Abstract
Dissecting the role of insulin in the complex regulation of triglyceride metabolism is necessary for understanding dyslipidemia and steatosis. Liver Insulin Receptor Knockout (LIRKO) mice show that in the physiological context of feeding, hepatic insulin signaling is not required for the induction of mTORC1, an upstream activator of the lipogenic regulator, SREBP-1c. Feeding induces SREBP-1c mRNA in LIRKO livers, though not to the extent observed in controls. A high fructose diet also partially induces SREBP-1c and lipogenic gene expression in LIRKO livers. Insulin signaling becomes more important in the pathological context of obesity, as knockdown of the insulin receptor in ob/ob mice, a model of Type 2 diabetes, using anti-sense oligonucleotides, abolishes the induction of SREBP-1c and its targets by obesity and ameliorates steatosis. Thus, insulin-independent signaling pathways can partially compensate for insulin in the induction of SREBP-1c by feeding but the further induction by obesity/Type 2 diabetes is entirely dependent upon insulin.
Introduction
In normal physiology, feeding triggers the release of insulin which suppresses glucose production and activates lipogenesis in the liver. In obesity and Type 2 diabetes, insulin fails to suppress glucose production, and hence, these conditions are considered states of insulin resistance. However, lipogenesis is paradoxically increased. Excessive hepatic lipogenesis promotes the development of dyslipidemia and atherosclerosis, the leading cause of death in diabetic patients, and the development of non-alcoholic fatty liver disease (NAFLD). NAFLD affects more than 40% of diabetic patients (Williamson et al., 2011), but effective therapies for it have yet to be found (Cusi, 2009). It has even been argued that excessive hepatic lipogenesis, by promoting ectopic lipid deposition in the muscle, could compromise glucose uptake and produce hyperglycemia itself (McGarry, 1992). Despite the prevalence of obesity and Type 2 diabetes, and the significant morbidity and mortality associated with these disorders, we have yet to define the factors that drive hepatic lipogenesis in the insulin resistant state.
At the molecular level, the increase in lipogenesis observed in insulin resistant states is due at least in part to dysregulation of the master transcriptional regulator of lipogenesis, sterol regulatory element binding protein (SREBP)-1c. SREBP-1c is capable of inducing the entire complement of genes necessary for the synthesis of monounsaturated fatty acids (Horton et al., 1998). Insulin activates SREBP-1c by at least two mechanisms: it increases SREBP-1c transcription (Foretz et al., 1999), and it increases the processing of SREBP-1c from an inactive membrane bound precursor to a soluble fragment capable of translocating to the nucleus to activate transcription(Yabe et al., 2003). Thus, SREBP-1c is decreased in insulin deficient states, such as fasting (Horton et al., 1998) and streptozotocin-diabetes (Shimomura et al., 1999), but increased in mouse models of insulin resistance, such as mice fed a high fat diet (Biddinger et al., 2005), ob/ob mice (Shimomura et al., 2000), and lipodystrophic mice (Shimomura et al., 2000).
There are two possible explanations for the increase in lipogenesis observed in the insulin resistant state. The first possibility is that insulin, despite its inability to control glucose homeostasis, retains its ability to stimulate lipogenesis (Reaven, 1988). This is conceivable because of the complex nature of insulin signaling (Biddinger and Kahn, 2006). Upon binding to its receptor, insulin triggers a branching cascade of signaling events to exert its myriad effects upon the cell. As suggested by Brown and Goldstein, the signaling pathways utilized by insulin to stimulate SREBP-1c could remain intact in obesity and Type 2 diabetes, even as the pathways that regulate glucose metabolism become resistant (Brown and Goldstein, 2008). Thus, insulin would fail to suppress glucose production, leading to hyperglycemia and a compensatory hyperinsulinemia. The hyperinsulinemia, acting through signaling pathways that remain sensitive to insulin, would drive SREBP-1c and lipogenesis to excess.
Alternatively, it is possible that in insulin resistant states, lipogenesis is driven by insulin independent pathways. Particular attention has recently focused on dietary carbohydrates. The excessive consumption of carbohydrates, particularly in the form of sweetened beverages, has risen in parallel with the prevalence of obesity, diabetes, and fatty liver disease in our society. Dietary carbohydrates, particularly fructose, drive lipogenesis (Mayes, 1993) and excessive fructose intake is correlated with elevations in serum (Mayes, 1993) and hepatic (Ouyang et al., 2008) triglyceride levels, as well as obesity and other features of the metabolic syndrome (Johnson et al., 2007). Whether carbohydrates increase lipogenesis directly, or indirectly, by stimulating insulin secretion, has been difficult to dissect. However, the fact that carbohydrate loads in humans can induce hypertriglyceridemia even in the presence of somatostatin, which inhibits insulin secretion, indicates that at least some of the carbohydrate effect may be independent of insulin signaling (Ginsberg et al., 1982). Consistent with this, SREBP-1c can be induced by carbohydrate feeding even in streptozotocin-treated mice, which are insulin deficient (Matsuzaka et al., 2004). Moreover, SREBP-1c is not the only transcriptional regulator of lipogenesis. For example, Carbohydrate Response Element Binding Protein (ChREBP), induces lipogenic gene expression primarily in response to glucose, and could potentially contribute to the insulin independent regulation of the lipogenic genes (Denechaud et al., 2008).
Using mice with knockdown or knockout of the insulin receptor, we have dissected the roles of insulin dependent versus insulin independent pathways in the control of SREBP-1c and hepatic triglycerides under different physiological and pathological conditions. The livers of Liver Insulin Receptor Knockout (LIRKO) mice are entirely unable to respond to insulin, due to a lack of the insulin receptor, but are nonetheless capable of responding to nutrients or other stimuli. We have previously shown that LIRKO mice have decreased levels of SREBP-1c and its targets, decreased plasma triglycerides, and decreased VLDL-triglyceride content (Biddinger et al., 2008). The fact that humans with insulin receptor mutations show a similar phenotype, with decreased lipogenesis, decreased serum triglycerides and decreased VLDL-triglyceride content, demonstrates the utility of the LIRKO model for studying the effects of insulin in vivo (Semple et al., 2009). In the present studies, we show that, surprisingly, insulin signaling is not required for the control of lipogenic gene expression and hepatic triglycerides under the normal physiological stimuli of fasting and feeding. Moreover, lipogenic gene expression is induced by a fructose diet even in the absence of hepatic insulin signaling. However, under the pathological conditions of Type 2 diabetes, modeled by ob/ob mice, insulin signaling is required for the induction of SREBP-1c and the lipogenic genes and contributes to steatosis.
Results
Regulation of the lipogenic program is largely preserved in LIRKO mice under fasted and ad libitum fed conditions
The lipogenic program is under complex control by multiple factors, including insulin, other hormones, and nutrients themselves. In normal physiology, these factors suppress lipogenesis in the fasted state and activate it in the fed state. To dissect the specific role of insulin in the control of the lipogenic program, we used LIRKO mice. LIRKO mice and their littermate controls were maintained on a standard chow diet and studied after a 24 hour fast, or in the ad libitum fed state.
Relative to their controls, LIRKO mice consumed similar amounts of food and were of normal weight (Supplemental Table 1). However, consistent with the lack of hepatic insulin signaling, LIRKO mice were hyperglycemic, despite marked hyperinsulinemia, and were unable to suppress the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (Pck1) with feeding (Table S1). On the other hand, the ability of feeding to increase plasma glucose and thyroid hormone levels, and suppress plasma glucagon levels was normal in LIRKO mice, though the suppression of corticosterone was blunted (Table S1).
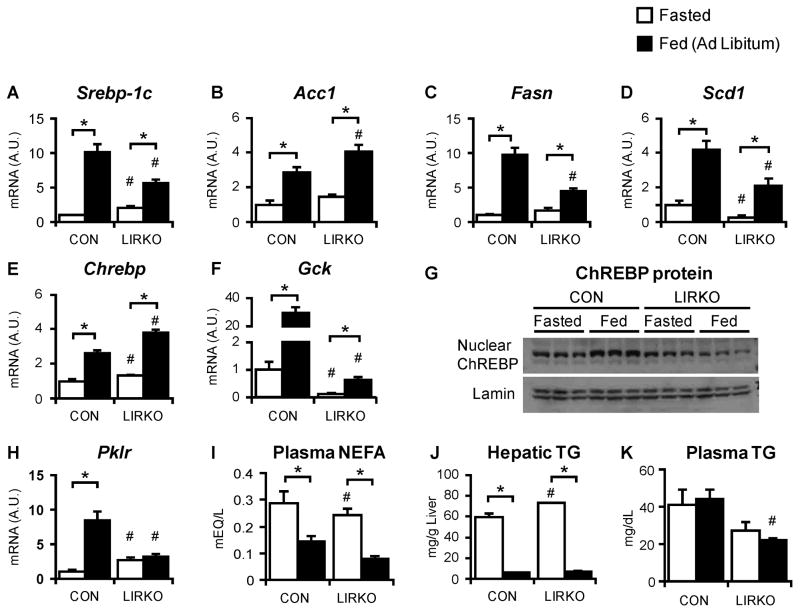
In control livers, feeding increased SREBP-1c mRNA levels by ten-fold (Fig. 1A). In LIRKO livers, SREBP-1c mRNA levels were increased three-fold with feeding, despite their complete absence of hepatic insulin signaling. The lipogenic genes, acetyl CoA carboxylase (Acc), fatty acid synthase (Fasn) and stearoyl CoA desaturase 1 (Scd1), showed a similar expression pattern with Acc, Fasn and Scd1 increased two- to ten-fold in both control and LIRKO mice (Fig. 1B–D). These data indicate that though insulin signaling is necessary for maximal induction, nutrients -either directly, or indirectly, via hormones other than insulin - are sufficient for the induction of the SREBP-1c and lipogenic gene transcripts.
Fig. 1. Regulation of lipogenic gene expression and triglycerides by ad libitum feeding is largely preserved in LIRKO livers.
LIRKO mice and their littermate controls (CON) were maintained on a chow diet and sacrificed after a 24 hour fast or in the ad libitum fed state. (A–F, H) Hepatic gene expression was measured using real time PCR. (G) ChREBP and lamin were measured by immunoblotting nuclear extracts (each lane represents a sample taken from an individual mouse liver). (I) Plasma non-esterified fatty acids, (J) hepatic triglycerides and (K) plasma triglycerides were measured at the time of sacrifice. Error bars represent S.E.M.; n=4–8; *p<0.05 versus non-fasted mice of the same genotype; #p<0.05 versus control mice in the same feeding state. See also Figure S1 and Table S1.
ChREBP is another lipogenic transcription factor; it is activated by glucose (Postic et al., 2004). ChREBP mRNA, in parallel with blood glucose levels (Table S1), was induced to a similar level in control and LIRKO mice by feeding (Fig. 1E), showing that the transcriptional regulation of ChREBP is independent of insulin. However, the activation of ChREBP requires its translocation to the nucleus, which in turn requires the metabolism of glucose to glucose-6-phosphate by glucokinase, an enzyme induced by insulin (Dentin et al., 2004). Thus, in control livers, feeding increased glucokinase expression by 50-fold, and this was associated with an increase in ChREBP protein in the nucleus (Fig. 1F, 1G). In contrast, feeding induced glucokinase only three-fold in LIRKO mice. Thus, glucokinase mRNA levels in the fed state were fifty-fold lower than controls, and LIRKO livers showed no increase in nuclear ChREBP. Consequently, pyruvate kinase (Pklr), a relatively specific target of ChREBP, was not induced by feeding in LIRKO livers, though it was induced almost ten-fold in control livers (Fig. 1H).
In the fasted state, non-esterified fatty acids are released from the adipocyte, taken up by the liver, and oxidized to generate ketones and fuel gluconeogenesis. Plasma non-esterified fatty acids were similar in control and LIRKO mice in the fasted state (Fig. 1I), and the uptake of free fatty acids was uncompromised in LIRKO hepatocytes (Fig. S1A). Direct measurements of fatty acid oxidation in LIRKO hepatocytes in vitro were normal (Fig. S1B, C), and the respiratory exchange ratio, which reflects whole body fatty acid oxidation, was normal (data not shown). Consistent with this hepatic triglycerides were normal in fasted LIRKO livers (Fig. 1J).
In the fed state, insulin and other hormones act at the level of the adipocyte to suppress lipolysis, and thereby decrease the flux of non-esterified fatty acids to the liver. Because insulin receptor expression is normal in the extrahepatic tissues of the LIRKO mouse (Fig S2C), the regulation of adipocyte lipolysis would be expected to be normal. Indeed, in response to feeding, LIRKO mice, like control mice, showed a three-fold reduction in plasma non-esterified fatty acids and a ten-fold reduction in hepatic triglycerides. Plasma triglycerides were lower in LIRKO mice than controls, consistent with the decreased triglyceride secretion reported previously (Biddinger et al., 2008), but did not change with fasting (Fig. 1K).
Insulin-independent activation of mTORC1 targets
The mTORC1 complex is an important regulator of SREBP-1c (Li et al., 2010; Porstmann et al., 2005; Jeon and Osborne, 2012). Insulin stimulates mTORC1 via a signaling cascade involving Akt. Upon activation, Akt becomes phosphorylated on residues Thr308 and Ser473 and in turn, activates mTORC1(Biddinger and Kahn, 2006).
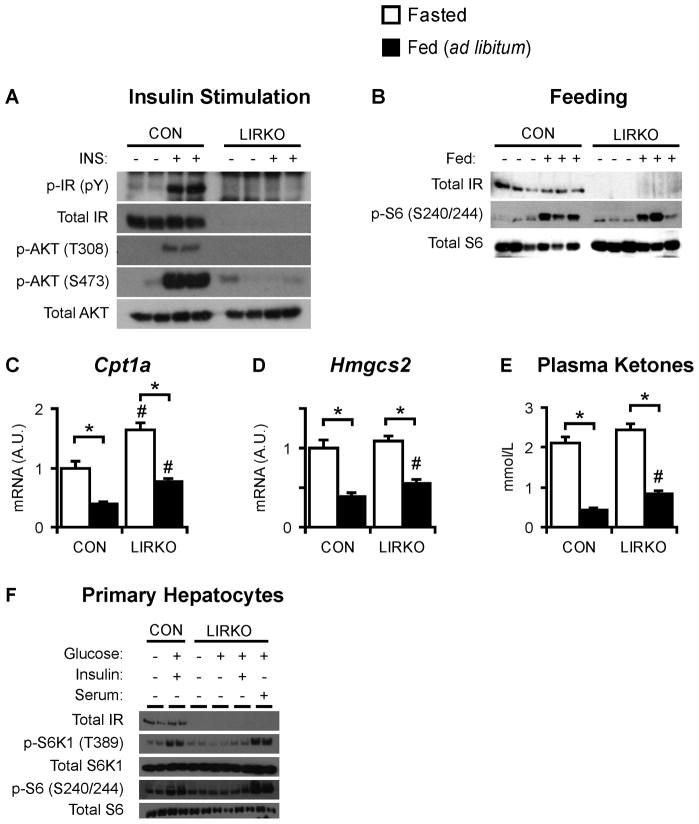
In fasted control livers, five minutes of insulin stimulation induced tyrosine phosphorylation of a 100 kDa protein, assumed to be the insulin receptor, and phosphorylation of Akt on Thr308 and Ser473 (Fig. 2A). In contrast, in LIRKO hepatocytes, insulin was unable to activate Akt either in vivo or in vitro (Fig. 2A, S2A). However, insulin signaling in LIRKO muscle and fat was not impaired (Fig. S2B, S2C).
Fig. 2. Insulin-independent activation of mTORC1.
(A) Control and LIRKO mice were fasted overnight, injected with either vehicle or 3 units of insulin via the inferior vena cava, and sacrificed five minutes later. Livers were subjected to immunoblotting. (B) Mice were fasted for 24 hours, or fasted for 24 hours and re-fed a high carbohydrate diet for one hour. Livers were subjected to immunoblotting. (C–E) Mice were sacrificed in the ad libitum fed state or after a 24 hour fast. (C,D) Hepatic gene expression was measured by real time PCR. (E) Ketone levels were measured in the blood at the time of sacrifice. (F) Hepatocytes were isolated from two month old female control and LIRKO mice. After plating, hepatocytes were incubated overnight in serum free medium containing low glucose (5 mM), and then stimulated for ten minutes with high glucose (25 mM), insulin (10 nM) and/or serum (10%). Error bars represent S.E.M.; n=4–8; *p<0.05 versus non-fasted mice of the same genotype; #p<0.05 versus control mice in the same feeding state. See also Figure S2.
Feeding activates mTORC1 in vivo (Sengupta et al., 2010). Phosphorylation of the ribosomal S6 protein, a well established marker of mTORC1 activation, was very low in the livers of fasted mice of either genotype (Fig. 2B). Consistent with prior reports, one hour of feeding robustly induced phospho-S6 levels in control livers (Sengupta et al., 2010). Surprisingly, feeding was also able to induce phospho-S6 in LIRKO livers, despite their inability to respond to insulin. This induction was abolished by the mTORC1 inhibitor, rapamycin (data not shown).
In addition to stimulating lipogenesis, mTORC1 is required for the inhibition of ketogenesis upon feeding (Sengupta et al., 2010). Upon feeding, mTORC1 is activated, Cpt1a and Hmgcs2, two genes required for ketone synthesis, are suppressed, and blood ketone levels fall. Thus, in the livers of ad libitum fed LIRKO mice, Cpt1a and Hmgcs2 mRNA levels and blood ketone levels are decreased two- to three-fold relative to the livers of fasted mice (Fig. 2C–E). Taken together, these data suggest that mTORC1 activation in response to feeding is intact in LIRKO livers.
Previous studies have shown that nutrients alone are not sufficient for the activation of mTORC1, and that growth factors must also be present (Sancak et al., 2008). The insulin and IGF-1 receptors activate an overlapping, if not identical, set of downstream targets. Thus, it was conceivable that IGF-1 receptor could compensate for the lack of insulin receptor in LIRKO hepatocytes. To test this, we measured IGF-1 receptor expression in LIRKO livers. IGF-1 receptor was undetectable in control livers, as previously reported (Czech, 1982), as well as LIRKO livers (Fig. S2D). Moreover, hepatocytes cultured from LIRKO livers failed to respond to IGF-1 (Fig. S2A).
Nonetheless, it remained possible that growth factors other than insulin/IGF-1 could play a permissive role in the activation of mTORC1 by nutrients in LIRKO hepatocytes. To test this, primary hepatocytes were isolated from control and LIRKO livers, and cultured overnight in media containing low glucose and no serum. Subsequent stimulation of control hepatocytes with high glucose and insulin produced phosphorylation of the mTORC1 target S6 kinase (S6K1), and its downstream target ribosomal S6 (Fig. 2F). LIRKO hepatocytes failed to respond to high glucose and insulin. However, stimulation of LIRKO hepatocytes with high glucose and serum, which contains numerous growth factors in addition to insulin, induced S6 kinase to the same level as control hepatocytes treated with high glucose and insulin. Thus, factors in the serum other than insulin can promote the activation of mTORC1.
Acute re-feeding fails to induce nuclear SREBP-1c protein in LIRKO livers
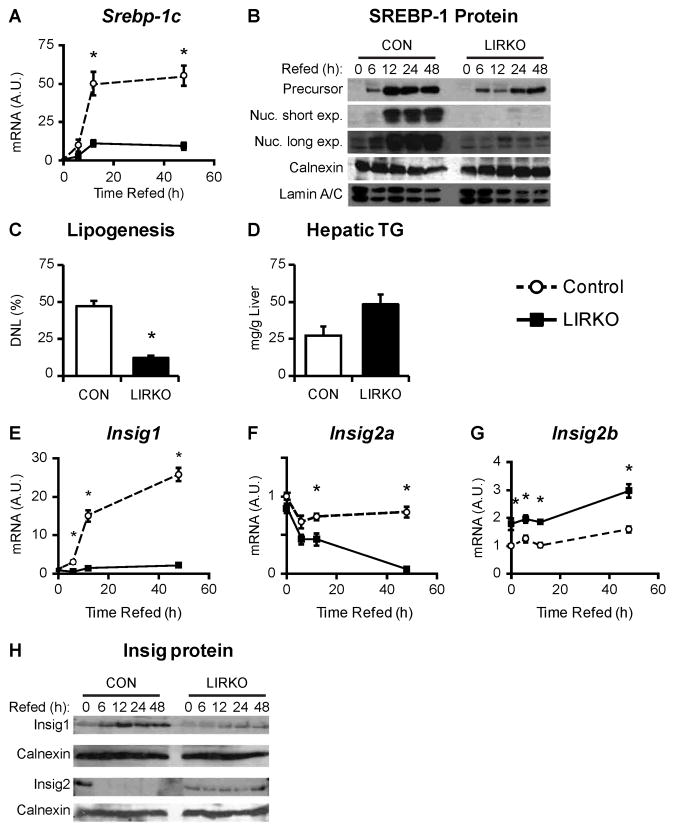
Re-feeding a high carbohydrate diet after fasting is a strong lipogenic challenge that is thought to be primarily driven by insulin and SREBP-1c (Horton et al., 1998). Thus, in contrast to the ad libitum fed state, the re-fed state is characterized by an exaggerated insulin response which induces SREBP-1c and lipogenesis to levels five-fold greater than those observed in the ad libitum fed state (Horton et al., 1998). To examine the effects of insulin and nutrients on SREBP-1c under the stress of re-feeding, we measured SREBP-1c expression in the livers of control and LIRKO mice fasted for 24 hours, and re-fed a high-carbohydrate diet for 6–48 hours. In control mice, SREBP-1c mRNA levels increased within six hours of re-feeding and reached a plateau by 12 hours (Fig. 3A). In LIRKO livers, the same temporal pattern was observed, but SREBP-1c mRNA levels were induced to levels only 20% of controls.
Fig. 3. LIRKO livers fail to accumulate nuclear SREBP-1c upon acute re-feeding of a high carbohydrate diet.
LIRKO mice and their littermate controls (CON) were fasted for 24 hours and then re-fed a high carbohydrate diet for 6–48 hours. Livers were used to prepare cDNA for real-time PCR analysis as well as microsomal and nuclear extracts. (A) SREBP-1c mRNA was measured using real-time PCR. (B) SREBP-1 precursor and nuclear protein levels were measured by immunoblotting microsomal and nuclear extracts, respectively. Calnexin and lamin are shown as loading controls. Each lane represents extracts prepared from equal aliquots of liver from three mice. (C) De novo lipogenesis was measured as the fraction of newly synthesized palmitate present 24 hours after re-feeding. (D) Hepatic triglycerides were measured after six hours of re-feeding. (E–G) Insig mRNA was measured using real time PCR. (H) Insig1 and Insig2 proteins were measured by immunoblotting microsomal extracts. Each lane represents extracts prepared from equal aliquots of liver from three mice. Error bars represent S.E.M.; n=4–8; *p<0.05 versus control mice. See also Figure S3.
SREBP-1c precursor and nuclear protein levels rose in parallel with mRNA levels in control mice, increasing after six hours of re-feeding and reaching a plateau by twelve hours (Fig. 3B). Precursor levels of SREBP-1 also increased in LIRKO livers in parallel with mRNA levels. Despite the increase in precursor SREBP-1, however, nuclear protein levels remained undetectable, even after 48 hours. Consistent with this, lipogenesis was markedly lower in re-fed LIRKO versus control mice (Fig. 3C). Interestingly, however, hepatic triglyceride levels after six hours of re-feeding tended to be increased (Fig. 3D), suggesting a lag in the removal of triglycerides from the LIRKO liver, perhaps due to a decrease in triglyceride secretion (Biddinger et al., 2008b).
The failure of LIRKO livers to accumulate the nuclear, i.e., active, form of SREBP1 upon re-feeding suggests that insulin is necessary for the processing of SREBP-1c. SREBP proteins are synthesized as inactive, membrane bound precursor proteins that become associated with SCAP, the SREBP cleavage-activating protein (reviewed in (Jeon and Osborne, 2012)). Insig proteins reside in the endoplasmic reticulum and bind to SCAP; in so doing, they retain the SCAP/SREBP complex in the endoplasmic reticulum, and prevent SREBP processing. When SCAP dissociates from the Insig proteins, the SCAP/SREBP complex can translocate to the Golgi. Two proteins which reside in the Golgi, Site 1 and Site 2 protease, can then cleave the SREBP precursor, releasing a soluble fragment that can translocate to the nucleus to activate transcription. There are two Insig proteins. Insig1 is thought to mediate feedback inhibition of the SREBPs, as Insig1 transcription is driven by the SREBP proteins (Engelking et al., 2004). Insig2 is encoded by two transcripts. Insig2a is the major Insig transcript in the fasted liver. It is induced by fasting and streptozotocin treatment, and suppressed by feeding and insulin treatment (Yabe et al., 2003). Insig2b is a ubiquitous transcript (Yabe et al., 2003).
LIRKO livers showed normal mRNA levels of SCAP, Site 1 and Site 2 protease (Fig. S3A), but marked derangements in Insig expression. In control livers, the increase in nuclear SREBP-1 observed with re-feeding was associated with an increase in Insig1 mRNA and protein (Fig. 3E, 3H). In LIRKO livers, the failure to induce nuclear SREBP-1c was associated with a failure to induce Insig1 mRNA and protein.
Insig2a mRNA decreased with re-feeding in control mice, as expected (Fig. 3F). Despite the lack of insulin signaling, Insig2a mRNA fell to an even greater extent with re-feeding in LIRKO livers. Insig2b mRNA levels were relatively stable with re-feeding in both control and LIRKO livers, though the expression in LIRKO livers was increased (Fig. 3G). Insig2 protein was also higher in LIRKO versus control livers (Fig. 3H).
Using an adenovirus encoding shRNA against Insig2, we knocked down Insig2 in the livers of non-fasted LIRKO mice (Fig. S3B). Compared with LIRKO mice injected with an adenovirus encoding a scrambled shRNA sequence, LIRKO mice injected with shInsig2 adenovirus showed very low levels of Insig2, indicating the virus was effective. Though there was slight trend for the shInsig2 adenovirus to increase nuclear levels of SREBP-1 in LIRKO livers, it was not to the level observed in the livers of their littermate controls. Thus, the failure of LIRKO livers to accumulate nuclear SREBP-1c is due to increased Insig2 protein as well as other factors.
Acute re-feeding fails to induce nuclear SREBP-1c protein in mice with knockdown of the insulin receptor
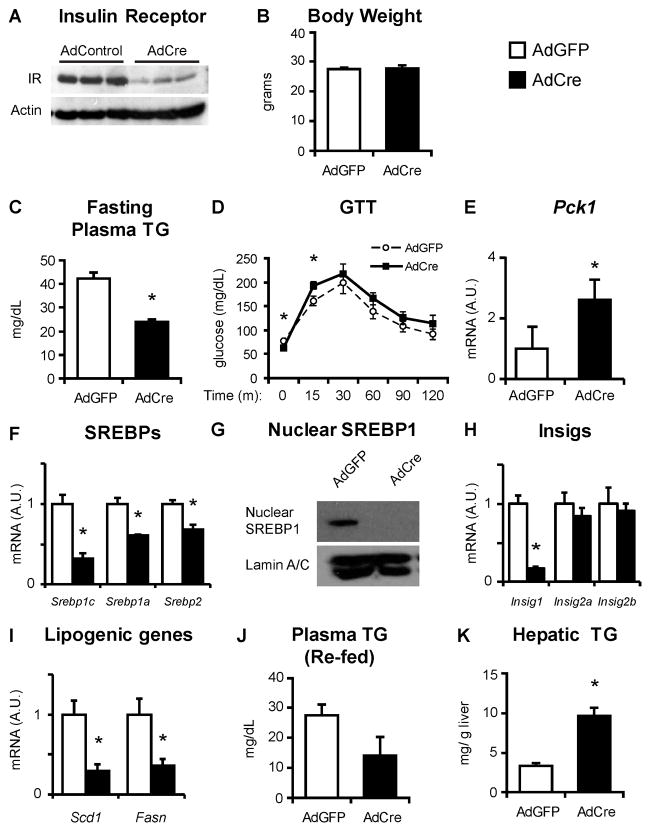
Because LIRKO mice lack hepatic insulin signaling from birth, it is conceivable that some of their phenotype is due to developmental defects or other chronic compensatory changes. Therefore, we knocked down the insulin receptor in 2–3 month old control mice, homozygous for the floxed allele of the insulin receptor, using adenoviral mediated expression of Cre recombinase (AdCre). AdCre mice showed a very similar phenotype to LIRKO mice. Compared to mice infected with adenovirus encoding green fluorescent protein (AdGFP), AdCre treated mice showed a 90% decrease in hepatic insulin receptor protein, normal body weights and a 50% decrease in fasting plasma triglycerides (Fig. 4A–C). AdCre treated mice also showed fasting hypoglycemia, presumably due to limited glycogen reserves, and hyperglycemia after the injection of glucose (Fig. 4D).
Fig. 4. Mice with knockdown of the insulin receptor fail to accumulate nuclear SREBP-1c upon acute re-feeding of a high carbohydrate diet.
Two month old mice homozygous for the floxed allele of the insulin receptor were injected with adenovirus encoding GFP or Cre recombinase, and studied two to three weeks later. Mice were fasted for 24 hours and re-fed a high carbohydrate diet for six hours prior to sacrifice. (A) Liver lysates were subjected to immunoblotting with antibodies against the insulin receptor. (B) Body weight was measured at the time of sacrifice. (C) Triglycerides were measured in plasma taken after a four hour fast. (D) Glucose tolerance testing was performed by fasting mice overnight and then injecting with 1 g/kg glucose, intraperitoneally. (E–F, H–I) RT PCR was used to measure hepatic gene expression. (G) SREBP-1 protein was measured in nuclear extracts (prepared from equal amounts of liver taken from at least three mice per group). (J) Plasma and (K) hepatic triglycerides measured in the re-fed state, at the time of sacrifice. Error bars represent S.E.M.; n=3–5, *p<0.05 versus mice injected with adenovirus encoding GFP. See also Figure S4.
The response of AdCre treated mice to re-feeding was almost identical to that of LIRKO mice. Thus, AdCre and AdGFP treated mice were fasted for 24 hours and re-fed for six hours with a high carbohydrate diet. Relative to AdGFP treated mice, AdCre treated mice showed increased Pck1 mRNA (Fig. 4E) and a 75% decrease in SREBP-1c mRNA. The other SREBP isoforms, SREBP-1a and SREBP-2, were decreased 30–40% in AdCre treated mice (Fig. 4F). Like LIRKO livers, the livers of AdCre treated mice showed markedly reduced nuclear SREBP-1, despite decreased Insig1 mRNA, and a 60–80% reduction in Fasn and Scd1(Fig. 4G–I). Neither AdCre (Fig. 4H) nor LIRKO (Fig. 3F) mice at the six hour re-feeding time point showed significant changes in Insig2a mRNA. Finally, AdCre treated mice in the re-fed state showed a trend towards reduced plasma triglycerides and increased hepatic triglycerides (Fig. 4J, K).
High-fructose feeding increases lipogenic gene expression in LIRKO mice
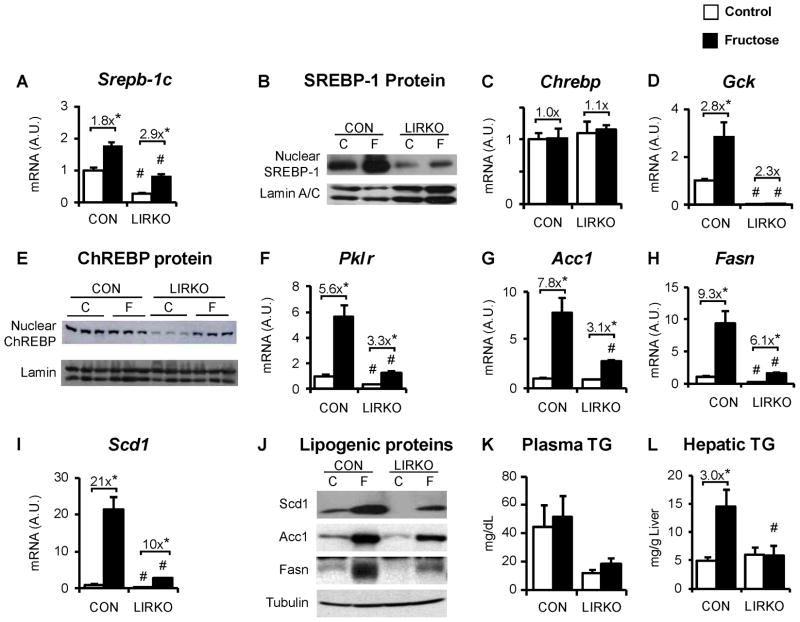
Dietary fructose is a strong stimulus for lipogenesis. Catalytic amounts of fructose activate glucokinase post-transcriptionally by promoting its dissociation from the glucokinase regulatory protein (Doiron et al., 1994; Mayes, 1993; Petersen et al., 2001). Fructose could therefore be particularly important in the insulin-independent induction of lipogenesis. Thus, LIRKO mice and their controls were subjected to a high-fructose (60% fructose) versus standard chow diet for one week. In control mice, the high fructose diet produced a 75% increase in Srebp-1c mRNA and an increase in nuclear SREBP-1c (Fig. 5A,B ). In LIRKO mice, fructose feeding produced a three-fold increase in Srebp-1c mRNA and a modest increase in nuclear SREBP-1c. Importantly, the levels of nuclear SREBP-1 protein in fructose-fed LIRKO livers were markedly lower than those found in even chow fed controls, indicating the dominant role played by insulin in the accumulation of nuclear SREBP-1.
Fig. 5. Effects of fructose feeding on LIRKO livers.
LIRKO mice and their littermate controls (CON) were placed on either a chow (C, Chow) or high fructose diet (F, 60% fructose by weight) for one week and were sacrificed in the non-fasted state. Livers were analyzed for gene expression and triglyceride content. (A, C–D, F–I) Gene expression was measured by real-time PCR. (B) SREBP-1 and (E) ChREBP proteins were measured by immunoblotting nuclear extracts. (J) Acc, Fasn and Scd1 were measured by immunoblotting liver lysates. (K) Plasma and (L) hepatic triglycerides were measured at the time of sacrifice. Error bars represent S.E.M.; n=4–8, *p<0.05 versus chow-fed mice of the same genotype; #p<0.05 versus control mice on the same diet; equal amounts of liver from three mice were pooled to prepare samples for immunoblotting SREBP-1, Acc, Fasn and Scd1; for ChREBP immunoblot, each lane represents an individual mouse liver. See also Figure S5.
In control livers, ChREBP mRNA was not induced by fructose feeding and glucokinase was increased more than two-fold; no consistent change in ChREBP nuclear protein was observed (Fig. 5C–5E). In LIRKO livers, neither ChREBP nor glucokinase mRNA was induced by fructose feeding. Nonetheless, fructose markedly increased the amount of nuclear ChREBP in LIRKO livers. In parallel, pyruvate kinase was increased by three-fold in LIRKO livers upon fructose feeding (Fig. 5F).
The induction of nuclear SREBP-1 in control livers and ChREBP in LIRKO livers was associated with an increase in lipogenic gene expression (Fig 5G–5I). In control mice, Scd1 was increased 20-fold and Acc, Fasn and pyruvate kinase were increased five- to ten-fold with fructose feeding. In LIRKO livers, Scd1, Acc, Fasn and pyruvate kinase increased three- to tenfold with fructose feeding.
In parallel, fructose feeding increased the protein levels of Scd1, Acc and Fasn in both control and LIRKO mice (Fig. 5J). Though lipogenic expression at the mRNA and protein levels were consistently lower in the LIRKO livers on either diet, fructose feeding induced lipogenic gene expression in fructose fed-LIRKO mice to levels similar to or higher than those found in control mice on the chow diet. This indicates the ability of a high fructose diet to drive lipogenic gene expression even in the complete absence of hepatic insulin signaling.
Plasma triglyceride levels were not significantly changed by the high fructose diet in either control or LIRKO mice (Fig. 5K). Hepatic triglycerides were increased three-fold in control mice subjected to the fructose diet (Fig. 5L). In contrast, LIRKO mice showed no change in hepatic triglycerides despite the increase in lipogenic gene expression. These data indicate that insulin is also necessary for some other aspect of triglyceride metabolism required for steatosis.
The acyl-CoA:diacylglycerol acyltransferase (DGAT) enzymes, which are encoded by two genes, catalyze the committed step in triglyceride biosynthesis and are important for the development of steatosis (Yen et al., 2008). Interestingly, LIRKO mice showed decreased levels of DGAT2 mRNA, which could contribute to the failure of LIRKO livers to accumulate hepatic triglycerides on the fructose diet (Fig. S5B). However, DGAT1 mRNA (Fig. S5A) and DGAT activity (Fig. S5C) were normal in LIRKO livers.
IR ASO therapy in ob/ob mice
In Type 2 diabetes, plasma insulin levels rise as the β-cells of the pancreas attempt to maintain glucose homeostasis. This increase in insulin levels could potentially drive SREBP-1c and the lipogenic genes. To test this directly, we used ob/ob mice. In these mice, leptin deficiency leads to hyperphagia, obesity and severe hyperinsulinemia. To block the effects of hyperinsulinemia on the liver, we knocked out the insulin receptor using chemically modified antisense oligonucleotides.
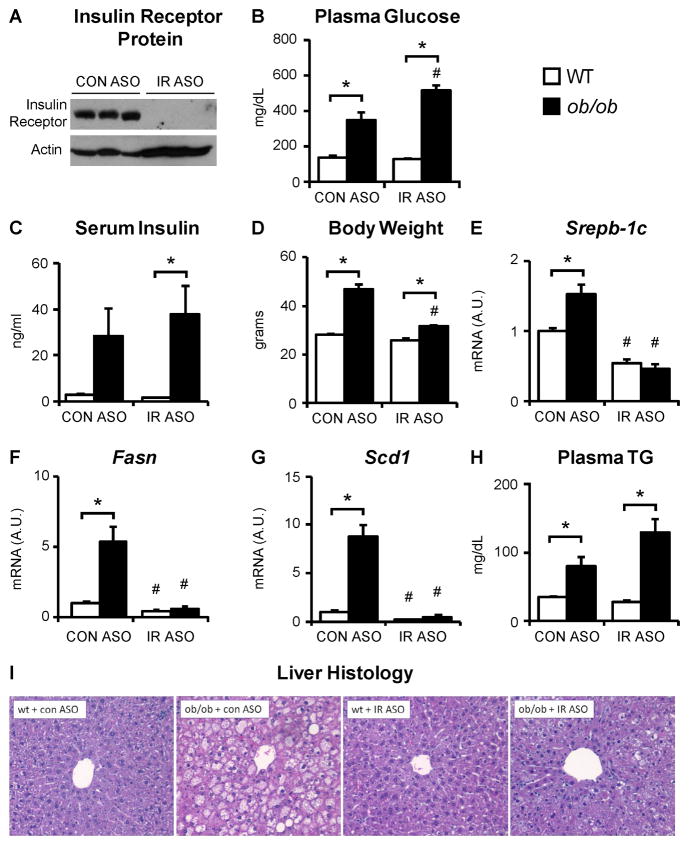
Wildtype and ob/ob mice on the C57Bl/6J background were treated with an antisense oligonucleotide against the insulin receptor (IR ASO) or a control antisense oligonucleotide (CON ASO). Treatment with the IR ASO reduced liver insulin receptor protein to near undetectable levels (Fig. 6A). There was also a decrease in insulin receptor content in the adipose tissue but this did not significantly alter phosphorylation or expression of hormone sensitive lipase, expression of adipocyte triglyceride lipase, plasma non-esterified free fatty acids or plasma adiponectin (Fig. S6A–D).
Fig. 6. The induction of lipogenic gene expression in ob/ob mice requires insulin signaling.
Five to six week old, male ob/ob mice and their lean controls (wt) were treated with chemically modified antisense oligonucleotides (ASO) for four weeks and sacrificed in the non-fasted state. (A) Insulin receptor expression was measured by immunoblotting liver lysates. (B) Blood glucose levels were measured after a four hour fast. (C) Plasma insulin and (D) body weights were measured at the time of sacrifice. (E–G) Livers were analyzed for gene expression using real-time PCR. (H) Plasma triglycerides were measured after a four hour fast. (I) Livers were subjected to hematoxylin and eosin staining. Error bars represent S.E.M.; n=3–6; *p<0.05 versus wt mice treated with the same ASO; #p<0.05 versus mice of the same genotype treated with control ASO. See also Figures S6 and S7.
In mice treated with the CON ASO, leptin deficiency produced hyperglycemia, raising fasting blood glucose levels from 136 mg/dL to 347 mg/dL (Fig. 6B). In mice treated with the IR ASO, the effect was exacerbated, as fasting blood glucose levels increased from 127 to 519 mg/dL, though Pck1 gene expression was not significantly changed (Fig. S6E). Leptin deficiency produced hyperinsulinemia during treatment with either the CON or IR ASO, even though the livers of mice treated with the IR ASO were unable to respond to the hyperinsulinemia (Fig. 6C). Consistent with the worsening of diabetes, ob/ob mice treated with the IR ASO accumulated less weight than ob/ob mice treated with the control ASO (Fig. 6D).
In the presence of the control ASO, leptin deficiency produced a 40% increase in SREBP-1c (Fig. 6E), a five-fold induction in Fasn (Fig. 6F), and an eight-fold induction in Scd1 (Fig. 6G). However, in the presence of the IR ASO, these effects were entirely abolished, and the expression of SREBP-1c, Fasn and Scd1 was similar in wildtype and ob/ob mice. Even the ability of leptin deficiency to induce glucokinase, ChREBP, and pyruvate kinase was abolished by treatment with the IR ASO (Fig. S6G–I). Consequently, in the presence of the CON ASO, leptin deficiency produced severe hepatic steatosis; in the presence of the IR ASO, the steatosis was markedly attenuated (Fig. 6I). The ability of leptin deficiency to increase plasma triglycerides, however, remained intact (Fig. 6H).
Another commonly used mouse model of Type 2 diabetes is diet-induced obesity (DIO). To determine whether the ablation of hepatic insulin signaling had similar effects in DIO mice, control and LIRKO mice were fed a high fat diet for 18 weeks. On the high fat diet, body weight and fat mass were similar in control and LIRKO mice, but LIRKO mice were more glucose intolerant (Fig. S7A–C). LIRKO mice also showed reduced levels of nuclear SREBP-1, lipogenic gene expression and hepatic triglycerides (Fig. S7D–G). Similarly, the injection of AdCre into DIO mice homozygous for the floxed allele of the insulin receptor also worsened glucose tolerance, but lowered nuclear SREBP-1c, lipogenic gene expression, and plasma triglycerides and tended to lower hepatic triglycerides (Fig. S4). Thus, the interruption of hepatic insulin signaling is sufficient to lower SREBP-1c and its targets in both the ob/ob and DIO models of Type 2 diabetes.
Discussion
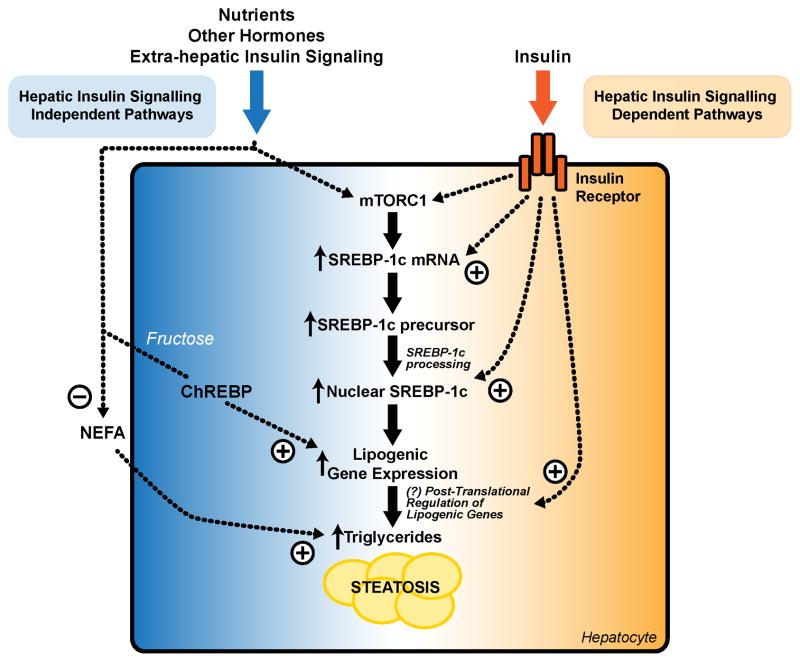
Insulin is one of several factors regulating SREBP-1c and hepatic triglyceride metabolism (Fig. 7). Our data, together with data in mice with liver-specific knockout of both IRS-1 and IRS-2 (Guo et al., 2009), or Akt2 (Leavens et al., 2009), show that feeding is able to activate mTORC1 and induce SREBP-1c mRNA via insulin-independent signaling pathways. The activation of ChREBP and lipogenic gene expression by fructose is also independent of hepatic insulin signaling, as is the regulation of non-esterified fatty acid flux from the adipocyte, an important driver of hepatic triglycerides. Hepatic insulin signaling is, however, required for the accumulation of nuclear SREBP-1c protein. Since SREBP-1c activates its own transcription, completing a feed-forward loop (Chen et al., 2004; Dif et al., 2006), insulin is also necessary for the full induction of SREBP-1c mRNA. These insulin-dependent signaling pathways are critical for the pathological induction of SREBP-1c and lipogenic gene expression observed in obesity.
Fig. 7. Interaction between insulin-dependent and insulin-independent signaling pathways in the control of SREBP-1c and hepatic triglycerides.
There are multiple nodes at which insulin dependent and independent signaling pathways can crosstalk in the regulation of SREBP-1c and hepatic triglycerides. Hepatic insulin signaling independent pathways involve the direct effects of nutrients, other hormones, and other tissues. These pathways are able to activate mTORC1 and increase SREBP-1c mRNA. In the case of fructose, they are also able to activate ChREBP. Moreover, insulin and other hormones, acting on the adipocyte, can regulate the flux of non-esterified fatty acids to the liver. Insulin-dependent signaling pathways are required for the processing of SREBP-1c, maximal lipogenic gene expression, and the induction of steatosis. The relative roles played by the insulin-dependent and insulin-independent signaling pathways vary in different physiological and pathophysiological states.
One important regulator of SREBP-1c is Insig2a. In vitro, insulin increases degradation of the Insig2a transcript (Yellaturu et al., 2009) but it may also increase Insig2a transcription, since activation of Liver X Receptor, a nuclear receptor that is induced by insulin, increases Insig2a mRNA (Hegarty et al., 2005). Our data add further complexity. First, Insig2a expression in vivo can be suppressed independently of hepatic insulin signaling, as Insig2a mRNA levels are decreased in the livers of re-fed LIRKO mice (Fig. 3F), Ad-Cre treated mice on the high-fat diet (Fig. S4F), and mice treated with IR ASO (Fig. S6K). Such in vivo regulation by feeding could be mediated by a factor other than insulin. One intriguing possibility is that in vivo Insig2a is suppressed by leptin, as Insig2a mRNA is increased by leptin deficiency (Kammoun et al., 2009), even after knockdown of the insulin receptor by ASO treatment (Fig. S6K). Second, insulin may regulate Insig2 post-transcriptionally, as Insig2 protein is increased in LIRKO livers despite lower levels of Insig2a mRNA. Moreover, insulin is required for other aspects of SREBP-1c processing, nuclear import or stability, as knockdown of Insig2 does not fully restore nuclear SREBP-1c protein in LIRKO livers (Fig. S3B).
The role of hepatic insulin signaling in the induction of SREBP-1c becomes more important in the setting of obesity/Type 2 diabetes, as modeled by the ob/ob mouse. That is, though feeding induces mRNA levels of SREBP-1c and its targets via insulin-independent signaling pathways, the further induction which occurs in obesity is entirely insulin dependent. Neither leptin deficiency, nor any of the other metabolic or hormonal changes associated with the Type 2 diabetic state is capable of inducing lipogenic gene expression in the absence of hepatic insulin signaling. This is consistent with concept of “selective insulin resistance.” Although the specific signaling pathways that remain sensitive to insulin in obesity remain unclear, continued insulin signaling through phosphatidylinositol 3-kinase (Anai et al., 1999) and its downstream targets mTORC1 (Um et al., 2004; Khamzina et al., 2005), as well as PKC-λ (Standaert et al., 2004), have been reported.
Both mTORC1 dependent and independent signals are necessary for the full activation of SREBP-1c (Yecies et al., 2011; Wan et al., 2011). We therefore propose a model of obesity in which over-nutrition, independently of insulin, drives mTORC1, while insulin signaling through a distinct pathway, such as PKC-λ, permits the accumulation of nuclear SREBP-1c and activation of its feed-forward transcriptional loop. However, our data do not rule out the possibility that insulin, in the setting of hyperinsulinemia and obesity, becomes an important driver of mTORC1, even though it is not required for the induction of mTORC1 by feeding.
Fructose can bypass the requirement for insulin in the regulation of the lipogenic genes, as the fructose diet increases Acc, Fasnand Scd1 mRNA and protein in LIRKO livers to levels equal to or higher than those found in chow-fed controls. This is due, in part, to activation of ChREBP, as nuclear ChREBP levels and expression of the ChREBP target, pyruvate kinase, are increased in the livers of fructose fed LIRKO mice. The activation of ChREBP requires glucokinase, which in turn requires insulin for its transcription (Dentin et al., 2004). However, fructose, which activates glucokinase post-transcriptionally by promoting its translocation from the nucleus to the cytosol, could drive ChREBP independently of insulin (Doiron et al., 1994; Mayes, 1993; Petersen et al., 2001).
In human Type 2 diabetes, hyperinsulinemia, dietary fructose, and other lipogenic stimuli are present. Our data suggest that identifying and targeting the specific signaling pathways by which insulin stimulates lipogenesis, as well as decreasing dietary fructose, could be extremely effective in reducing SREBP-1c and ameliorating hepatic steatosis in Type 2 diabetes.
Experimental Procedures
A detailed description of the Experimental Procedures can be found in the Supplemental Information.
Animals, Diets and Treatments
Generation and genotyping of LIRKO (Cre+/−, IR lox/lox) mice and their littermate controls (Cre−/−, IR lox/lox) has been described previously (Michael et al., 2000). LIRKO mice were maintained on a mixed genetic background. Unless otherwise indicated, the mice used in these experiments were male, eight to ten weeks of age, and sacrificed at 2 p.m. For fasting and re-feeding studies, mice were sacrificed under the following conditions: ad libitum fed; after a 24 hour fast; or after a 24 hour fast followed by re-feeding a high carbohydrate diet (TD. 88122, Harlan Teklad Diets). For adenoviral mediated knockdown of the insulin receptor, two month old mice homozygous for the floxed allele of the insulin receptor were injected via tail vein with 5 × 109 pfu of adenovirus encoding Cre or GFP (Viraquest), and sacrificed 21 days later. For the fructose feeding experiments, mice were fed ad libitum with a 60% fructose diet for one week (TD. 00202, Harlan Teklad Diets). For the antisense oligonucleotide mediated knockdown of the insulin receptor, C57Bl/6J mice and ob/ob mice(Jackson Labs) were given 50 mg/kg IP of the chemically modified ASO each week for five weeks and sacrificed one day after the final dose. All animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at Children’s Hospital Boston.
Gene Expression Analysis
Gene expression was measured using real-time PCR. Results were normalized to the house keeping gene, Tbp, and the value of the control group was set to 1.
Western Blotting
Microsomal and nuclear protein extracts for measurements of SREBP-1 (Horton et al., 1998), microsomal extracts for the measurements of the Insig proteins (Engelking et al., 2004), and nuclear extracts for the measurements of ChREBP (Miao et al., 2009) were prepared as previously described, and subjected to western blotting.
Phenotypic and Histological Characterization
Blood glucose and ketone levels were measured using a glucometer and ketone meter. Plasma insulin (ALPCO) was measured using a commercial kit. Plasma measurements of non-esterified fatty acids (Wako Chemicals) and total triglycerides (ThermoScientific) were made using colorimetric assays. Hepatic triglycerides were measured as previously described (Biddinger et al., 2008). Hematoxylin and eosin staining of the liver was performed by the Dana-Farber/Harvard Cancer Center Rodent Histopathology Core.
De novo Lipogenesis
Mice were fasted for 24 hours, re-fed a high carbohydrate diet for 24 hours, and then sacrificed. One hour prior to re-feeding, mice were injected IP with 24 μl/g body weight of deuterated normal saline, and their drinking water was replaced with 4% D2O. The fraction of newly synthesized palmitate was measured using gas chromatography-electron impact ionization mass spectrometry as previously described (Leavens et al., 2009).
Primary Hepatocytes
Primary hepatocytes were isolated from eight to ten week old female control and LIRKO mice as previously described (Biddinger et al., 2008). After plating the hepatocytes, they were cultured overnight in DMEM (5mM glucose), without serum or insulin. They were then stimulated for ten minutes with DMEM (5 or 25mM glucose), with or without 10 nM insulin, or 10% fetal bovine serum.
Statistics
Differences between groups were assessed by Student’s t-test using the Bonferroni correction for multiple testing. Bars and error bars correspond to the mean and SEM, respectively.
Supplementary Material
Highlights.
Hepatic insulin signaling is not required for activation of mTORC1 by feeding.
SREBP-1c mRNA is partially induced by feeding in absence of insulin.
Induction of SREBP-1c by obesity is entirely dependent upon insulin signaling.
Acknowledgments
We would like to thank Dr. Delphine Eberlé, Dr. David Cohen, Dr. Joseph Majzoub, Dr. Peter Crawford, and Dr. Diane Fingar for helpful discussion. We also thank Dr. C. Ronald Kahn for the LIRKO mice and Dr. Jay Horton for antibodies. This work was supported by the Joslin Diabetes Specialized Assay Core (5P30DK36836), Einstein Stable Isotope and Metabolomics Core (P60DK020541), the Cell Biology Core at the UCSF Liver Center (P30DK026743), as well as the following grants: NSF Graduate Research Fellowship, DK08369, HL10965, DK056084, DK05608, and DK05813.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anai M, Funaki M, Ogihara T, Kanda A, Onishi Y, Sakoda H, Inukai K, Nawano M, Fukushima Y, Yazaki Y, Kikuchi M, Oka Y, Asano T. Enhanced insulin-stimulated activation of phosphatidylinositol 3-kinase in the liver of high-fat-fed rats. Diabetes. 1999;48:158–169. doi: 10.2337/diabetes.48.1.158. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141–149. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- Czech MP. Structural and functional homologies in the receptors for insulin and the insulin-like growth factors. Cell. 1982;31:8–10. doi: 10.1016/0092-8674(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J, Postic C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest. 2008;118:956–964. doi: 10.1172/JCI34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- Dif N, Euthine V, Gonnet E, Laville M, Vidal H, Lefai E. Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem J. 2006;400:179–188. doi: 10.1042/BJ20060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doiron B, Cuif MH, Kahn A, Diaz-Guerra MJ. Respective roles of glucose, fructose, and insulin in the regulation of the liver-specific pyruvate kinase gene promoter. J Biol Chem. 1994;269:10213–10216. [PubMed] [Google Scholar]
- Engelking LJ, Kuriyama H, Hammer RE, Horton JD, Brown MS, Goldstein JL, Liang G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J Clin Invest. 2004;113:1168–1175. doi: 10.1172/JCI20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci U S A. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN, Jacobs A, Le NA, Sandler J. Effect of somatostatin-induced suppression of postprandial insulin response upon the hypertriglyceridemia associated with a high carbohydrate diet. J Clin Invest. 1982;70:1225–1233. doi: 10.1172/JCI110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV, White MF. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty BD, Bobard A, Hainault I, Ferre P, Bossard P, Foufelle F. From The Cover: Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory elementbinding protein-1c. Proc Natl Acad Sci U S A. 2005;102:791–796. doi: 10.1073/pnas.0405067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab. 2012;23:65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Tomita S, Sekiya M, Hasty A, Nakagawa Y, Sone H, Toyoshima H, Ishibashi S, Osuga J, Yamada N. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53:560–569. doi: 10.2337/diabetes.53.3.560. [DOI] [PubMed] [Google Scholar]
- Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50:1263–1268. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30:398–408. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture: Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O’Rahilly S, Savage DB. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the edinburgh type 2 diabetes study. Diabetes Care. 2011;34:1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100:3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt Stimulates Hepatic SREBP1c and Lipogenesis through Parallel mTORC1-Dependent and Independent Pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellaturu CR, Deng X, Park EA, Raghow R, Elam MB. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP). SREBP-1c complex. J Biol Chem. 2009;284:31726–31734. doi: 10.1074/jbc.M109.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.