Abstract
Moral sentiment has been hypothesized to reflect evolved adaptations to social living. If so, individual differences in moral values may relate to regional variation in brain structure. We tested this hypothesis in a sample of 70 young, healthy adults examining whether differences on two major dimensions of moral values were significantly associated with regional gray matter volume. The two clusters of moral values assessed were “individualizing” (values of harm/care and fairness), and “binding” (deference to authority, in-group loyalty, and purity/sanctity). Individualizing was positively associated with left dorsomedial prefrontal cortex volume, and negatively associated with bilateral precuneus volume. For binding, a significant positive association was found for bilateral subcallosal gyrus and a trend to significance for the left anterior insula volume. These findings demonstrate that variation in moral sentiment reflects individual differences in brain structure and suggest a biological basis for moral sentiment, distributed across multiple brain regions.
Introduction
Moral conduct is central to the well-being and cohesion of societies, and thus delineating the origins of morality represents both an important scientific and social goal. Central constructs in the moral lexicon are harm reduction/care and fairness/justice (Gilligan, 1982; Kohlberg, 1969), alongside more recent additions such as sentiment concerning deference to authority, loyalty to one’s in-group, and concerns over purity/sanctity (Haidt, 2009; Haidt & Graham, 2009). With regards to the etiology of moral sentiment, and in contrast to social learning approaches to moral behavior (Bandura, 1991), evolutionary models suggest that these moral foundations reflect deep-rooted adaptations to social living (Haidt & Kesebir, 2010). With this in mind, it is plausible that individual differences in brain structure may reflect variation in moral sentiment. Accordingly, in the current study we sought to test this hypothesis using voxel-based morphometry in a large sample of healthy, young adults.
Moral values are commonly conceived to relate to sentiment concerning the rights of the individual (Gilligan, 1982; Kohlberg, 1969). Indeed, neuroscientific research seeking to uncover the neural bases of moral sentiment has typically focused on these constructs, such as in the runaway trolley paradigm, where participants are required to make tradeoffs between harming a single individual to save several others (Cushman, Young, & Hauser, 2006) and experimental economics games, which assess motivations concerning interpersonal fairness vs. self-interest (e.g. Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003). Nonetheless, while benevolence and universalism are uncontroversial components of the moral lexicon, recent work has suggested that this framework is incomplete. Drawing from cross-cultural studies of social and moral systems (e.g. Haidt et al., 1993; Shweder, Mahapatra, & Miller, 1987), Graham et al. (2011) argued that morality extends “beyond the individual-based concerns of harm and fairness, involving concerns about spiritual purity and degradation (even for acts that involve no harm), concerns about proper hierarchical role fulfillment, and moral expectations of loyalty to the local or national group (p. 367).
With these observations in mind, Haidt and Graham (2009) suggested that moral behaviour varies according to five core foundations: 1) harm (minimizing harm to others), 2) fairness (maximizing fairness to all), 3) ingroup loyalty (the importance of the ingroup), 4) authority (respect for status and hierarchy), and 5) purity (avoiding impure or disgusting acts/entities). Empirical tests of these five moral foundations have provided psychometric support for the structure of this model (Graham et al., 2011): Confirmatory factor analyses supported a five-correlated factors solution, although evidence for the existence of two super-ordinate moral factors was also noted: “individualizing” – the aggregate score on harm and fairness, and “binding” - the aggregate score on authority, ingroup loyalty, and purity (Graham, Haidt, & Nosek, 2009).
Theorizing within an evolutionary framework, Haidt and Graham (2009) suggested that “natural selection prepared the human mind to easily learn to detect and respond to (at least) five sets of patterns in the social world” (pp. 380-81). If humans evolved a capacity to detect and adhere to a small and specific set of social patterns, the question arises as to which neural processes and structures underlie this capacity. While most prior neuroscience research has focused on deficits in moral reasoning in patients with brain lesions (Koenigs et al., 2007), or functional activation correlates of moral sentiment or reasoning (Greene, Sommerville, Nystrom, Darley, & Cohen, 2001), recent work has demonstrated that regional variation in brain volume is also linked to individual differences in psychological traits (cf. Kanai & Rees, 2011). Utilizing this volumetric approach, here we examined whether regional variation in brain structure was associated with differences on the two higher-order moral factors of “binding” – the aggregate score of authority, in-group loyalty, and purity – and “individualizing” – the aggregate score of harm and fairness, as well as with these component factors (Graham et al., 2011).
Although extensive work has examined the neural bases of social cognition, no neuroscientific work to our knowledge has directly addressed these particular moral constructs. Moreover, of prior relevant work, it is apparent that both individualizing and binding could each plausibly be associated with similar neural correlates. For example, insula cortex is active when others feel pain (Saarela et al., 2007; Singer et al., 2006), and as such is conceivably linked to individualizing. Insula function, however, is also associated with disgust sensitivity (Calder, Keane, Manes, Antoun, & Young, 2000; Wright, He, Shapira, Goodman, & Liu, 2004), an emotion closely related to purity (Rozin & Fallon, 1987), thus also linking this region with binding. Similarly, the amygdala appears to play a role in operant conditioning (Davis & Whalen, 2001), which in turn plays an important role in normative behavior (Blair, 2007), and thus links this structure to binding. However, Haruno and Frith (2010) have reported amygdala activation in situations where selfish acts are committed, potentially linking this region with individualizing. Further complicating matters, regions typically linked with mentalizing (e.g. medial prefrontal cortex, temporal-parietal junction, temporal poles; Amodio & Frith, 2006), thus facilitating empathy, might at first thought appear to correspond to individualizing; however, empathy is not necessarily synonymous with sympathy (Decety & Michalska, 2010) and moral sentiments reflecting binding concerns may also rely on the capacity to understand the mental states of others. Finally, while much recent work has examined the neural bases of social cognition (Amodio & Frith, 2006), the vast majority of this research has involved functional neuroimaging: Limited work to date has assessed regional gray matter correlates of the social mind and it is not yet clear whether functional imaging data correspond well to volumetric assessments (Kanai & Rees, 2011).
In line with the limited directly relevant prior work in this domain, as noted above, while we expected frontal regions in particular to show links to our moral measures (in line with extensive work linking these regions to social cognition; Carrington & Bailey, 2009; Amodio & Frith, 2006), here we remained largely agnostic with regards to specific regions, instead focusing our analyses on (partial) whole-brain corrected tests (see Methods for full detail). We analyzed structural MRI volumes collected from a large (n = 70) sample of healthy, young adults. We used voxel-based morphometry (VBM) methods to characterize any differences in regional brain structure correlated with individual differences in moral values, using appropriate correction for multiple comparisons.
Methods
Participants
Seventy healthy participants (42 females; mean age = 24.6 ± 3.76 years) were recruited from the local community of University College, London. The study was approved by the local ethical committee and written informed consent was obtained from all participants.
Moral Values Measures
Moral values were assessed using the 32-item Moral Foundations Questionnaire (MFQ) measuring harm/care, fairness, in-group loyalty, authority deference, and purity/sanctity, along with a 2-item validity scale (Graham et al., 2011). Examples of MFQ scale items are as follows: “Compassion for those who are suffering is the most crucial virtue” (Harm); “When the government makes laws, the number one principle should be ensuring that everyone is treated fairly” (Fairness); “It is more important to be a team player than to express oneself” (Ingroup loyalty); “Respect for authority is something all children need to learn” (Authority); “People should not do things that are disgusting, even if no one is harmed” (Purity). The MFQ was administered on-line via a web browser to facilitate data storage and scoring.
MRI Data Acquisition
MR images were acquired on a 1.5-T Siemens Sonata MRI scanner (Siemens Medical, Erlangen, Germany). High-resolution anatomical images were acquired using a T1-weighted 3-D Modified Driven Equilibrium Fourier Transform (MDEFT) sequence (TR = 12.24 ms; TE = 3.56 ms; field of view = 256 × 256 mm; voxel size = 1 × 1 × 1 mm).
Voxel-based Morphometry (VBM)
The MR images were first segmented for gray matter (GM) and white matter (WM) using the segmentation tools in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Subsequently, we performed Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) in SPM8 for inter-subject registration of the GM images. To ensure that local gray matter volumes were conserved after spatial transformation, the image intensity of each voxel was modulated by the Jacobian determinants of the deformation fields. Registered images were then smoothed with a Gaussian kernel (FWHM = 8 mm) and transformed to MNI stereotactic space using affine and non-linear spatial normalisation implemented in SPM8 for multiple regression analysis.
The gender and age of the participants as well as whole-brain gray matter volume were included as covariates in the design matrix, thus regressing out effects correlated with these variables. We conducted all statistical analyses using a mask volume that comprised the entire brain with the exception of the cerebellum and the occipital lobe, which were excluded from analysis. The mask volume was constructed from a probabilistic MNI structural atlas (Mazziotta et al., 2001). Within this mask volume, clusters were initially identified as contiguous groups of voxels that exceeded an uncorrected threshold of voxel-wise p < 0.001. We then employed a threshold of p(corr) < 0.05 corrected for multiple comparisons across the entire mask volume at a cluster level using non-stationary correction (Hayasaka, Phan, Liberzon, Worsley, & Nichols, 2004) to identify regions in which gray matter volume significantly correlated with moral values.
Results
Binding: VBM Correlates
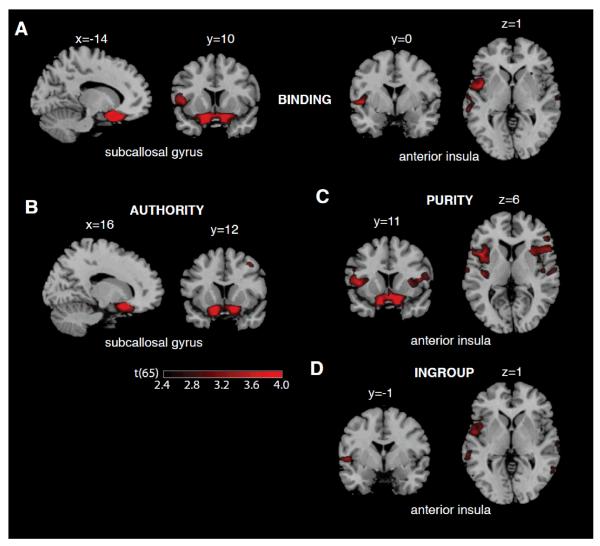
Binding was positively and significantly correlated with the gray matter volume of bilateral subcallosal gyrus (p(corr) < 0.01, r = 0.57, t(65) = 5.56) and marginally with gray matter volume of the left anterior insula (p(corr) = 0.057, r = 0.45, t(65) = 4.03; see Table 1 and Figure 1). We did not find any cluster that exhibited a significant (corrected) negative correlation with binding.
Table 1.
Summary of the gray matter volume associations with moral values.
| Moral Foundation | Area | H | MNI coordinates of peak voxel |
Correlation (Pearson’s r) |
t(65) | Cluster size (mm3) |
p(corr) | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Binding | subcallosal gyrus | L/R | −14 | 10 | −24 | 0.57 | 5.56 | 7854 | <0.01 ** |
| anterior insula | L | −46 | 0 | 1 | 0.45 | 4.03 | 1110 | 0.057 n.s. | |
| Authority | subcallosal gyrus | L/R | 16 | 12 | −15 | 0.49 | 4.51 | 6581 | <0.01 ** |
| Purity | subcallosal gyrus | L/R | −15 | 20 | −21 | 0.55 | 5.32 | 6891 | <0.01 ** |
| anterior insula | L | −44 | 11 | 6 | 0.45 | 4.01 | 1417 | 0.04 * | |
| anterior insula | R | 45 | 12 | 12 | 0.44 | 3.96 | 1667 | 0.10 n.s. | |
| Disgust (part of purity) | anterior insula | R | 42 | 13 | 9 | 0.48 | 4.36 | 3605 | <0.01 ** |
| In-group | anterior insula | L | −52 | −1 | 1 | 0.40 | 3.49 | 3510 | 0.20 n.s. |
| Individualizing | DMPFC | L | −8 | 44 | 43 | 0.47 | 4.33 | 813 | 0.04 * |
| Precuneus | L/R | 9 | −42 | 45 | −0.47 | 4.32 | 759 | 0.07 n.s. | |
| Harm/care | Precuneus | L/R | 10 | −45 | 46 | −0.47 | 4.30 | 2204 | 0.03 * |
| postcentral gyrus | L | −64 | −16 | 33 | −0.52 | 4.86 | 1623 | 0.04 * | |
| DMPFC | L | −8 | 44 | 43 | 0.43 | 3.87 | 283 | 0.30 n.s. | |
| Fairness | DMPFC | L | −9 | 44 | 42 | 0.44 | 3.91 | 283 | 0.058 n.s. |
Note: p(corr) denotes the p values corrected for multiple comparison at the cluster level across the volume of interest (see Methods for details). Significant results are denoted by * (p < 0.05, corrected) and ** (p < 0.01, corrected); n.s. indicates non-significant results; DMPFC = dorsomedial prefrontal cortex; H = hemisphere; L = left; R = right; L/R = left and right.
Figure 1.
Regions where gray matter volume was associated with Binding (A), Authority (B), In-group loyalty (C), and Purity/sanctity (C) values are overlaid on a T1-weighted standard MNI template (Collins et al., 1998); x, y, and z values denote the position of the sagittal, coronal and transverse slices, respectively; t values are overlaid for regions that showed significant positive correlation in the VBM analyses (see the main text and Table 1). The overlays are thresholded at p < 0.01 (uncorrected for display purposes).The color bar illustrates the corresponding t values.
Binding Subscales: VBM Correlates
Within the subscales of the binding foundations, authority scores were positively correlated with the gray matter volume of bilateral subcallosal gyrus (p(corr) < 0.01, r = 0.49, t(65) = 4.51; see Figure 1). The purity score also correlated significantly with the same region of bilateral subcallosal cortex (p(corr) < 0.01, r = 0.55, t(65) = 5.32) and with gray matter volume of the left anterior insula (left insula, p(corr) = 0.04, r = 0.45, t(65) = 4.01; see Figure 1). The purity score exhibited a trend of correlation with gray matter volume of the right insula but this was not significant (p(corr) = 0.10, r = 0.44, t(65) = 3.96). Items related to feelings of disgust primarily drove the correlation in the right insula with purity: the right anterior insula was significantly correlated with the sum-score of the disgust-only items (p(corr) = 0.01, r = 0.48, t(65) = 4.36).
We did not find any brain region whose gray matter volume significantly correlated with in-group loyalty: the numerically highest correlation was found in the left anterior insula but this did not survive correction (p(corr) = 0.20, r = 0.40, t(65) = 3.49).
Individualizing: VBM Correlates
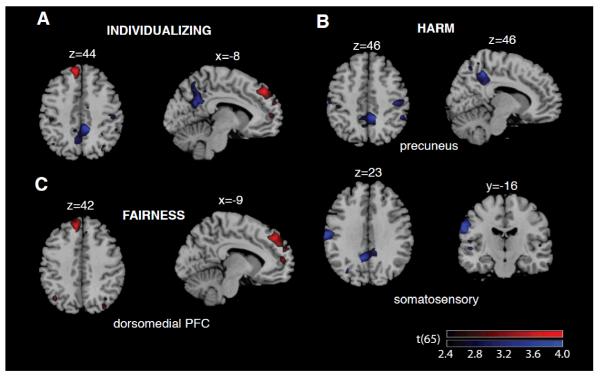
Individualizing was positively correlated with gray matter volume of the left dorsomedial prefrontal cortex (DMPFC; p(corr) = 0.04, r = 0.47, t(65) = 4.33; see Table 1 and Figure 2) and showed a marginal negative correlation with precuneus (p(corr) = 0.077, r = −0.47, t(65) = 4.32).
Figure 2.
Regions where gray matter volume was associated with Individualizing (A), Harm/care (B) and Fairness (C) values are overlaid on a T1-weighted standard MNI template (Collins et al., 1998); x, y, and z values denote the position of sagittal, coronal, and transverse slices, respectively; red and blue, respectively, indicate regions where volumes exhibited positive and negative correlations with their respective moral value trait in the VBM analysis (see the main text and Table 1). The overlays are thresholded at p <0.01 (uncorrected for display purposes). The color bars illustrate the corresponding t values.
Individualizing Subscales: VBM Correlates
Harm/care showed a significant negative correlation with the gray matter volume of the precuneus (p(corr) = 0.03, r = −0.47, t(65) = 4.30) and left postcentral gyrus (somatosensory cortex; p(corr) = 0.04, r = −0.52, t(65) = 4.86; see Figure 2). Harm/care showed the same positive effect for DMPFC volume as for the overall individualizing domain, but this did not reach corrected levels of significant (p(corr) = 0.30, r = 0.43, t(65) = 3.87). Fairness also showed a trend of positive correlation with the DMPFC (p(corr) = 0.058, r = 0.44, t(65) = 3.91; see Figure 2).
Political Orientation and Individualizing/Binding
Both individualizing and binding are associated with political orientation (Graham et al 2009; Lewis & Bates, 2011). To examine their relationship in our current dataset, we therefore also generated statistical overlays showing brain regions associated with political orientation reported in a previous study (Kanai et al., 2011: see Supplementary Figure 1) together with regions associated with individualizing and binding. These data are reported in full in the Supplementary Materials. In summary, however, no common brain region was observed linking the moral domains and political orientation.
Discussion
The current study, utilizing a large sample of young, healthy adults, is the first (to our knowledge) to report associations between regional brain volumes and moral values. The findings indicate that scores on individualizing (sensitivity to harm/care and fairness) and binding (reflecting in-group loyalty, deference to authority, and concerns about purity) are associated with specific brain structures, supporting the idea that these regions constitute an evolved substrate assisting the detection and adherence to each of a core set of social patterns. Specifically, here we find evidence for a positive association between individualizing and left dorsomedial prefrontal cortex (DMPFC) volume and a (marginal) negative association with bilateral precuneus volume. We also observed a positive association between binding and bilateral subcallosal gyrus volume as well as a (marginal) positive association with left anterior insula volume.
The specific associations reported here are broadly consistent with previous research on affective and socio-cognitive processing. For example, the insula is typically activated during the processing of disgust stimuli (Wright et al., 2004), and lesions to this area create deficits in the detection and experience of disgust (Calder et al., 2000). Positive association of anterior insula volume to purity may suggest, then, that this disgust substrate also influences responses in the moral domain of binding; however, it should be borne in mind that the insula is associated with multiple psychological processes (cf. Craig, 2009; Menon & Uddin, 2010) that may also account for this pattern of association. Similarly, the finding that DMPFC and precuneus volumes were significantly associated with the moral values of harm/care and fairness suggests that the functions of mentalizing/empathy processing previously associated with these regions (Gallagher et al., 2000; Green et al., 2001; Saxe & Kanwisher, 2003) may be linked to differences in the moral domain of concern for the individual, although further work establishing direction of causality is required.
The (negative) association between left postcentral gyrus (somatosensory cortex) volume and the sub-scale harm/care are in keeping with recent work indicating a common-coding somatosensory system for self- and other-representation (Bufalari, Aprile, Avenanti, Di Russo, & Aglioti, 2007; Lamm, Fischer, & Decety, 2007), which may facilitate empathy for other conspecifics. In contrast, the association of subcallosal gyrus volume with binding does not obviously reflect findings from previous work in the social and moral neuroscience literature and thus represents a novel behavioral correlate for this brain region.
While the anatomical correlates outlined above provide insights into the biological bases of human sensitivity to important social and moral patterns, they also highlight additional research questions. Firstly, it is unclear, at a mechanistic level, why individual differences in brain volume should correlate with psychological traits in general (cf. Kanai & Rees, 2011), and, now, in moral traits more specifically. Moreover, we observed a positive association between DMPFC volume and individualizing, but a negative association between precuneus volume and individualizing. With regards to gray matter correlates in general, it is possible that greater volume reflects enhanced computational efficiency, perhaps as a function of greater neuronal density (Kanai & Rees, 2011). However, while this interpretation can account for positive associations it is less clear how to account for negative associations, such as that observed here with individualizing and precuneus volume: One possibility is that reduced volume reflects efficient cortical pruning (i.e. the removal of redundant synapses and neurons), which in turn can also generate increased computational efficiency. Determining the precise mechanisms underlying the correlation between brain structure and moral concerns reported here, though, remains beyond the scope of these data, although such work will be valuable and thus is recommended.
Secondly, with the current design we are not able to determine the direction of causality underlying the observed relationships between brain structure and moral values. Future work utilizing genetically informative designs, such as the classical twin study or discordant twin design (Martin, Boomsma, & Machin, 1997), may help to resolve this issue, as has been demonstrated in studies of brain volume and general intelligence (Toga & Thompson, 2005). In addition, longitudinal studies of brain development and change will also be valuable to further tease apart the direction of causal influences underlying such associations (Hedman, van Haren, Schnack, Kahn, & Hulshoff Pol, 2011). Thirdly, while prior work has robustly linked the moral domains of binding and individualizing to political orientation (e.g. Graham et al., 2009; Lewis & Bates, 2011), the current study identified a distinct pattern of gray matter correlates for these moral domains than those recently identified for political orientation (i.e. the amygdala and anterior cingulate cortex; Kanai et al., 2011: also see Supplementary Materials). This disparity may reflect a lack of statistical power to detect modest common effects, although associations between political orientation and both individualizing and binding are typically moderate (i.e. some way from unity; e.g. Lewis & Bates, 2011) and so the current results may reflect distinct neural correlates for moral values from those underlying political orientation. Further work in larger samples, along with more targeted hypotheses (in line with findings reported here), is recommended.
Some relevant brain regions, such as the anterior cingulate cortex (with prior links to fairness; e.g. Singer et al., 2006), did not show significant associations to any of the traits examined in this study. Limited power (particularly as we performed near whole-brain corrected analyses) to detect weak associations may be one explanation for this null finding. In addition, we examined only young adults: As regional brain volumes change throughout life (Gogtay et al., 2004; Good et al., 2001) it is not possible to determine whether the associations detected here generalize to other age or socio-demographic groups. Finally, it is also possible that for some regions, functional activation is not reflected in structural differences (or vice-versa), at least with regards to macroscopic volume. Understanding which functions are linked to individual differences in gray matter volume and which are not, in a systematic and principled way, would thus be of great value.
In summary, we examined whether individual differences in regional brain volume were predictive of variation in moral values. Several brain regions were significantly associated with moral values, including the dorsomedial prefrontal cortex and precuneus to values concerning harm reduction and fairness, and insula cortex and subcallosal gyrus to values concerning purity and authority deference. These findings demonstrate that variation in moral sentiment corresponds to individual differences in brain structure and suggest that moral values possess deep-rooted biological bases distributed across distinct brain regions.
Supplementary Material
Acknowledgements
This work was funded by the Wellcome Trust (GR).
References
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social cognitive theory of moral thought and action. In: Kurtines WM, Gewirtz JL, editors. Handbook of moral behavior and development: Theory, research, and applications. Vol. 1. Erlbaum; Hillsdale NJ: 1991. pp. 45–103. [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30:2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, et al. Design and construction of a realistic digital brain phantom. IEEE Transactions on Medical Imaging. 1998;17:463–46. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cushman F, Young L, Hauser M. The role of conscious reasoning and intuitions in moral judgment: Testing three principles of harm. Psychological Science. 2006;17:1082–1089. doi: 10.1111/j.1467-9280.2006.01834.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010;13:886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gilligan C. In a different voice: Psychological theory and women’s development. Harvard University Press; Cambridge, MA: 1982. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Laituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometrix study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Graham J, Haidt J, Nosek BA. Liberals and conservatives rely on different sets of moral foundations. Journal of Personality and Social Psychology. 2009;96:1029–1046. doi: 10.1037/a0015141. [DOI] [PubMed] [Google Scholar]
- Graham J, Nosek BA, Haidt J, Iyer R, Koleva S, Ditto PH. Mapping the moral domain. Journal of Personality and Social Psychology. 2011;101:366–85. doi: 10.1037/a0021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Haidt J, Koller S, Dias M. Affect, culture, and morality, or is it wrong to eat your dog? Journal of Personality and Social Psychology. 1993;65:613–628. doi: 10.1037//0022-3514.65.4.613. [DOI] [PubMed] [Google Scholar]
- Haidt J. The new synthesis in moral psychology. Science. 2007;316:998–1002. doi: 10.1126/science.1137651. [DOI] [PubMed] [Google Scholar]
- Haidt J, Graham J. Planet of the Durkheimians, where community, authority, and sacredness are foundations of morality. In: Jost J, Kay AC, Thorisdottir H, editors. Social and Psychological Bases of Ideology and System Justification. Oxford University Press; New York: 2009. pp. 371–401. [Google Scholar]
- Haidt J, Kesebir S. Morality. In: Fiske S, Gilbert D, editors. Handbook of Social Psychology. 5th Edition 2010. [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hedman AM, van Haren NEM, Schnack HD, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: A review of 56 longitudinal magnetic resonance imaging studies. Human Brain Mapping. 2011 doi: 10.1002/hbm.21334. Article published online ahead of print. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kanai R, Feilden T, Firth C, Rees G. Political orientations are correlated with brain structure in young adults. Current Biology. 2011;21:677–680. doi: 10.1016/j.cub.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:865–866. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlberg L. Stage and sequence: The cognitive-developmental approach to socialization. In: Goslin DA, editor. Handbook of socialization theory and research. Rand McNally; Chicago: 1969. pp. 347–480. [Google Scholar]
- Lamm C, Fischer M, Decety J. Predicting the actions of others taps into one’s own somatosensory representations - An fMRI study. Neuropsychologia. 2007;45:2480–2491. doi: 10.1016/j.neuropsychologia.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Lewis GJ, Bates TC. From left to right: how the personality system allows basic traits to influence politics via characteristic moral adaptations. British Journal of Psychology. 2011;102:546–558. doi: 10.1111/j.2044-8295.2011.02016.x. [DOI] [PubMed] [Google Scholar]
- Martin N, Boomsma DI, Machin G. A twin-pronged attack on complex traits. Nature Genetics. 1997;17:387–391. doi: 10.1038/ng1297-387. [DOI] [PubMed] [Google Scholar]
- Haruno M, Frith CD. Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nature Neuroscience. 2010;13:160–161. doi: 10.1038/nn.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94:32–41. [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another’s face. Cerebral Cortex. 2007;17:230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Shweder RA, Mahapatra M, Miller JG. Culture and moral development. In: Kagan J, Lamb S, editors. The emergence of morality in young children. The University of Chicago Press; Chicago: 1987. [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annual Review of Neuroscience. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15:2347–2351. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.