamin D intake.
Figure 2. Relationship between total body bone mineral content (BMC) and total and central adiposity in overweight adolescents.
(A) Fat mass vs. total body BMC, (B) Visceral adipose tissue (VAT) vs. total body BMC and (C) subcutaneous abdominal adipose tissue (SAAT) vs. total body BMC. Relationship are adjusted for age, sex, race, height and far-free soft tissue mass. N = 143
Table II displays the stepwise multiple linear regression that was conducted to examine the independent association of age, sex, race, height, FFST mass, VAT, HOMA-IR, HDL-cholesterol and energy intake with total body BMC. Sex (5.2%), race (1.3%), height (1.2%), FFST mass (69.2%) and HOMA-IR (4.1%) explained a total of 81% of the variance in total body BMC, with no contribution by age, VAT, HDL-cholesterol and energy intake.
Table 2.
Multiple linear regression model for the dependent variable total body BMC in the total sample of overweight adolescents (N = 143)
| Independent variable | b ± SE | R2 | P valuea |
|---|---|---|---|
| Intercept | −1548.9 ± 760.7 | ||
| Age | NS | ||
| Sexb | −281.9 ± 70.1 | 5.2% | 0.001 |
| Race | 133.3 ± 49.2 | 1.3% | 0.030 |
| Height | 10.5 ± 5.0 | 1.2% | 0.040 |
| FFST mass | 44.1 ± 3.1 | 69.2% | <0.001 |
| Visceral adipose tissue | NS | ||
| HOMA-IRc | −250.4 ± 97.9 | 4.1% | 0.012 |
| HDL-cholesterol | NS | ||
| Energy intake | NS | ||
| Total R2 | 81.0% | ||
b, unstandardized coefficient; SE, standard error; R2, percentage of variability in total BMC (bone mineral content) that is attributable to the regression equation. NS, not significant (P > 0.05).
Tests of significance were determined by stepwise linear regression at P < 0.05.
Sex coded such that male adolescents =1 and female adolescents = 2.
Race coded such that white adolescents =1 and black adolescents = 2.
HOMA-IR, homeostasis model assessment of insulin resistance
DISCUSSION
Our findings suggest that if cardiometabolic risk factors are present alongside being overweight, it could have a negative effect on bone. More importantly, it is possible to hypothesize that if the extra weight from fat mass provides no additional influence to bone, an overweight adolescent with metabolic abnormalities may be more susceptible to fracture because traumatic impact forces generally scale with body weight. However, further work is required to establish this connection.
Even in adults, the associations of metabolic syndrome with bone mass have not been extensively explored, and the results have been mixed. Whereas some authors have reported greater bone mass in adults with metabolic syndrome,26 others have shown the opposite relationship.12, 13 These conflicting reports may be due, in part, to the heterogeneous samples studied (e.g. age, co-morbid disease status and medication use) and potentially of greater importance, the lack of consideration of the generally larger body size in metabolic syndrome patients compared with controls. If the latter is not considered, the effect of body size on bone mass could lead to misinterpretations when comparing individuals of different stature and body composition. In this study, we sought to minimize the confounding effects of body size on bone mass by making comparisons solely between overweight adolescents, and by statistically controlling for height and FFST mass, thus separating the effect of fat mass on bone.
Recent studies have challenged the traditionally accepted view that obesity is beneficial to the growing skeleton;6–9 however, it is apparent that a phenotype, beyond total fat accumulation, is needed to explain the complex relationship between developing bone and obesity. Given that HOMA-IR in this study explained a significant proportion of the variance in total body bone mass, it is plausible that insulin resistance may explain, in part, the association between obesity and suboptimal bone mass. Evidence to support this notion was first introduced by Afghani el al27 in a cohort of overweight Hispanic-American children in which total body BMC was inversely associated with markers of insulin resistance, as determined by oral glucose tolerance test. Similarly, in a recent study of overweight prepubertal children,28 our group observed lower bone mass in the children with pre-diabetes compared with the those with normal glucose levels. Taken together, these studies indicate that abnormal glucose regulation has a negative effect on the growing skeleton. The mechanism for the potential negative effect of insulin resistance on bone development is currently unknown; however, some hypotheses include increased calcium excretion,29 increased concentrations of advanced glycation end-products in collagen,30 disruption in the growth hormone-insulin-like growth factor axis,31 and increased inflammation.32
It is also possible that obesity during childhood promotes both low bone mass accrual and risk for diabetes through events that are mechanistically associated. Recent animal data have uncovered the presence of a “bone-fat-pancreas” axis that regulates energy homeostasis, coordinates energy partitioning between bone and adipose tissue, and impacts insulin sensitivity. In mice lacking the gene for osteocalcin, a bone-derived protein and well-known biomarker of bone formation, Lee et al33 observed phenotypes of glucose intolerance, insulin resistance, and visceral obesity. When recombinant osteocalcin was administered to the animals, improvements in glucose tolerance and insulin secretion were observed.33 In a mouse model lacking the gene for the insulin receptor in osteoblast (Ob-ΔIR), Fulzele et al34 demonstrated lower postnatal bone mass acquisition in the Ob-ΔIR mice compared with controls. Along with decreased bone formation, the Ob-ΔIR mice displayed marked peripheral adiposity and insulin resistance that was accompanied by decreased osteocalcin concentrations. When recombinant osteocalcin was administered, the researchers observed improvements in glucose tolerance and insulin sensitivity.34
The novel relationships described above between osteocalcin and glucose-insulin metabolism appear to be regulated via leptin,35 an adipocyte-derived hormone that is strongly and positively correlated with fat mass levels.36 Animal studies reveal that leptin treatment elicits a bimodal response, where low doses of leptin can stimulate bone formation and prevent bone loss, but higher concentrations of leptin actually suppress bone formation and increase bone resorption.37 Thus, one way in which high levels of adiposity may inhibit the accumulation of bone mass during growth is via hyperleptinemia. Though the basic mechanisms underlying this biphasic effect are unclear, they may involve leptin receptor downregulation, which is known to occur with increases in endogenous leptin due to increased food intake or adiposity.38 Given that obesity, insulin resistance and type 2 diabetes are related disorders of energy metabolism, further investigation of the bone-fat-pancreas axis is warranted.
Another significant finding in this study was that the influence of adiposity on bone mass may depend on the manner in which the fat mass accumulates. Given that metabolic abnormalities are more strongly associated with visceral, rather than subcutaneous, adiposity, 39 it is possible that the type of fat (visceral vs. subcutaneous) could affect developing bone distinctly, which could be another potential explanation for the conflicting pediatric data of the bone-fat relationship. In pediatric investigations, visceral adiposity, measured by either MRI or computed tomography, has shown to be inversely associated with bone mass.28, 40, 41 In this study, we found an inverse relationship not only between VAT and bone mass, but also between waist circumference and bone mass. We did not observe a significant relationship between SAAT and bone mass. Because waist circumference is frequently employed as a surrogate for visceral adiposity and is reported to be more closely associated with negative health outcomes than SAAT or BMI,39 it is possible that waist circumference may have important clinical utility as an easy and inexpensive screening tool for assessment of bone health. Whether waist circumference can be used to predict bone health and risk of skeletal fracture, however, must be validated by subsequent prospective studies. Nevertheless, our data along with the four aforementioned pediatric investigations suggest that increased visceral adiposity, which is more clinically relevant for metabolic abnormalities than increased total body adiposity, could play an adverse role in bone health.
Modifiable factors such as physical activity and diet not only play an important role in obesity and metabolic abnormality progression but also impact skeletal development. Thus, it is possible that differences in bone mass found between groups could be attributed to dissimilarities in physical activity and diet. In this study, we did not find significant differences in physical activity between groups; however, the Healthy group performed, on average, twice as much vigorous physical activity (5.9 vs. 2.9 minutes) than the ≥2 CMR group. Including vigorous physical activity as a covariate in our analysis did not change any of our bone outcomes (data not shown). Weight-bearing activity, in general, is known to be an important determinant of peak bone mass; however, the specific type of exercise, intensity and duration that will provide the optimal stimulus for peak bone mineral accretion and metabolic disease diminution still requires further investigation. With regard to dietary intake, the groups reported no significant differences in energy, macronutrient and micronutrient intakes. It is important to note, however, that we found a positive relationship between energy intake and bone mass in the total sample. Given that our group recently reported inverse relations between adiposity and energy intake,42 it would seem that trying to limit energy intake in overweight youth may run counter to the demands of skeletal growth. Therefore, interventions in overweight children and adolescents may be more effective if we increase the emphasis on vigorous physical activity and reduce the degree to which we advise overweight youth to restrict their energy intake.
Our study has several limitations. First, because of our cross-sectional study design, the associations between bone mass and CMR and VAT do not prove causality. Thus, other factors such as environmental and inherited may have influenced our findings. Another limitation was that we utilized only total body DXA-derived bone measurements. Although specific regional DXA-derived bone data would have provided additional information, the original intent of this study was not designed to study bone health. Three-dimensional imaging techniques such as peripheral quantitative computed tomography would afford more definitive information on volumetric BMD and bone geometry and the effect of metabolic abnormalities on the individual cortical and trabecular compartments. Nevertheless, total body BMC is considered one of the preferred bone measurements for the assessment of bone status in youth, due to its reproducibility, low radiation, and lack of areal density-related errors.21 More importantly, total body BMC has been shown to be a good predictor of childhood fracture risk.43 Another important limitation to note is that our small sample size did not allow separate analysis of data by males and females or by whites and blacks. Some studies have reported sex and race differences of not only skeletal development but also of visceral fat accumulation and glucose-insulin metabolism.18, 44 Although determining the effects of metabolic abnormalities on bone mass by sex and race was not the objective of this study, future work in the area is warranted.
Acknowledgments
Funded by NIH grants HL077230 and HL64157.
A special thanks goes to the study subjects and their parents who made this research possible.
Abbreviations
- CMR
Cardiometabolic risk factors
- DXA
Dual-energy X-ray absorptiometry
- BMC
Bone mineral content
- aBMD
Areal bone mineral density
- FFST
Fat-free soft tissue
- MRI
Magnetic resonance imaging
- VAT
visceral adipose tissue
- SAAT
Subcutaneous abdominal adipose tissue
- HOMA-IR
Homeostasis model assessment of insulin resistance
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- PA
Physical activity
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Norman K Pollock, Email: npollock@mcg.edu.
Paul J Bernard, Email: pbernard@mcg.edu.
Bernard Gutin, Email: bernardgutin@yahoo.com.
Catherine L Davis, Email: cadavis@mcg.edu.
Haidong Zhu, Email: hzhu@mcg.edubr.
Yanbin Dong, Email: ydong@mcg.edu.
References
- 1.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimitri P, Wales J, Bishop N. Fat and Bone in Children - Differential Effects of Obesity on Bone Size and Mass According to Fracture History. J Bone Miner Res. 2009;25:527–536. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- 3.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 4.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducher G, Bass SL, Naughton GA, Eser P, Telford RD, Daly RM. Overweight children have a greater proportion of fat mass relative to muscle mass in the upper limbs than in the lower limbs: implications for bone strength at the distal forearm. Am J Clin Nutr. 2009;90:1104–1111. doi: 10.3945/ajcn.2009.28025. [DOI] [PubMed] [Google Scholar]
- 6.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–576. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 8.Wosje KS, Khoury PR, Claytor RP, Copeland KA, Kalkwarf HJ, Daniels SR. Adiposity and TV viewing are related to less bone accrual in young children. J Pediatr. 2009;154:79–85. e72. doi: 10.1016/j.jpeds.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollock NK, Laing EM, Hamrick MW, Baile CA, Hall DB, Lewis RD. Bone and fat relationships in postadolescent black females: a pQCT study. Osteoporos Int. 2010 doi: 10.1007/s00198–010–1266–6. [DOI] [PubMed] [Google Scholar]
- 10.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 11.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey, 2001–2006. Arch Pediatr Adolesc Med. 2009;163:371–377. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 12.Hwang DK, Choi HJ. The relationship between low bone mass and metabolic syndrome in Korean women. Osteoporos Int. 2010;21:425–431. doi: 10.1007/s00198-009-0990-2. [DOI] [PubMed] [Google Scholar]
- 13.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 14.Tanko LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. 2003;32:8–14. doi: 10.1016/s8756-3282(02)00918-3. [DOI] [PubMed] [Google Scholar]
- 15.Petit MA, Paudel ML, Taylor BC, Hughes JM, Strotmeyer ES, Schwartz AV, et al. Bone Mass and Strength in Older Men with Type 2 Diabetes: The Osteoporotic Fractures in Men Study. J Bone Miner Res. 2010;25:285–91. doi: 10.1359/jbmr.090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balint E, Szabo P, Marshall CF, Sprague SM. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001;28:21–28. doi: 10.1016/s8756-3282(00)00426-9. [DOI] [PubMed] [Google Scholar]
- 17.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 18.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 20.Tanner J. Growth and Adolescence. 2. Oxford, UK: Blackwell Scientific Publications; 1962. [Google Scholar]
- 21.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 24.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 25.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 26.Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based U.S. sample. J Clin Endocrinol Metab. 2007;92:4161–4164. doi: 10.1210/jc.2007-0757. [DOI] [PubMed] [Google Scholar]
- 27.Afghani A, Cruz ML, Goran MI. Impaired glucose tolerance and bone mineral content in overweight latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28:372–378. doi: 10.2337/diacare.28.2.372. [DOI] [PubMed] [Google Scholar]
- 28.Pollock NK, Bernard PJ, Wenger K, Misra S, Gower BA, Allison JD, et al. Lower bone mass in prepubertal overweight children with pre-diabetes. J Bone Miner Res. 2010 doi: 10.1002/jbmr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNair P, Madsbad S, Christensen MS, Christiansen C, Faber OK, Binder C, et al. Bone mineral loss in insulin-treated diabetes mellitus: studies on pathogenesis. Acta Endocrinol. 1979;90:463–472. doi: 10.1530/acta.0.0900463. [DOI] [PubMed] [Google Scholar]
- 30.Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K, et al. Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol. 1997;8:260–270. doi: 10.1681/ASN.V82260. [DOI] [PubMed] [Google Scholar]
- 31.Argente J, Caballo N, Barrios V, Pozo J, Munoz MT, Chowen JA, et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in prepubertal children with exogenous obesity: effect of short- and long-term weight reduction. J Clin Endocrinol Metab. 1997;82:2076–2083. doi: 10.1210/jcem.82.7.4089. [DOI] [PubMed] [Google Scholar]
- 32.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 33.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 37.Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology. 2007;148:3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- 38.Martin RL, Perez E, He YJ, Dawson R, Jr, Millard WJ. Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein downregulation. Metabolism: clinical and experimental. 2000;49:1479–1484. doi: 10.1053/meta.2000.17695. [DOI] [PubMed] [Google Scholar]
- 39.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 40.Afghani A, Goran MI. Racial differences in the association of subcutaneous and visceral fat on bone mineral content in prepubertal children. Calcif Tissue Int. 2006;79:383–388. doi: 10.1007/s00223-006-0116-1. [DOI] [PubMed] [Google Scholar]
- 41.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stallmann-Jorgensen IS, Gutin B, Hatfield-Laube JL, Humphries MC, Johnson MH, Barbeau P. General and visceral adiposity in black and white adolescents and their relation with reported physical activity and diet. Int J Obes. 2007;31:622–629. doi: 10.1038/sj.ijo.0803587. [DOI] [PubMed] [Google Scholar]
- 43.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:e291–297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 45.The Quest Diagnostics manual: endocrinology test selection and interpretation. 4. San Juan Capistrano (CA): Nichols Institute; 2007. [Google Scholar]
- 46.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 47.Physical activity guidelines advisory committee report: Part A: executive summary 2008. Nutr Rev. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 48.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for energy, carbohydrates, fiber, fat, fatty acid, cholesterol, protein, amino acids. Washington, DC: The National Academies Press; 2002. [Google Scholar]
- 49.Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Flouride. Washington DC: National Academy Press; 1997. [PubMed] [Google Scholar]