Abstract
We previously reported that apolipoprotein (Apo) E-deficient, ApoB48-containing (E−/B48) lipoproteins inhibited expression of lysosomal hydrolase and transformed mouse peritoneal macrophages (MPMs) into foam cells. The present study examined the effect of 2-aminopurine (2-AP), an inhibitor of eukaryotic initiation factor (eIF)-2α phosphorylation, on E−/B48 lipoprotein-induced changes in gene expression and foam cell formation. Our data demonstrated that E−/B48 lipoproteins enhanced phosphorylation of eIF-2α in macrophages. Incubation of MPMs with E−/B48 lipoproteins inhibited the translation efficiency of mRNAs encoding lysosomal acid lipase, cathepsin B, and cation-dependent mannose 6 phosphate receptor, with a parallel reduction in the level of these proteins. Addition of 2-AP to the culture media alleviated the suppressive effect of E−/B48 lipoproteins on lysosomal hydrolase mRNA translation, increased macrophage degradation of E−/B48 lipoproteins, and inhibited foam cell formation. Transfection of MPMs with a nonphosphorylatable eIF-2α mutant also attenuated the suppressive effect of E−/B48 lipoproteins on expression of lysosomal acid lipase, associated with a reduced accumulation of cellular cholesterol esters. This is the first demonstration that ApoE-deficient lipoproteins inhibit lysosomal hydrolase synthesis and transform macrophages into foam cells through induction of eIF-2α phosphorylation.
Individuals with apolipoprotein (Apo) E deficiency develop hyperlipidemia and atherosclerosis (Mahley et al., 1999). Likewise, ApoE-deficient (ApoE−/−) mice manifest elevated plasma ApoB48-containing lipoproteins and develop atherosclerosis in a manner that resembles the human disease (Piedrahita et al., 1992; Guo et al., 2002). The presence of foam cells in the arterial intima is a hallmark of the early stages of atherosclerosis (Shashkin et al., 2005). Previous findings from our laboratory demonstrated that incubation of mouse peritoneal macrophages (MPMs) with ApoE-deficient, ApoB48-containing (E−/B48) lipoproteins resulted in intralysosomal lipoprotein accumulation, leading to foam cell formation (Wu et al., 2007). We also demonstrated that the degradation rate of E−/B48 lipoproteins in MPMs declined over time. Moreover, E−/B48 lipoproteins reduced the protein levels of lysosomal acid lipase, cathepsin B, and cation-dependent mannose 6 phosphate receptor (MPR46) (Wu et al., 2007). Lysosomal acid lipase is the sole hydrolase responsible for cleavage of cholesteryl esters delivered to the lysosomes (Zschenker et al., 2006), whereas cathepsin B is one of the lysosomal proteases responsible for degradation of the endocytic proteins, including the protein components of lipoproteins (O’Neil et al., 2003). MPR46 is a protein that delivers hydrolases to endosomes/lysosomes. Thus, reduction in lysosomal hydrolases may be a mechanism by which E−/B48 lipoproteins trigger foam cell formation.
Phosphorylation of eukaryotic translation initiation factor (eIF)-2α has been shown to regulate gene expression in response to various physiological and pathological stimuli (for review, see Roybal et al., 2005). In mammalian cells, eIF-2α phosphorylation is mediated by four different kinases, depending on the stimuli (for review, see Wek et al., 2006): heme deprivation or oxidative or heat stress activate heme-regulated inhibitor or EIF2AK1 (Wek et al., 2006); virus infection activates double-stranded RNA-activated protein kinase; amino acid deprivation activates general control non-derepressible-2 or EIF2AK4; and endoplasmic reticulum (ER) stress activates RNA-dependent protein kinase-like endoplasmic reticulum kinase (PERK) or EIF2AK3.
ER stress classically results from accumulation of unfolded or misfolded proteins in the ER lumen. Activation of the signaling network in response to ER stress is known as the unfolded protein response (UPR). It is interesting to note that atherosclerotic lesions in the aorta root of ApoE−/− mice show UPR, as reflected by increased PERK phosphorylation in the lesions (Zhou et al., 2004, 2005). Under physiological conditions, PERK is associated with the ER protein Bip [also known as glucose-regulated protein (GRP) 78]; this interaction keeps PERK in an inactive state. When the ER is overloaded with newly synthesized proteins or is stimulated by agents that cause accumulation of unfolded protein, GRP78/Bip preferentially associates with the unfolded proteins, and PERKs are released from the ER. The released PERKs are autophosphorylated, leading to increased catalyst activity, and activated PERK phosphorylates eIF-2α. Phosphorylated eIF-2α subsequently inhibits global protein synthesis, preventing further influx of nascent proteins into an already saturated ER lumen. Paradoxically, eIF-2α phosphorylation induces ATF4 translation and subsequent ATF4-mediated gene expression.
Data from the present report demonstrate that E−/B48 lipoprotein-induced foam cell formation is associated with activation of an eIF-2α phosphorylation-mediated signaling pathway, as reflected by increased PERK and eIF-2α phosphorylation, inhibition of global protein synthesis, and increased levels of ATF4 protein. Furthermore, incubation of macrophages with 2-aminopurine (2-AP), an eIF-2α kinase inhibitor, or transfection of these cells with a nonphosphorylatable eIF-2α mutant inhibits E−/B48 lipoprotein-induced cellular lipid accumulation, providing evidence for a causal role of eIF-2α phosphorylation in E−/B48 lipoprotein-induced foam cell formation.
Materials and Methods
Animals
Wild-type (WT) C57BL/6, ApoE−/−, and ApoB48/48/ApoE−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME). ApoE−/− mice were generated by Piedrahita et al. (1992) and were backcrossed to C57BL/6 for over 10 generations. ApoB48/48/ApoE−/− mice were obtained by crossbreeding ApoE−/− mice with ApoB48/48 mice. The ApoB48/48 mice, which express only ApoB48 and not ApoB100, were generated by Farese et al. (1996). All mice were studied at 3 to 4 months of age and were fed a chow diet containing approximately 5% fat and 19% protein by weight (Harlan Teklad, Madison, WI). All procedures were approved by the Institutional Animal Care and Use Committees of Meharry Medical College.
Antibodies
Antibodies against mouse PERK, eIF-2α, ATF4, cathepsin B, MPR46, scavenger receptor (SR)-A, CD36, GRP78, and β-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and those against lysosomal acid lipase (LAL), calreticulin, and phosphorylated eIF-2α were obtained from Abcam Inc. (Cambridge, MA). SR-B1 and phosphorylated PERK antibodies were obtained from Cell Signaling Technology Inc. (Danvers, MA) and Novus Biologicals, Inc. (Littleton, CO), respectively.
Mouse Peritoneal Macrophages and Lipoproteins
MPMs were prepared from ApoE−/− mice that had received an i.p. injection of 3 ml of 3% thioglycollate broth 4 days earlier as described previously (Wu et al., 2007). Cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C under 5% CO2 in 75-mm culture flasks (Corning Inc., Corning, NY).
Wild-type and E−/B48 lipoproteins were prepared from wild-type and ApoB48/48/ApoE−/− mice, respectively, as described previously (Wu et al., 2007). Mouse plasma was overlaid with a potassium bromide gradient solution (density 1.063) and centrifuged at 120,000 rpm for 2 h. The mixture of VLDL and LDL was collected and dialyzed in phosphate-buffered saline (PBS; pH 7.4) containing 10 mM EDTA for 48 h at 4°C, and filtered through a 0.45-µm filter (Wu et al., 2007).
Macrophage Association and Degradation of 125I-Labeled Lipoproteins
E−/B48 lipoproteins were iodinated with 125I as described previously (Wu et al., 2005). MPMs grown in six-well plates were pretreated at 37°C for 1 h with 2 mM 2-AP or culture medium alone as control, before addition of 20 µg/ml 125I-labeled E−/B48 lipoproteins. Culture medium and cell lysates were collected at the indicated incubation time. The amount of 125I-labeled lipoprotein that was degraded or associated with MPMs was determined as described previously (Wu et al., 2007). The amount of lipoprotein associated with MPMs is a measure of the lipoproteins bound to the cell surface and internalized into the endosomes and lysosomes (Wu et al., 2007).
Measurement of Cellular Cholesterol
MPMs grown in 75-mm culture flasks were pretreated with 2 mM 2-AP or culture medium for 1 h and then incubated with 20 µg/ml lipoproteins for 48 h followed by a 12-h equilibrium in lipoprotein-free medium. For 2-AP treatment, 2 mM 2-AP was maintained in the culture medium for the entire incubation period. After removing the culture medium, MPMs were scraped into 1 ml of distilled water. Lipids were extracted, and cellular total cholesterol and free cholesterol were determined using an enzymatic kit (Wako Chemicals, Richmond, VA). The level of esterified cholesterol was calculated as the difference between total cholesterol and unesterified cholesterol (Wu et al., 2005).
Localization of Endocytic Lipids by Confocal Microscopy
E−/B48 lipoproteins were fluorescently labeled by incubation with 10 µl of 3 mg/ml DiIC18 (dissolved in dimethyl sulfoxide) per 0.5 mg/ml lipoproteins as described previously (Wu et al., 2007). MPMs grown on chamber slides were pretreated with 2 mM 2-AP or culture medium alone at 37°C for 1 h. A mixture of 0.2 mg/ml DiI-labeled lipoproteins and 2 mg/ml unlabeled lipoproteins was added and incubated for 48 h followed by 12-h equilibrium in a lipoprotein-free medium. The cells were then incubated for 1 h with 75 nM LysoTracker Green (Invitrogen, Carlsbad, CA), which permeates the cellular membrane and is commonly used to localize lysosomes. After incubation, the cells were rinsed three times with PBS and fixed for 10 min with 2% paraformaldehyde at 4°C. Fluorescence images for DiI and LysoTracker were obtained with a Nikon TE2000 C1 confocal system equipped with UV-visible lasers (Nikon Instruments Inc., Melville, NY) as described previously (Wu et al., 2007).
Western Blot Analysis of Macrophage Protein Expression
MPMs grown in 75-mm culture flasks were treated with 2-AP and lipoproteins as described above. Cells were washed twice with ice-cold PBS and lysed in an M-PER mammalian protein extraction reagent (Pierce Chemical, Rockford, IL) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and a mixture of phosphatase inhibitors (50 mM NaF, 200 µM Na3VO4, 10 mM Na3PO4, and 100 µM EDTA). The lysate was centrifuged at 14,000g at 4°C for 5 min. The resulting supernatant, containing 5 to 60 µg of proteins, based on the abundance of the studied proteins, was separated on 10 or 12% SDS-polyacrylamide gels, and the proteins were transferred to polyvinylidene difluoride membranes. After blocking with 5% bovine serum albumin, the polyvinylidene difluoride membrane was sequentially incubated with antibodies against indicated proteins and horseradish peroxidase-conjugated secondary antibodies. After incubation with an ECL plus Western blotting detection system (Amersham Biosciences, Chalfont St. Giles, UK), protein fluorescence on the membrane was detected using a laser scanner (Typhoon 9410; Amersham Biosciences).
Metabolic Labeling to Measure Global Protein and Specific Protein Synthesis
MPMs were incubated for 14 h with 0.5 µCi/ml 35S-labeled methionine/cysteine (PerkinElmer Life and Analytical Sciences, Waltham, MA) in 5 ml of methionine/cysteine-free culture medium in the presence or absence of 20 µg/ml E−/B48 lipoproteins and 2 mM 2-AP. For determination of global protein synthesis, cells were washed with ice-cold PBS and lysed with 10% ice-cold trichloroacetic acid. The lysates were filtered on glass fiber discs under vacuum, and the radioactivity on the discs was counted with a Packard Tri-Carb 2300TR liquid scintillation analyzer (PerkinElmer Life and Analytical Sciences).
For determination of specific protein synthesis, cells were sonicated, and the lysate was subjected to immunoprecipitation with the appropriate antibodies. In brief, the lysate was centrifuged at 12,000 rpm for 10 min at 4°C to remove cell debris, and the supernatant was precleaned with protein A-agarose beads (Roche Molecular Biochemicals). Extracts were incubated with the indicated antibodies at 4°C for 1 h and then with fresh agarose beads at 4°C for 1 h. The reactant was centrifuged at 14,000 rpm for 30 s, and the pellets were washed with a lysis buffer containing 50 mM Tris-Cl, 150 mM NaCl, and 1% NP-40. Dissociated proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis and exposed to a PhosphorImager (Typhoon 9410; Amersham Biosciences).
Polysomal Fractionation
MPMs were treated with 2-AP and lipoproteins or culture medium alone as described above, washed twice with ice-cold PBS, and harvested by scraping in a hypotonic solution containing 10 mM Tris-Cl and 5 mM MgCl2 and 0.5% Ivory detergent (Daskal et al., 1976). The lysate was centrifuged at 12,000 rpm for 10 min at 4°C to remove cell debris and nuclei, and the supernatant was layered on a 10-ml continuous sucrose gradient (5–40% sucrose in 15 mM MgCl2, 15 mM Tris-Cl, and 0.3 M NaCl). After 6 h of centrifugation at 41,000 rpm at 4°C in an SW41-Ti rotor (Beckman Coulter, Fullerton, CA), the absorbance at 254 nm was measured continuously as a function of the gradient depth in an UV monitor (UPC900; Amersham Biosciences). The position of nonpolysomal ribosomes and polysomes in the polysome profile was identified by calibration with 70S Escherichia coli ribosomes centrifuged in parallel gradients. The area under the curve representing polysomes was integrated and normalized to the total area under the curve. The percentage derived from this calculation is defined as the overall translation efficiency, which is a reflection of de novo protein synthesis (Koritzinsky et al., 2006).
Isolation of Total and Polysome-Associated RNA
MPMs treated with 2-AP and lipoproteins or culture medium alone were lysed in hypotonic solution, and the lysate was centrifuged as described above. Half of the resulting supernatant was collected for extraction of total RNA using RNAeasy plus mini kits (QIAGEN, Valencia, CA). To isolate polysome-associated RNAs, the remaining supernatant was layered over a 1.35 M sucrose solution, and centrifuged at 55,000 rpm for 80 min at 4°C with a 70Ti Beckman fixed angle rotor (Daskal et al., 1976). The pellet containing polysomes was collected, and polysome-associated RNA was extracted using a QIAGEN RNeasy plus mini kit.
Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis
Quantitative RT-PCR was performed using an iCycler system (Bio-Rad, Hercules, CA). After DNase treatment, each RNA sample was reverse-transcribed and PCR-amplified in triplicate using an iScript one-step RT-PCR kit (Bio-Rad) in a total reaction volume of 20 µl. Reactions were performed with 300 nM each primer and 1 to 10 ng of total RNA depending on the abundance of the RNA studied. Primers for the indicated genes were designed in our laboratory or in commercial laboratories according to information in GenBank. A standard curve of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was generated by 10-fold serial dilutions of macrophage RNA. The relative quantity of the target genes was estimated for each sample by amplifying the target genes simultaneously with the GAPDH standard curve. GAPDH is a suitable gene for normalization because it has shown little variation after treatment with E−/B48 lipoproteins and 2-AP.
Construction and Transfection of a Nonphosphorylatable eIF2α Mutant
It has been established that expression of an eIF-2α mutant, in which Ser51 of eIF-2α is substituted with an alanine, attenuates the repressive effect of endoplasmic reticulum stress on protein synthesis (Donzé et al., 1995). In this report, an eIF2α-S51A mutant was generated as described by Donzé et al. (1995). In brief, an eIF-2α expression construct in lentiviral vector (pSIREN-RetroQ) (Clontech, Mountain View, CA) was generated by amplifying the eIF-2α cDNA using RT-PCR (forward 5′-gacCTCGAGatgccggggctaagttgtaga-3′, backward 5′-gacAAGCTTatcttcagctttggcttc-3′) and inserting the amplified cDNA into the BamHI and EcoRI sites of the lentiviral vector. The Ser51 of eIF-2α was replaced with alanine using the GeneEditor site-directed mutagenesis system (Promega, Madison, WI) and the specific oligonucleotide, 5′-gaattagccagacgacg-3′. Mouse macrophages (Raw 264.7) obtained from American Type Culture Collection (Manassas, VA), were cultured to approximately 70% confluence and infected with lentiviral vectors (control) and vectors encoding eIF2α-S51A. Infection efficiency was monitored by the green fluorescence protein tagged in the vectors. The transfected cells were treated with lipoproteins, and the protein and cholesterol levels were determined using Western blot and enzymatic assays as described above.
Statistical Analysis
Data were reported as mean ± S.E.M., and data distribution was examined by the Shapiro-Wilk normality test. Comparisons between treatment and control groups were performed with Student’s t test, and differences were considered significant at a P value <0.05. All statistical analyses were performed using Stastix software (Analytical Software, Tallahassee, FL).
Results
The Effect of E−/B48 Lipoproteins and 2-AP on PERK-eIF2α-ATF4 Signaling
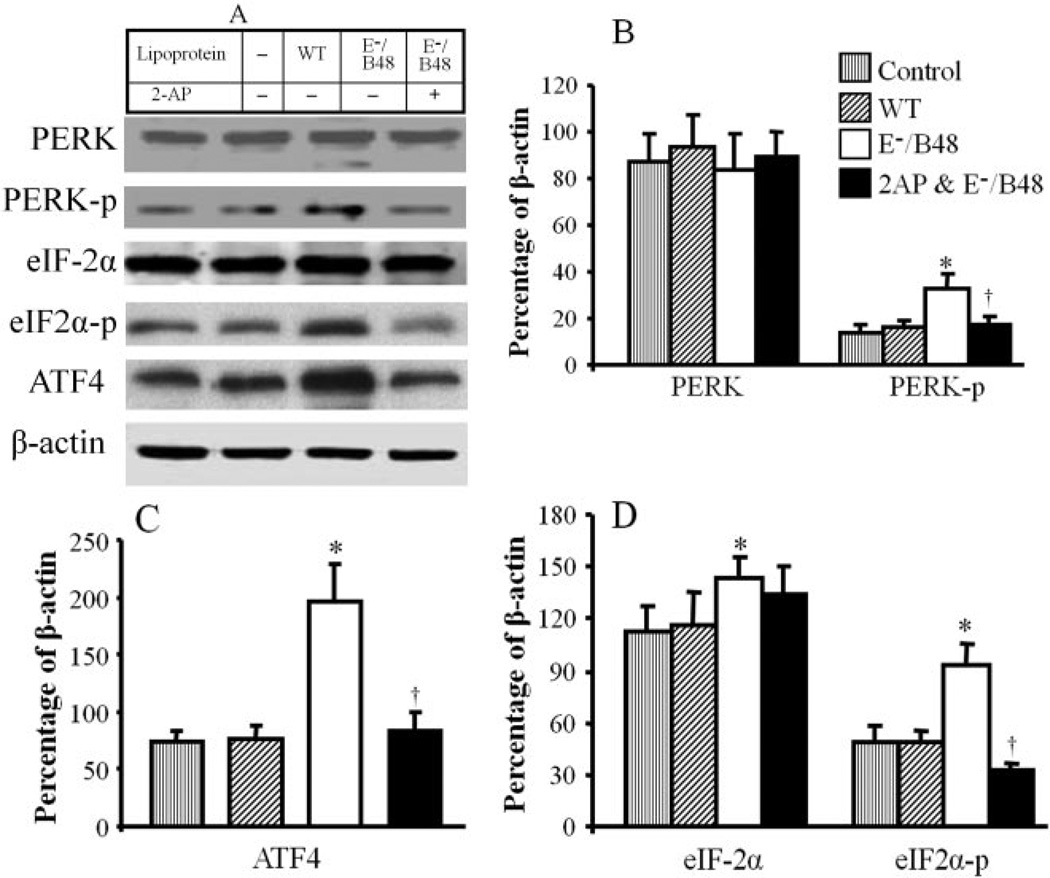
We previously reported that E−/B48 lipoproteins transformed macrophages into foam cells (Wu et al., 2007). Activation of UPR has been detected in atherosclerotic lesions of ApoE−/− mice (Zhou et al., 2005). To investigate whether E−/B48 lipoprotein-induced foam cell formation is associated with UPR activation, we examined the effect of E−/B48 lipoproteins on the PERK-eIF2α-ATF4 signaling pathway, one of the UPR signaling pathways. We observed that incubation of MPMs with wild-type lipoproteins did not significantly affect the phosphorylation and protein level of PERK, eIF-2α, and ATF4. In contrast, addition of E−/B48 lipoproteins to the culture medium induced PERK and eIF-2α phosphorylation in MPMs, starting from 30-min incubation, reaching peaks within 2 h and persisting as long as the E−/B48 lipoproteins existed in the medium (data not shown). Figure 1 shows the effect of E−/B48 lipoproteins on PERK and eIF-2α expression and phosphorylation at 24 h. Incubation of MPMs with E−/B48 lipoproteins elevated levels of both phosphorylated and total eIF-2α protein (Fig. 1, A and D). However, there was a greater increase in the level of phosphorylated eIF-2α protein, significantly increasing the ratio of phosphorylated versus total eIF-2α proteins in the E−/B48 lipoprotein-treated cells compared with the untreated controls (66 ± 8 versus 43 ± 5%, P < 0.05) (Fig. 1). Incubation of MPMs with E−/B48 lipoproteins also increased the phosphorylation level of PERK (Fig. 1, A and B) and the protein level of ATF4 (Fig. 1, A and C). These observations indicate that E−/B48 lipoproteins activate the macrophage PERK-eIF2α-ATF4 signaling pathway in vitro. Data in Fig. 1, A to D, also show that incubation of MPMs with 2 mM 2-AP inhibited E−/B48 lipoprotein-induced phosphorylation of PERK and eIF-2α and reduced the protein level of ATF4. This inhibitory response was not a toxic effect of 2-AP on macrophages because 2 mM 2-AP did not reduce cellular ATP levels (data not shown). In addition, this dose of 2-AP did not alter the phosphorylation and protein level of extracellular signal-regulated kinase 1/2 and P38 mitogen-activated kinase in a time period of 10 min to 24 h (data not shown).
Fig. 1.
The effect of 2-AP on E−/B48 lipoprotein-activated PERK-eIF2−-ATF4 signaling. MPMs were incubated with 20 µg/ml WT or E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone (control) as described under Materials and Methods. The total protein levels of PERK, eIF-2α and ATF4, and the level of phosphorylated PERK (PERK-p) and eIF-2α (eIF2α-p) were determined by Western blot analysis (Fig. 1A), and the results were expressed as a percentage of β-actin expression (B–D). Values represent the mean ± S.E.M. of five separate experiments. *, P < 0.05 compared with control; †, P < 0.05 compared with E−/B48 lipoprotein treatment alone.
The Effect of 2-AP on E−/B48 Lipoprotein Catabolism
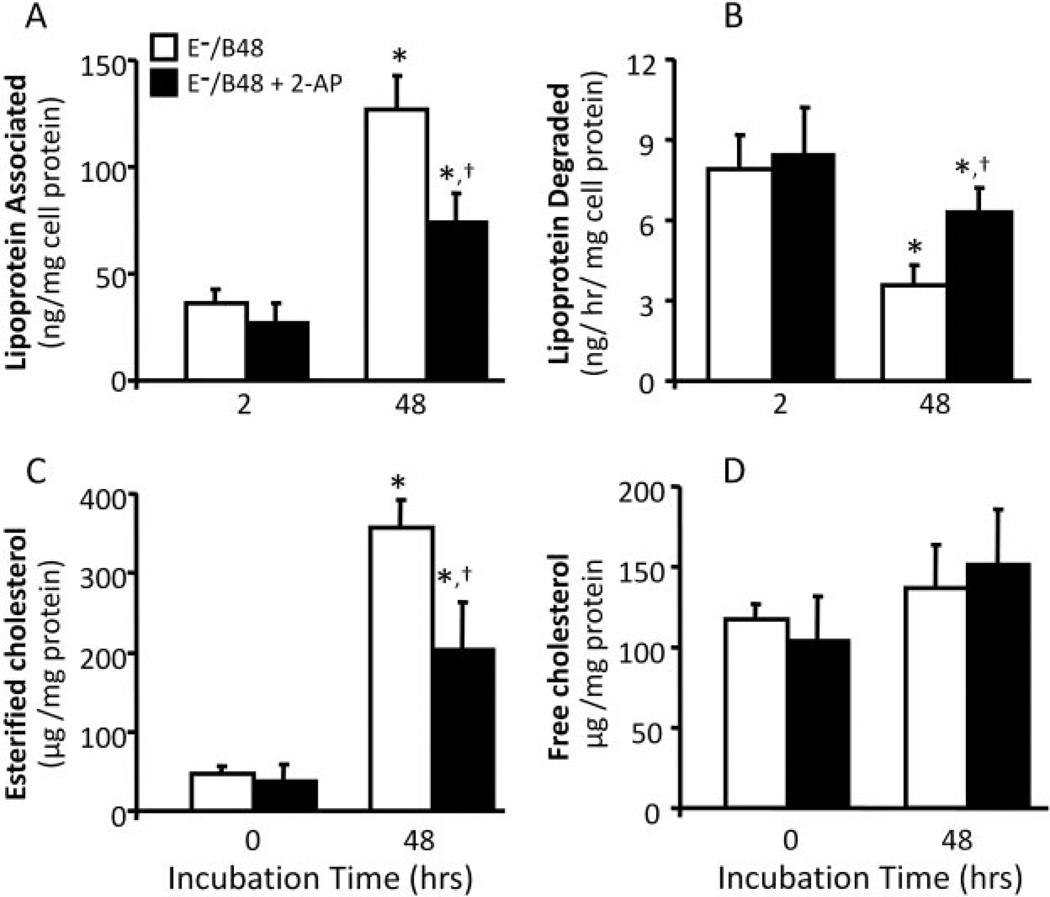
Having demonstrated an inductive role of E−/B48 lipoproteins in eIF-2α phosphorylation, we next examined whether 2-AP, an inhibitor of eIF-2α phosphorylation, enhances E−/B48 lipoprotein catabolism in macrophages. As shown in Fig. 2, incubation of MPMs with wild-type lipoproteins did not result in significant accumulation of lipoproteins (Fig. 2A), and the degradation rate of wild-type lipoproteins remained unchanged over 48 h (Fig. 2B). In contrast, E−/B48 lipoproteins induced a time-dependent increase in lipoprotein accumulation in MPMs, with a greater than 4-fold increase in the level of macrophage-associated lipoproteins over a 2- to 48-h incubation period (Fig. 2A). In parallel, the average degradation rate of E−/B48 lipoproteins in MPMs decreased in a time-dependent manner, decreasing more than 2-fold over a 2 to 48-h incubation period (Fig. 2B). Accordingly, the total amount of E−/B48 lipoproteins degraded by MPMs within 48 h was significantly reduced compared with the amount of degraded wild-type lipoproteins in the same time period (164 ± 23 versus 427 ± 56 ng/mg proteins). Data in Fig. 2, A and B, also show that 2-AP significantly enhanced E−/B48 lipoprotein degradation and suppressed E−/B48 lipoprotein accumulation. These observations suggest that eIF-2α phosphorylation plays a role in both the reduced degradation and the increased accumulation of E−/B48 lipoproteins in MPMs. Furthermore, treatment with 2-AP significantly decreased the level of esterified cholesterol (Fig. 2C) but did not significantly alter the free cholesterol level in E−/B48 lipoprotein-treated MPMs (Fig. 2D), suggesting a causal relationship between eIF-2α phosphorylation and esterified cholesterol accumulation in macrophages.
Fig. 2.
The effect of 2-AP on E−/B48 lipoprotein catabolism in MPMs. MPMs were treated with 20 µg/ml wild-type or E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone. Lipoprotein association, lipoprotein degradation in MPMs, and macrophage cholesterol contents were determined as described under Materials and Methods. The lipoprotein degradation rate was calculated by dividing the cumulative lipoprotein degradation within 2 and 48 h by 2 or 48, respectively. Values for each time point represent the mean ± S.E.M. of five experiments. *, P < 0.05 compared with 2 (top) or 0 (bottom) h; †, P < 0.05 compared with E−/B48 lipoprotein treatment alone.
The Effect of 2-AP on E−/B48 Lipoprotein-Induced Intralysosomal Lipid Accumulation
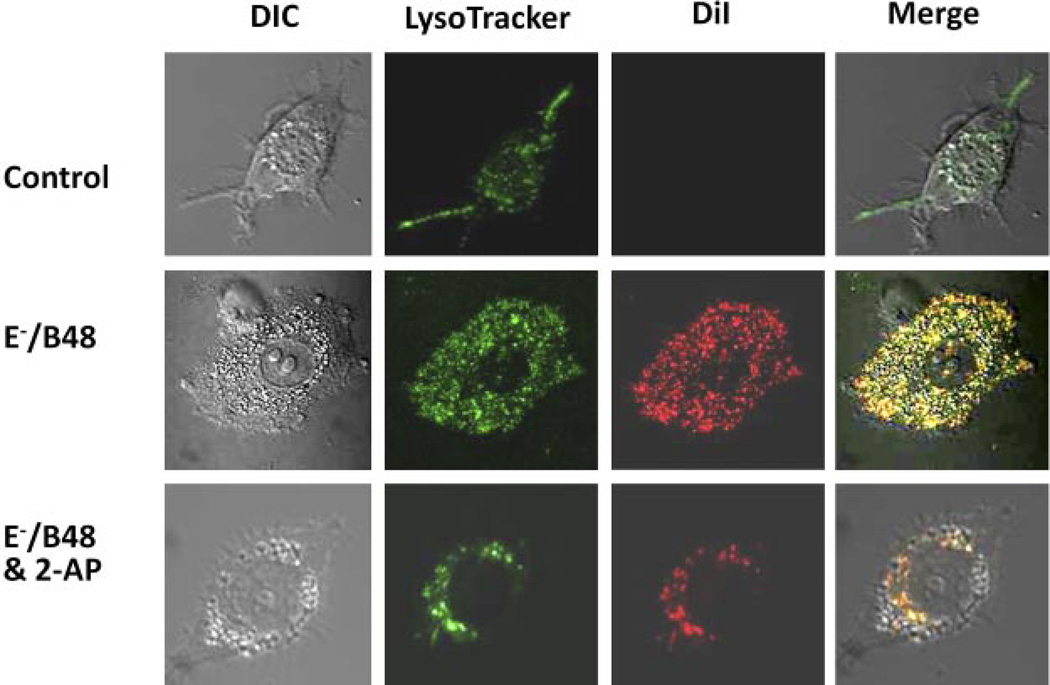
We previously reported that incubation of MPMs with E−/B48 lipoproteins induces lipid droplet accumulation in the cytoplasm. In some cells, E−/B48 lipoproteins caused the cytoplasm to be almost entirely accumulated lipids, giving these cells a foam cell-like appearance (Wu et al., 2007). In contrast, treatment of MPMs with wild-type lipoproteins did not induce lipid droplet accumulation (Wu et al., 2007). In the present study, we used a confocal microscopy to localize the accumulated lipids in MPMs and to assess the effect of 2-AP on this localization. Both untreated and lipoprotein-treated MPMs showed cytoplasmic lysosomal staining with LysoTracker (Fig. 3); however, the extent of staining was markedly greater in cells treated with E−/B48 lipoproteins than in untreated cells. Untreated cells did not show DiI staining, whereas E−/B48 lipoprotein-treated MPMs were significantly stained by DiI (Fig. 3). Furthermore, most of the DiI-stained lipid droplets colocalized with LysoTracker labeling (Fig. 3), suggesting that the lipids accumulated in the cytoplasm were located in the lysosomes. Treatment of MPMs with 2-AP markedly reduced the level of staining with DiI, suggesting that phosphorylation of eIF-2α may play a role in E−/B48 lipoprotein-induced intralysosomal lipid accumulation (Fig. 3).
Fig. 3.
The effect of 2-AP on E−/B48 lipoprotein-induced lipoprotein accumulation in MPMs. MPMs were treated with 20 µg/ml DiI-labeled E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone (control). After staining with LysoTracker, confocal microscopy images were captured as described under Materials and Methods. The merged images are an overlay of the differential interference contrast (DIC) images and the fluorescence images of intracellular DiI and LysoTracker staining. Colocalization of DiI and LysoTracker staining indicates lipoprotein accumulation in the lysosomes.
2-AP Attenuates the Suppression of Overall Protein Synthesis by E−/B48 Lipoprotein
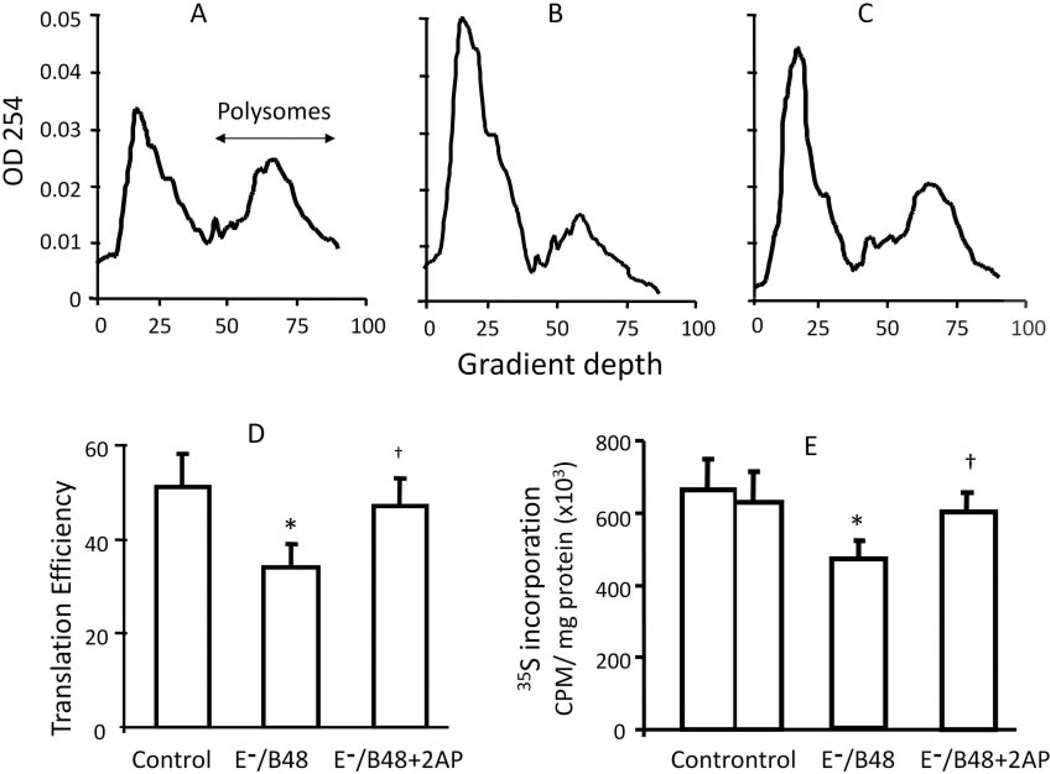
A hallmark of eIF-2α phosphorylation is the subsequent reduction in global protein synthesis caused by reduced initiation of mRNA translation (Wek et al., 2006). To test whether E−/B48 lipoproteins inhibit global protein synthesis, we examined the effect of E−/B48 lipoproteins on the association of mRNA with polysomes. Polysomes, in which actively translated mRNA is simultaneously associated with two or more ribosomes, were separated from monomer ribosomes, ribosomal subunits, and nontranslated mRNAs using a standard protocol outlined under Materials and Methods (Daskal et al., 1976). The representative polysome profiles in Fig. 4, A to C, show that incubation of MPMs with E−/B48 lipoproteins caused a large reduction in high-molecular weight polysomes and a corresponding increase in free ribosomes and ribosomal subunits and that these E−/B48 lipoprotein-induced changes were reversed upon addition of 2-AP. To quantitatively assess overall mRNA translation, we calculated the percentage of the RNAs associated with polysomes, which is a reflection of de novo protein synthesis (Scheuner et al., 2005; van den Beucken et al., 2006). As shown in Fig. 4D, overall mRNA translation was reduced from 51 to 34% after E−/B48 lipoprotein treatment, and addition of 2-AP to the culture prevented or reversed this E−/B48 lipoprotein-induced reduction.
Fig. 4.
The effect of 2-AP on the inhibition of de novo protein synthesis by E−/B48 lipoprotein in macrophages. A to D, overall mRNA translation efficiency. MPMs were incubated with 20 µg/ml E−/B48 lipoproteins (B), 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP (C), or culture medium alone (A). Polysomal and nonpolysomal RNAs were separated on sucrose gradients, and the optical density at 254 nm was plotted as a function of depth in the gradient as described under Materials and Methods. Translation efficiency was expressed as the percentage of the integrated area under the polysome fraction of the curve relative to the total area under both the polysome and nonpolysome fractions (D). E, radiolabeling of newly synthesized proteins. MPMs were incubated with 20 µg/ml E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone (control) in the presence of 0.5 µCi/ml 35S-labeled methionine/cysteine. After 14-h incubation, the radioactivity incorporated into newly synthesized proteins was analyzed as described under Materials and Methods. Values represent the mean ± S.E.M. of four separate experiments. *, P < 0.05 compared with control; †, P < 0.05 compared with E−/B48 lipoprotein treatment alone.
To independently confirm the suppressive effect of E−/B48 lipoproteins on global protein synthesis, we measured the incorporation of 35S-labeled amino acids into newly synthesized proteins. As shown in Fig. 4E, incubation of MPMs with E−/B48 lipoproteins reduced protein synthesis by approximately 32%, and addition of the eIF-2α kinase inhibitor 2-AP prevented or reversed the inhibitory effects of E−/B48 lipoproteins. Taken together, these data suggest that E−/B48 lipoproteins inhibit overall mRNA translation and reduce protein synthesis in MPMs by inducing phosphorylation of eIF-2α. In contrast, wild-type lipoproteins do not affect either overall mRNA translation or new protein synthesis in MPMs (Fig. 4, B, E, and F).
2-AP Selectively Attenuates E−/B48 Lipoprotein-Induced Changes in Expression of Specific Proteins
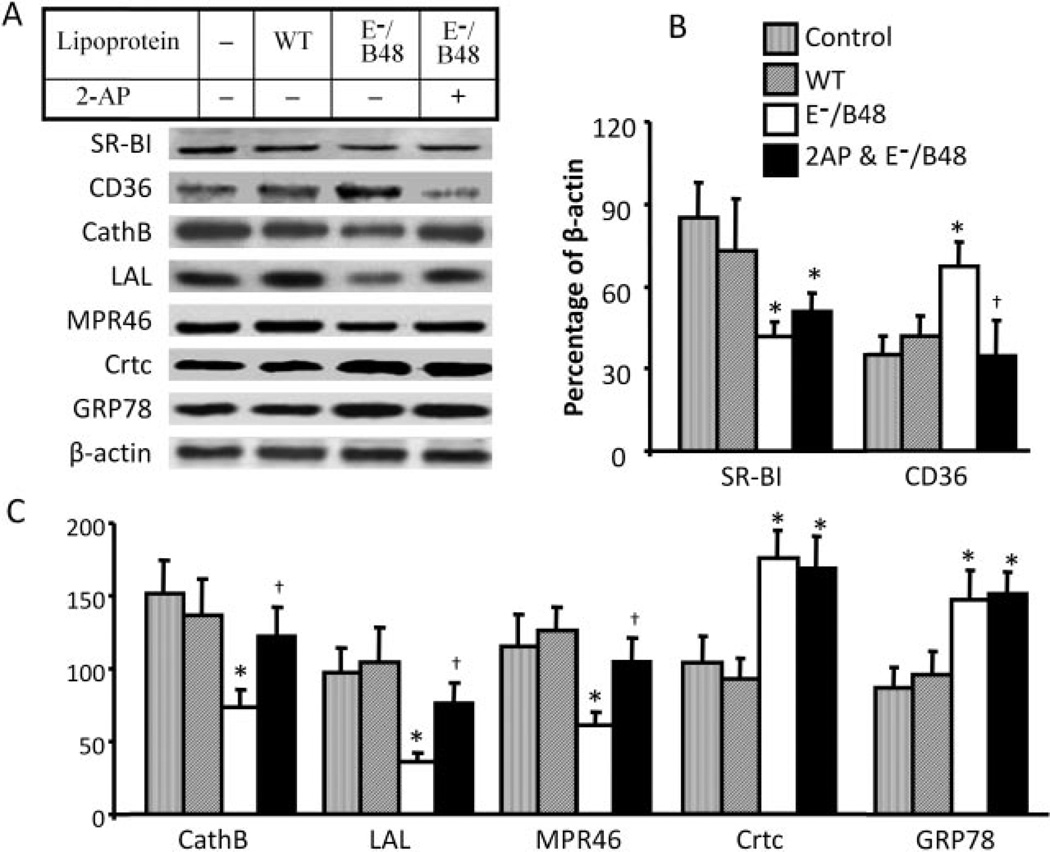
Because the regulation of protein expression is highly gene-specific, we proposed that the effect of E−/B48 lipoproteins and UPR on protein expression might vary between proteins. Thus, we selectively measured the steady-state level of SRs, lysosomal function-related proteins, and protein chaperones. As shown in Fig. 5, E−/B48 lipoprotein-induced changes in expression of scavenger receptors are receptor-specific; the protein level of CD36 was increased after treatment with lipoproteins, whereas the level of SR-BI was decreased. Furthermore, 2-AP selectively attenuated the increase in CD36 level but did not significantly affect the suppression of SR-BI expression (Fig. 5, A and B).
Fig. 5.
The effect of 2-AP on E−/B48 lipoprotein-induced changes in specific protein expression. MPMs were incubated with 20 µg/ml WT or E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone (control) as described under Materials and Methods. The protein levels of SR-BI, CD36, cathepsin B (CathB), LAL, MPR46, calreticulin (Crtc), and GRP78 were determined by Western blot analysis. Expression levels are expressed as a percentage of their immunoblot intensity relative to β-actin. Values represent the mean ± S.E.M. of five separate experiments. *, P < 0.05 compared with control; †, P < 0.05 compared with E−/B48 lipoprotein treatment alone.
Data in Fig. 5 also show that E−/B48 lipoproteins significantly reduced the protein level of cathepsin B, lysosomal acid lipase, and MPR46 and that 2-AP attenuated the suppressive effect of E−/B48 lipoproteins on cathepsin B, lysosomal acid lipase, and MPR46 (Fig. 5, A and C). In contrast, E−/B48 lipoproteins increase the protein levels of chaperones calreticulin and GRP78, which are target genes of ATF6; this increase is not altered by addition of 2-AP (Fig. 5). These findings indicate that E−/B48 lipoproteins activate both the eIF-2α phosphorylation-mediated and the ATF6-mediated pathways of UPR signaling and that 2-AP selectively suppresses the eIF-2α phosphorylation-mediated pathway, confirming that the effects of 2-AP are not caused by nonselective inhibition of a variety of cellular processes. Treatment of MPMs with wild-type lipoproteins did not induce significant changes in expression of these proteins (Fig. 5).
2-AP Attenuates E−/B48 Lipoprotein-Induced Changes in mRNA Expression
To determine whether the changes in expression of specific proteins shown in Figs. 1 and 5 result from changes in mRNA expression, we examined the effect of E−/B48 lipoproteins on the steady-state level of mRNAs encoding lysosomal function-related proteins, scavenger receptors, ER stress-related proteins, and housekeeping genes. As shown in Table 1, E−/B48 lipoproteins did not significantly alter the mRNA level of β-actin and GAPDH, although they significantly elevated the 18S rRNA level. For this study, we calculated the level of mRNA expression for each gene by normalizing to GAPDH mRNA levels.
TABLE 1. Selective regulation of the steady-state level and translation efficiency of specific mRNAs.
MPMs were incubated with 20 µg/ml E-/B48 lipoproteins and 20 µg/ml E-/B48 lipoproteins plus 2 mM 2-AP or culture medium alone (control), and levels of the indicated mRNAs and 18S rRNA were determined by quantitative RT-PCR as described under Materials and Methods. The steady-state level of each mRNA was expressed relative to the mRNA level of the GAPDH housekeeping gene. The level of polysome-associated mRNA was normalized with respect to the steady-state level of that particular mRNA in the whole cell. Values represent the mean ± S.E.M. of four experiments.
| Total Cellular mRNAs | Polysome-Associated mRNAs | |||||
|---|---|---|---|---|---|---|
| Control | E-/B48 | E-/B48 + 2-AP | Control | E-/B48 | E-/B48 + 2-AP | |
| Lysosomal proteins | ||||||
| LAL | 0.36 ± 0.05 | 0.37 ± 0.04 | 0.33 ± 0.04 | 1.04 ± 0.22 | 0.70 ± 0.11* | 1.16 ± 0.14† |
| Cathepsin B | 0.92 ± 0.09 | 0.83 ± 0.14 | 0.85 ± 0.17 | 1.05 ± 0.25 | 0.69 ± 0.08* | 1.17 ± 0.15† |
| MPR46 | 0.68 ± 0.07 | 0.70 ± 0.16 | 0.66 ± 0.09 | 1.08 ± 0.10 | 0.66 ± 0.09* | 1.03 ± 0.06† |
| Scavenger receptors | ||||||
| ApoB48R | 0.12 ± 0.01 | 0.17 ± 0.02* | 0.13 ± 0.02 | 1.55 ± 0.17 | 1.12 ± 0.13* | 1.27 ± 0.16† |
| CD36 | 0.16 ± 0.02 | 0.37 ± 0.04* | 0.21 ± 0.04† | 1.26 ± 0.18 | 0.83 ± 0.12* | 1.16 ± 0.14† |
| SR-B-1 | 0.18 ± 0.02 | 0.08 ± 0.01* | 0.10 ± 0.02* | 1.21 ± 0.14 | 0.85 ± 0.09* | 1.19 ± 0.14† |
| ER stress proteins | ||||||
| GRP78 | 2.99 ± 0.46 | 9.51 ± 0.61* | 8.42 ± 0.63* | 1.99 ± 0.27 | 1.19 ± 0.09* | 1.39 ± 0.19* |
| Calreticulin | 0.56 ± 0.07 | 2.02 ± 0.14* | 1.84 ± 0.11* | 1.71 ± 0.19 | 1.08 ± 0.09* | 1.30 ± 0.19* |
| eIF-2α | 0.36 ± 0.05 | 0.96 ± 0.09* | 0.87 ± 0.26* | 1.16 ± 0.11 | 0.67 ± 0.08* | 0.78 ± 0.10* |
| PERK | 0.27 ± 0.01 | 0.25 ± 0.05 | 0.24 ± 0.05 | 1.24 ± 0.15 | 0.86 ± 0.17* | 1.33 ± 0.18† |
| ATF4 | 0.45 ± 0.06 | 0.48 ± 0.06 | 0.48 ± 0.08 | 0.98 ± 0.11 | 1.27 ± 0.12* | 0.78 ± 0.05† |
| Housekeeping proteins | ||||||
| 18S | 1.54 ± 0.19 | 3.95 ± 0.52* | 3.47 ± 0.29 | 2.41 ± 0.35 | 0.72 ± 0.09* | 1.07 ± 0.09*,† |
| GAPDH | 1.02 ± 0.09 | 1.05 ± 0.19 | 0.97 ± 0.12 | 1.31 ± 0.18 | 0.92 ± 0.13* | 1.13 ± 0.08 |
| β-Actin | 0.54 ± 0.08 | 0.56 ± 0.08 | 0.49 ± 0.07 | 1.74 ± 0.23 | 1.34 ± 0.09* | 1.84 ± 0.16† |
P < 0.05 compared with control.
P < 0.05 compared with E-/B48 lipoprotein treatment alone.
Under control conditions, the abundance of mRNAs in MPMs varies between genes; the GRP78 and ApoB48R mRNA levels are approximately 3-fold higher and 8-fold lower, respectively, than the GAPDH mRNA level (Table 1). It is noteworthy that the effect of E−/B48 lipoproteins on mRNA levels was highly gene specific and did not always correlate with the changes in protein levels reported in Figs. 1 and 5. For example, incubation of MPMs with E−/B48 lipoproteins did not alter the mRNA levels of lysosomal acid lipase, cathepsin B, MPR46, and ATF4 (Table 1), despite a significant effect on expression of their protein products (Figs. 1 and 5). In contrast, E−/B48 lipoproteins up-regulated ApoB48R mRNA (Table 1) but did not significantly alter the ApoB48R protein level (Wu et al., 2007). However, for some genes, the changes in protein and mRNA levels did occur in parallel; E−/B48 lipoproteins significantly elevated mRNA levels of CD36, calreticulin, GRP78, and eIF-2α and reduced the mRNA level of SR-BI, consistent with the observed changes in the corresponding protein products and suggesting that these genes are transcriptionally regulated in response to E−/B48 lipoproteins. It is interesting to note that addition of 2-AP attenuated the up-regulation of CD36 mRNA by E−/B48 lipoprotein but did not significantly affect mRNA levels of calreticulin, GRP78, eIF-2α, and SR-BI, suggesting that a mechanism independent of eIF-2α phosphorylation regulates the expression of calreticulin, GRP78, eIF-2α, and SR-BI mRNAs in response to E−/B48 lipoproteins.
2-AP Attenuates E−/B48 Lipoprotein-Induced Changes in Gene-Specific Translation
To investigate the effect of E−/B48 lipoproteins on de novo synthesis of individual proteins, we evaluated the translation efficiency of individual mRNAs whose protein levels were altered by E−/B48 lipoprotein treatment. Because actively translated mRNAs are associated with polysomes, the abundance of a particular mRNA in polysomes reflects its translation efficiency (Table 1). Incubation of MPMs with E−/B48 lipoproteins reduced the translation efficiency of most of the mRNAs tested by 30 to 50%, consistent with the reduction in overall mRNA translation efficiency shown in Fig. 4. A reduction in mRNA translation efficiency may, at least in part, be responsible for the reduced protein levels of some genes, such as lysosomal acid lipase, cathepsin B, and MPR46, because their steady-state mRNA levels did not change in response to E−/B48 lipoprotein treatment. The addition of 2-AP attenuated the reduction in mRNA translation efficiency of these genes observed after treatment with E−/B48 lipoprotein (Table 1), returning these proteins to the normal level (Fig. 5). Data in Table 1 also show that E−/B48 lipoproteins reduced the translation efficiency of calreticulin, GRP78, eIF-2α, and ApoB48R; however, the protein levels of these genes either increased or remained unchanged, possibly due to elevated steady-state mRNA levels of these genes in response to E−/B48 lipoprotein treatment. The mRNA translation efficiency of ATF4 increased in response to E−/B48 lipoproteins, as indicated by increased polysome association of ATF4 mRNA, despite unaltered steady-state levels of the mRNA encoding ATF4 (Table 1). The addition of 2-AP blocked E−/B48 lipoprotein-induced polysomal association of ATF4 mRNA, thereby reducing its translation efficiency (Table 1).
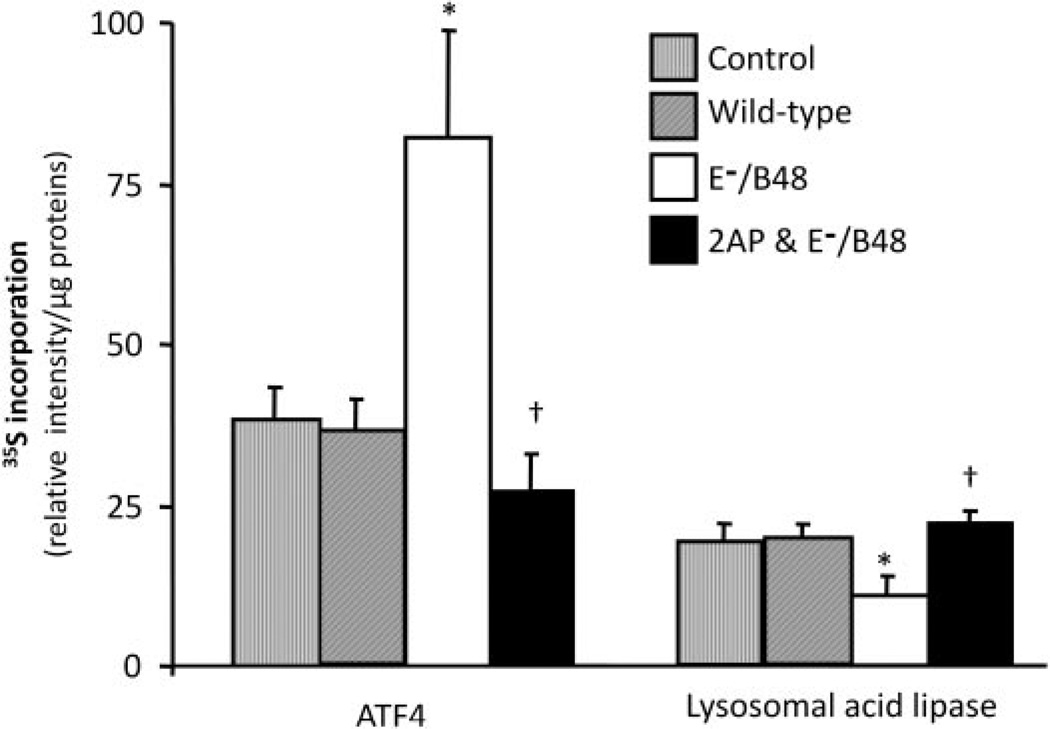
The data in Fig. 6 are derived from parallel studies of ATF4 and lysosomal acid lipase using 35S-metabolic labeling as a complementary strategy to assess mRNA translation levels in MPMs. Treatment of MPMs with wild-type lipoproteins did not alter the synthesis of ATF4 and lysosomal acid lipase. However, E−/B48 lipoproteins significantly increased ATF4 protein synthesis and significantly decreased incorporation of 35S-labeled methionine and cysteine into lysosomal acid lipase proteins. Treatment with 2-AP attenuated the changes in both ATF4 and lysosomal acid lipase synthesis induced by E−/B48 lipoproteins.
Fig. 6.
The effect of 2-AP on E−/B48 lipoprotein-induced changes in gene-specific translation. MPMs were incubated with 0.5 µCi/ml 35S-labeled methionine/cysteine in the presence of 20 µg/ml wild-type or E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone (control). After 14-h incubation, the indicated proteins were immunoprecipitated, resolved by SDS-polyacrylamide gel electrophoresis, and the intensity of the protein bands was quantified as described under Materials and Methods. Values represent the mean ± S.E.M. of three experiments. *, P < 0.05 compared with control; †, P < 0.05 compared with E−/B48 lipoprotein treatment alone.
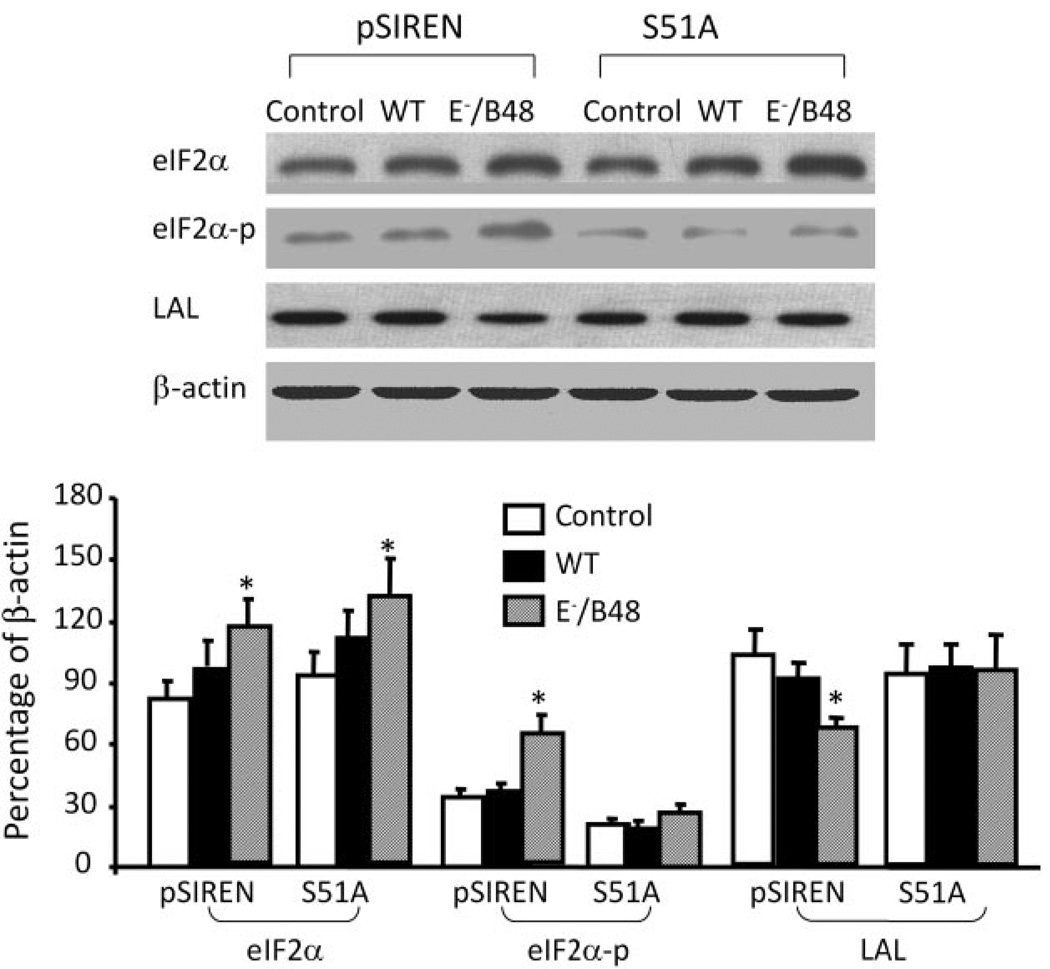
The eIF2α-S51A Mutant Attenuates E−/B48 Lipoprotein-Induced Changes in Protein Expression and Cholesterol Contents
To exploit a complementary strategy to test the causal relationship between eIF-2α phosphorylation and lipid accumulation in macrophages, MPMs were transfected with eIF2α-S51A, which cannot serve as a substrate for eIF-2α kinases. Data in Fig. 7 show that E−/B48 lipoproteins significantly enhance the phosphorylation of eIF-2α and increase the protein level of eIF-2α in the MPMs transfected with control vectors but do not elevate eIF-2α phosphorylation in the cells transfected with eIF2α-S51A, despite increasing levels of eIF-2α protein. These data are consistent with the observations that 2-AP inhibits E−/B48 lipoprotein-induced eIF-2α phosphorylation but does not affect elevation of eIF-2α protein levels (Fig. 1). Data in Fig. 7 also demonstrate that E−/B48 lipoproteins reduce the protein levels of lysosomal acid lipase in the control vector-transfected cells and that transfection of macrophages with eIF2α-S51A mutant attenuates E−/B48 lipoprotein-induced suppressive effects on lysosomal acid lipase. These findings are consistent with the interpretation that induction of eIF-2α phosphorylation is a mechanism by which E−/B48 lipoproteins down-regulate expression of the gene for cholesterol ester degradation in mouse macrophages.
Fig. 7.
The effect of eIF2α-S51A mutant on E−/B48 lipoprotein-induced changes in specific protein expression. Raw 264.7 cells stably transfected with eIF2α-S51A or an empty vector (pSIREN) were treated with 20 µg/ml WT or E−/B48 lipoproteins or medium alone as a control (ctrl) for 24 h. Total eIF2α, eIF2α-p, LAL, and β-actin were measured by immunoblot analysis. The eIF2α, eIF2α-p, and LAL levels were expressed as the percentage of their immunoblot intensities relative to β-actin. Values represent the mean ± S.E.M. of five separate experiments. *, P < 0.05 compared with control.
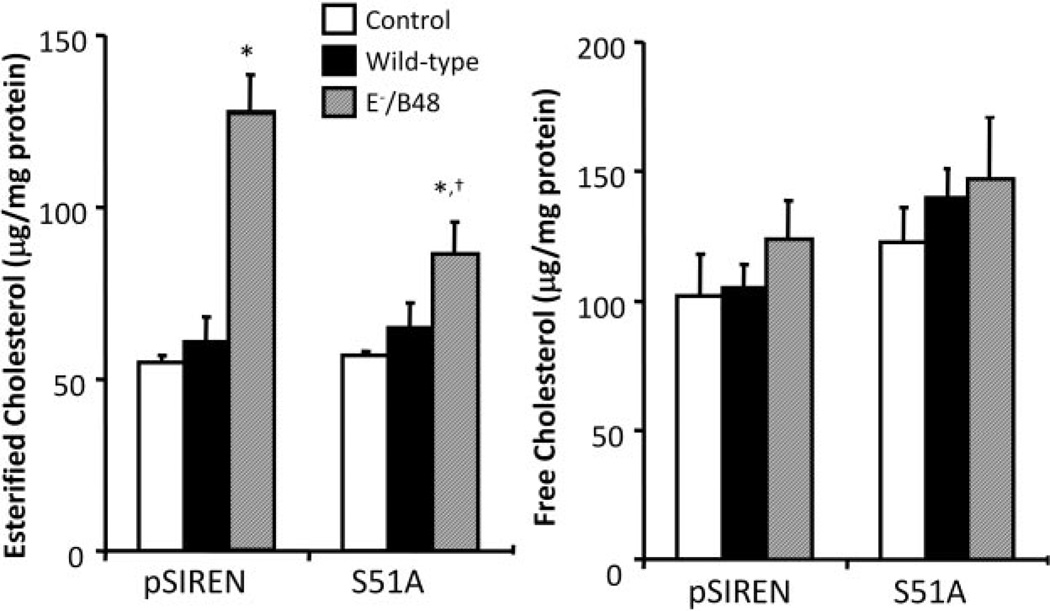
Data in Fig. 8 show that treatment of the control vector-transfected MPMs with E−/B48 lipoproteins significantly increases esterified cholesterol levels but does not significantly alter the free cholesterol levels. In contrast, MPMs transfected with eIF2α-S51A did not respond to E−/B48 lipoprotein treatment to significantly increase cellular cholesterol content (Fig. 8). These data provide another independent line of evidence that inhibition of the ability of eIF-2α to be phosphorylated prevents E−/B48 lipoprotein-induced cholesterol ester accumulation in macrophages, suggesting a causal relationship between eIF-2α phosphorylation and cholesterol accumulation during foam cell formation.
Fig. 8.
The effect of eIF2α-S51A mutant on E−/B48 lipoprotein-induced cholesterol accumulation in MPMs. Raw 264.7 cells were stably transfected with eIF2α-S51A or an empty vector (pSIREN). Cells were incubated with 20 µg/ml WT, E−/B48 lipoproteins, or culture medium alone as a control for 24 h. After a 12-h equilibration in a lipoprotein-free medium, macrophage lipids were extracted, and cellular cholesterol levels were determined. Values represent the mean ± S.E.M. of five experiments. *, P < 0.05 compared with control.
Discussion
ApoE−/− mice develop atherosclerotic lesions that are histologically similar to those observed in humans. These lesions begin as early subintimal foam cell deposits and progress to advanced fibroproliferative plaques (Reddick et al., 1994; Yang et al., 2004). It is interesting to note that all stages of atherosclerotic lesions developed in the aorta root of ApoE−/− mice show increased expression of phosphorylated PERK (Zhou et al., 2004, 2005), one of four eIF-2α kinases that phosphorylate eIF-2α on Ser51 (Zhang and Kaufman, 2006). The present study provides the first evidence that E−/B48 lipoproteins enhance phosphorylation of PERK and eIF-2α in mouse macrophages and that this phosphorylation is reduced by 2-AP. It has been reported that 2-AP inhibits other kinases in addition to eIF-2α kinases, such as mitogen-activated protein kinases (MAPKs) (Zhou et al., 2003; Hosoi et al., 2006); however, our preliminary studies indicated that E−/B48 lipoproteins were unable to stimulate MAPKs, including extracellular signal-regulated kinase 1/2 and P38 MAPKs, and that 2-AP did not alter the protein level and phosphorylation of these MAPKs in the absence and presence of E−/B48 lipoproteins (data not shown). Therefore, we attribute the effects of 2-AP observed in our study to the inhibition of eIF-2α kinases, such as PERK.
Another major finding of the present study is that inhibition of eIF-2α phosphorylation by 2-AP increases macrophage degradation of E−/B48 lipoproteins and decreases E−/B48 lipoprotein-induced intralysosomal lipid accumulation, indicating a causal role of eIF-2α phosphorylation in foam cell formation. Our data also demonstrate that inhibition of foam cell formation by 2-AP is paralleled by elevated protein levels of lysosomal acid lipase and cathepsin B in E−/B48 lipoprotein-treated cells. Our interpretation of these findings is that the interaction of E−/B48 lipoproteins with macrophages activates eIF-2α kinase(s) such as PERK, which phosphorylate eIF-2α. Phosphorylated eIF-2α down-regulates lysosomal hydrolases and reduces degradation of the lipid and protein components of E−/B48 lipoproteins, leading to lipoprotein accumulation in the lysosomes. Through inhibition of eIF-2α phosphorylation, 2-AP alleviates the suppressive effect of E−/B48 lipoproteins on lysosomal hydrolases and reduces lipoprotein and cholesterol ester accumulation in the macrophages. This postulation is confirmed by the studies using eIF2α-S51A as a tool to inhibit eIF2α phosphorylation. Our data demonstrate that transfection of MPMs with eIF2α-S51A attenuates the suppressive effect of E−/B48 lipoproteins on lysosomal acid lipase, accompanied by reduced cellular cholesterol ester levels. Thus, our data suggest that eIF-2α kinases are a possible therapeutic target for prevention or reduction of E−/B48 lipoprotein-induced foam cell formation.
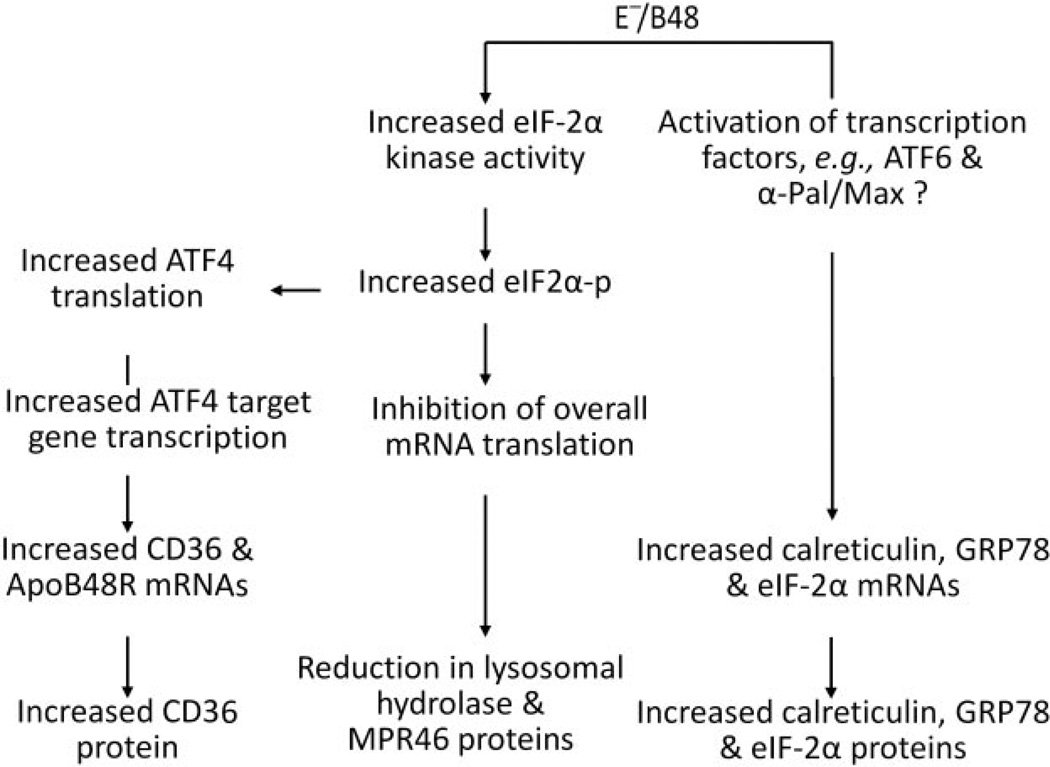
The schematic in Fig. 9 shows the proposed role of UPR signaling pathways in macrophage gene expression in response to E−/B48 lipoprotein. One hallmark consequence of eIF-2α phosphorylation is inhibition of overall mRNA translation, reducing global protein synthesis (Roybal et al., 2005). Indeed, E−/B48 lipoprotein-induced eIF-2α phosphorylation is associated with a reduction in overall mRNA translation. Correspondingly, the translation efficiency of all the mRNAs tested, except ATF4 mRNA, was reduced in E−/B48 lipoprotein-treated macrophages. For some genes, decreased mRNA translation efficiency may explain the reduced protein level; for example, E−/B48 lipoprotein decreases both the translation efficiency and the protein level of lysosomal acid lipase, cathepsin B, and MPR46, whereas the steady-state levels of the mRNAs encoding these proteins are unchanged. These observations imply that a reduction in levels of these proteins in the E−/B48 lipoprotein-treated MPMs is not due to a reduced availability of their mRNAs but results, at least in part, from reduced translation efficiency (Fig. 9). 2-AP attenuates the reduction in mRNA translation efficiency of these genes induced by E−/B48 lipoprotein and restores normal protein levels.
Fig. 9.
Putative mechanism through which E−/B48 lipoproteins regulate gene expression. MPMs were incubated with 20 µg/ml E−/B48 lipoproteins, 20 µg/ml E−/B48 lipoproteins plus 2 mM 2-AP, or culture medium alone (control), and levels of the indicated mRNAs and 18S rRNA were determined by quantitative RT-PCR as described under Materials and Methods. The steady-state level of each mRNA was expressed relative to the mRNA level of the GAPDH housekeeping gene. The level of polysome-associated mRNA was normalized with respect to the steadystate level of that particular mRNA in the whole cell. Values represent the mean ± S.E.M. of four experiments. *, P < 0.05 compared with control; †, P < 0.05 compared with E−/B48 lipoprotein treatment alone.
Another hallmark consequence of eIF-2α phosphorylation is a selective increase in ATF4 translation (Roybal et al., 2005). Our data demonstrate that E−/B48 lipoproteins selectively enhance ATF4 mRNA translation despite suppressing the translation efficiency of other mRNAs. This increase in ATF4 mRNA translation efficiency may account for the increased ATF4 protein level because the steady-state level of ATF4 mRNA is comparable in E−/B48 lipoprotein-treated macrophages and untreated control cells. ATF4 is the only transcription factor known to be directly induced by eIF-2α phosphorylation, and a number of ATF4 target genes have been described in mammalian cells (Roybal et al., 2005). In this study, we observed that E−/B48 lipoproteins significantly increased the mRNA levels of several genes, including CD36, ApoB48R, eIF-2α, GRP78, and calreticulin. However, the effects of 2-AP on these genes are different; 2-AP reduces the induction of CD36 and ApoB48R mRNAs by E−/B48 lipoprotein but does not significantly affect changes in mRNA expression of eIF-2α, GRP78, and calreticulin. These findings suggest that E−/B48 lipoproteins increase CD36 and ApoB48R mRNA levels in macrophages through activation of the eIF2α-ATF4 signaling pathway (Fig. 9). In contrast, the lack of effect of 2-AP on the induction of eIF-2α, GRP78, and calreticulin mRNA by E−/B48 lipoproteins implies that the expression of these genes is regulated via mechanisms other than the eIF2α-ATF4 pathway. It has been reported that the promoter regions of GRP78 and calreticulin contain the endoplasmic reticulum stress-responsive element; therefore, their transcription can be activated by ATF6 (Fig. 9) (Okada et al., 2002; Lee et al., 2003). It is known that expression of eIF-2α is primarily regulated at the transcriptional level by transcription factors α-Pal (Shors et al., 1998) and Max (Shors et al., 1998), which bind to the eIF-2α promoter either as a Max/Max homodimer or as a Max/α-Pal heterodimer. It is possible that the reduction in global protein synthesis after E−/B48 lipoprotein treatment activates α-Pal and/or Max to increase eIF2α expression as a compensatory response (Fig. 9).
In summary, the data presented here provide the first demonstration that E−/B48 lipoprotein-induced foam cell formation is associated with enhanced phosphorylation of PERK and eIF-2α. This phosphorylation seems causal for foam cell formation because treatment of MPMs with the eIF-2α kinase inhibitor 2-AP or transfection the cells with an eIF-2α nonphosphorylatable mutant attenuates the suppressive effect of E−/B48 lipoproteins on lysosomal hydrolases and inhibits lipoprotein and cholesterol ester accumulation. Therefore, these data indicate that E−/B48 lipoproteins regulate gene expression and induce foam cell formation through activation of eIF-2α phosphorylation. These findings also implicate inhibition of signaling pathways involving eIF-2α phosphorylation as a possible therapeutic target for treatment or prevention of atherosclerosis.
Acknowledgments
We thank Lee Limbird for critical reading of the manuscript and useful suggestions.
This study was supported by National Institutes of Health Grants K01HL076623 and G12RR003032 (to H.Y.), U54NS041071 (to J.S.G.), and R01ES014471 (to Z.G.).
ABBREVIATIONS
- Apo
apolipoprotein
- MPM
mouse peritoneal macrophage
- E−/B48
apolipoprotein E-deficient, ApoB48-containing
- MPR46
cation-dependent mannose 6 phosphate receptor
- eIF
eukaryotic initiation factor
- ER
endoplasmic reticulum
- PERK
RNA-dependent proteinkinase-like endoplasmic reticulum kinase
- UPR
unfolded protein response
- GRP
glucose-regulated protein
- ATF
activating transcription factor
- 2-AP
2-aminopurine
- SR
scavenger receptor
- LAL
lysosomal acid lipase
- PBS
phosphate-buffered saline
- RT
reverse transcription
- PCR
polymerase chain reaction
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MAPK
mitogen-activated protein kinase
- WT
wild type
References
- Daskal I, Ramirez SA, Ballal RN, Spohn WH, Wu B, Busch H. Detergent lysis for isolation of intact polysomes of Nivikoff hepatoma ascites cells. Cancer Res. 1976;36:1026–1034. [PubMed] [Google Scholar]
- Donzé O, Jagus R, Koromilas AE, Hershey JW, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Jr, Veniant MM, Cham CM, Flynn LM, Pierotti V, Loring JF, Traber M, Ruland S, Stokowski RS, Huszar D, et al. Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc Natl Acad Sci U S A. 1996;93:6393–6398. doi: 10.1073/pnas.93.13.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZM, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Matsunami N, Nagahama T, Okuma Y, Ozawa K, Takizawa T, Nomura Y. 2-Aminopurine inhibits leptin receptor signal transduction. Eur J Pharmacol. 2006;553:61–66. doi: 10.1016/j.ejphar.2006.09.044. [DOI] [PubMed] [Google Scholar]
- Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Rall SC., Jr Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): questions, quandaries, and paradoxes. J Lipid Res. 1999;40:1933–1949. [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil J, Hoppe G, Hoff HF. Phospholipids in oxidized low density lipoproteins perturb the ability of macrophages to degrade internalized macromolecules and reduce intracellular cathepsin B activity. Atherosclerosis. 2003;169:215–224. doi: 10.1016/s0021-9150(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick RL, Zhang S, Maeda N. Atherosclerosis in mice lacking Apo E: evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280:20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Vander MD, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- Shashkin P, Dragulev B, Ley K. Macrophage differentiation to foam cells. Curr Pharm Des. 2005;11:3061–3072. doi: 10.2174/1381612054865064. [DOI] [PubMed] [Google Scholar]
- Shors ST, Efiok BJ, Harkin SJ, Safer B. Formation of alpha-Pal/Max heterodimers synergistically activates the eIF2-alpha promoter. J Biol Chem. 1998;273:34703–34709. doi: 10.1074/jbc.273.52.34703. [DOI] [PubMed] [Google Scholar]
- van den Beucken T, Koritzinsky M, Wouters BG. Translational control of gene expression during hypoxia. Cancer Biol Ther. 2006;5:749–755. doi: 10.4161/cbt.5.7.2972. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wu D, Yang H, Xiang W, Zhou L, Shi M, Julies G, Laplante JM, Ballard BR, Guo Z. Heterozygous mutation of ataxia-telangiectasia mutated gene aggravates hypercholesterolemia in apoE-deficient mice. J Lipid Res. 2005;46:1380–1387. doi: 10.1194/jlr.M400430-JLR200. [DOI] [PubMed] [Google Scholar]
- Wu DF, Sharan C, Yang H, Goodwin JS, Zhou L, Grabowski GA, Du H, Guo Z. Apolipoprotein E-deficient lipoproteins induce foam cell formation by down-regulation of lysosomal hydrolases in macrophages. J Lipid Res. 2007;48:2571–2578. doi: 10.1194/jlr.M700217-JLR200. [DOI] [PubMed] [Google Scholar]
- Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006:69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol Sci. 2003;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- Zhou J, Werstuck GH, Lhotak S, de Koning AB, Sood SK, Hossain GS, Moller J, Ritskes-Hoitinga M, Falk E, Dayal S, et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- Zschenker O, Illies T, Ameis D. Overexpression of lysosomal acid lipase and other proteins in atherosclerosis. J Biochem. 2006;140:23–38. doi: 10.1093/jb/mvj137. [DOI] [PubMed] [Google Scholar]