Abstract
Vitamin D deficiency is associated with negative health outcomes, including infections. Vitamin D modulates inflammation and down-regulates the expression of calprotectin, a molecule which influences neutrophil functions and which has been linked to oral candidiasis (OC), the most prevalent oral lesion in human immunodeficiency virus (HIV). We hypothesized a positive association between vitamin D deficiency and OC, and that this effect was partially modulated by calprotectinemia. Plasma calprotectin and serum 25 (OH) vitamin D levels were measured in stored samples from 84 HIV-seropositive Chicago women enrolled in the Oral Substudy of the Women’s Interagency HIV Study (WIHS). OC and vitamin D deficiency were diagnosed in, respectively, 14 (16.7%) and 46 (54.8%) of those studied. Vitamin D deficiency was positively associated with OC (p = 0.011) and with higher calprotectinemia (p = 0.019) in univariate analysis. After adjustment for CD4, HIV viral load, HIV treatment, and tobacco and heroin/methadone use, vitamin D deficiency remained a significant predictor of OC (OR 5.66; 95% confidence interval 1.01-31.71). This association weakened after adjustment for calprotectinemia, supporting a role for calprotectinemia as a moderator of this effect. These findings support studies to examine the effect of vitamin D status on calprotectinemia, neutrophil functions, and opportunistic mucosal infections in HIV.
Keywords: innate immunity, women’s health, mucosal immunity, immune suppression, yeast infection, metabolic disease
Introduction
Oral lesions associated with HIV infection were identified early in the HIV pandemic as clinical signs of immune suppression (Klein et al., 1984). Oral candidiasis (OC), hairy leukoplakia (HL), and oral warts, conditions associated, respectively, with yeast organisms such as Candida albicans, the Epstein-Barr virus (EBV), and the Human Papillomavirus (HPV), are the most commonly observed oral mucosal lesions in HIV (Greenspan et al., 1992).
Therapies targeting the HIV life cycle, especially HIV protease inhibitors, a component of highly active antiretroviral therapy (HAART), have resulted in remarkable beneficial effects, including immune reconstitution, HIV viral suppression, reduced incidence of opportunistic infections, and ultimately increased survival rates (Powderly et al., 1998). The introduction of HAART in industrialized countries and in the developing world also resulted in a sharp decline in the prevalence and incidence of most opportunistic oral mucosal lesions (Aguirre et al., 1999). OC remains the most commonly observed oral lesion associated with HIV even in the HAART era, with cohorts reporting a prevalence of OC around the 20% range (Sroussi et al., 2007; Gaitan-Cepeda et al., 2010).
A comprehensive understanding of the immune dysregulation resulting in oral opportunistic infections such as OC is lacking. Results from the Women’s Interagency HIV Study (WIHS) confirmed earlier findings from others that OC was correlated with immunosuppression and HIV replication (Greenspan et al., 2000). Researchers have reported other risk factors for OC such as tobacco smoking (Sroussi et al., 2007), oral sex (Greenspan et al., 2000), a direct effect of HIV medication on fungal organisms (De Bernardis et al., 2004), and abnormal levels of antimicrobial immune regulatory factors in saliva, such as calprotectin (Kleinegger et al., 2001). As effective HIV treatment succeeds at increasing or stabilizing CD4 cell counts and improves long-term HIV virological control, other risk factors for oral lesions associated with HIV may therefore emerge.
WIHS recently reported an association between vitamin D deficiency and bacterial vaginosis, a common mucosal infection in HIV-infected women (French et al., 2011). Vitamin D deficiency is common among women independent of their HIV status, especially among African American and Hispanic women (Adeyemi et al., 2011). Bacterial vaginosis, similarly to oral candidiasis, results from an overgrowth of commensal organisms and may signal the presence of an imbalance in normal mucosal flora, possibly linked to defective immune surveillance mechanisms. The possible role played by vitamin D deficiency in oral mucosal infections (Sun, 2010), including OC, in the general population and in the context of HIV-related immune suppression, is unknown.
Calprotectin is an antimicrobial and immune regulatory protein complex (Perera et al., 2010) expressed constitutively by oral keratinocytes and neutrophils (Ross and Herzberg, 2001). High levels of circulating calprotectin have been documented in HIV and correlate with HIV disease exacerbation (Strasser et al., 1997). Moreover, a familial syndrome of hypercalprotectinemia which includes recurrent infections has been described (Sampson et al., 2002), and an association between high salivary calprotectin and OC was reported (Sweet et al., 2001). The relationship between calprotectinemia and a higher risk of OC in HIV patients is unknown and was investigated herein.
In previous work, we demonstrated that calprotectin down-regulated neutrophil recruitment (Sroussi et al., 2006) and inhibited neutrophil oxidative functions (Sroussi et al., 2010). Others indicated that vitamin D reduced the expression of calprotectin genes in human oral keratinocytes in vitro (Haussler et al., 2010). Whether deficiency in vitamin D levels affects calprotectin concentration in the circulation is unknown. Vitamin D deficiency may result in an exaggerated expression of calprotectin which would inhibit neutrophil recruitment and functions, resulting in an increased risk for opportunistic infections such as OC.
In the present study, we explored the relationship among vitamin D, calprotectinemia, and OC. Our hypothesis was that vitamin D deficiency would be associated with a diagnosis of OC and higher calprotectinemia, and that calprotectinemia would partially modulate the association between vitamin D and OC.
Materials & Methods
Sample
This study conforms to the Strobe guidelines. The WIHS is an ongoing prospective cohort study of HIV-infected and uninfected at-risk women enrolled at six sites: Chicago, San Francisco Bay Area (SF), Brooklyn and Bronx/Manhattan, New York, Washington, DC (DC), and Los Angeles (LA). The WIHS cohort was designed to reflect the demographics of the HIV epidemic among women in the United States. Details of cohort recruitment, retention, and demographics have been previously described (Barkan et al., 1998). Briefly, participants undergo semi-annual visits that include an interviewer-administered structured questionnaire, a physical examination, and collection of blood and gynecologic specimens. From study inception in 1995 through 2004, a subset of WIHS participants was enrolled in a nested study of oral health. Informed consent was obtained from all participants in accordance with the US Department of Health and Human Services guidelines and the institutional review boards of participating institutions.
The current study included 84 HIV-infected Chicago WIHS participants. The study sample was selected for the availability of vitamin D data from a previous study (French et al., 2011) and concurrent data from oral soft-tissue examination. Stored plasma was used to measure calprotectin. Data were collected from July 1995 through March 2004.
Measures and Statistical Analysis
This study is in compliance with the Strobe guidelines for cohort studies (http://www.strobe-statement.org). Detailed description of the measures and statistical analysis are available in the Appendix.
Results
The study sample did not differ significantly from the overall Chicago HIV-seropositive WIHS cohort on any of the characteristics shown (data not shown) (Barkan et al., 1998).
Of the total 84 women in the sample, 14 (16.7%) were diagnosed with OC. The prevalence of OC by vitamin D status indicated that vitamin D–sufficient (n = 15), –insufficient (n = 23), and –deficient (n = 34) women had, respectively, a 0%, 8.7%, and 26.1% prevalence rate of OC (Table 1) [chi-square = 6.99 (d.f. = 2), p < 0.05].
Table 1.
Characteristics of the Study Sample
| Characteristic | Total % (n) 100% (84) | Oral Candidiasis 17% (14) | No OC 83% (70) | p-value (2-sided chi-square) |
|---|---|---|---|---|
| Race | 0.128 | |||
| Black Non-Hispanic | 69 (58) | 79 (11) | 67 (47) | |
| Hispanic | 13 (11) | 21 (3) | 11 (8) | |
| White | 18 (15) | 0 (0) | 21 (15) | |
| HIV Risk Category | 0.089 | |||
| Injection drug | 45 (38) | 43 (6) | 46 (32) | |
| Heterosexual | 42 (35) | 29 (4) | 44 (31) | |
| Blood transfusion | 4 (3) | 14 (2) | 1 (1) | |
| Other/Unknown | 10 (8) | 14 (2) | 9 (6) | |
| Cigarette smoker | 54 (45) | 77 (10) | 50 (35) | 0.074 |
| Marijuana smoker | 18 (15) | 15 (2) | 19 (13) | 0.767 |
| Heroin/methadone user | 10 (8) | 15 (2) | 9 (6) | 0.456 |
| Oral sex | 23 (19) | 7 (1) | 26 (18) | 0.129 |
| CD4 | 0.034 | |||
| < 200 | 35 (29) | 64 (9) | 29 (20) | |
| 200-499 | 37 (31) | 14 (2) | 42 (29) | |
| >= 500 | 28 (23) | 21 (3) | 29 (20) | |
| Undetectable HIV RNA viral load | 12 (10) | 7 (1) | 13 (9) | 0.526 |
| Viral load | 0.218 | |||
| <= 4,000 | 42 (34) | 21 (3) | 46 (31) | |
| > 4,000 - <= 50,000 | 35 (29) | 43 (6) | 34 (23) | |
| > 50,000 | 23 (19) | 36 (5) | 21 (14) | |
| Antiretroviral Use | 74 (62) | 71 (10) | 74 (52) | 0.824 |
| HAART | 12 (10) | 21 (3) | 10 (7) | 0.228 |
| Season (Fall/Winter) | 60 (50) | 64 (9) | 59 (41) | 0.691 |
| Vitamin D–deficient | 55 (46) | 86 (12) | 49 (34) | 0.011 |
| Mean (SD) | Mean (SD) | Mean (SD) | t test p-value | |
| Age, yrs; mean (s.d.) | 40 (7) | 41 (7) | 40 (7) | 0.387 |
| HIV yrs since known infection | 7 (4) | 6 (4) | 7 (4) | 0.627 |
| Volume unstimulated saliva (mL) | 1.5 (1.4) | 1.5 (1.9) | 1.5 (1.3) | 0.895 |
Demographic, behavioral, and clinical characteristics of the women in the sample, and their association with OC, are presented in Table 1. CD4 cell counts by category were significantly associated with OC in univariate analysis; 64% (n = 9) of women with OC had a CD4 at or below 200, compared with 29% (n = 20) of women without OC [chi-square = 6.79 (d.f. = 2), p < 0.05]. A significantly higher proportion of women with vitamin D deficiency had OC than those without [86% (n = 12) vs. 49% (n = 34), p = 0.011].
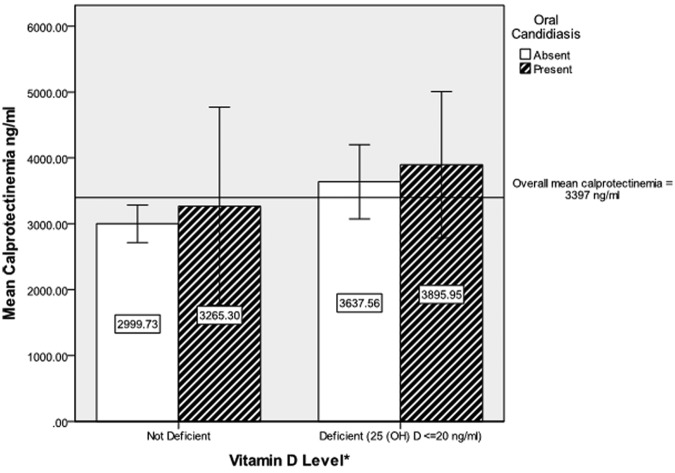
No significant association was found between OC status and plasma calprotectin when calprotectin was analyzed as a continuous variable or as a dichotomous variable (data not shown) defined as hypercalprotectinemia (> 3500 ng/mL) and normal (< 3500 ng/mL) (Fig. 1). However, a significantly higher plasma concentration of calprotectin was associated with vitamin D deficiency [mean 3,705 ng/mL vs. 3,014 ng/mL, t = −2.25 (d.f. = 81), p < 0.05].
Figure.
Univariate relationships among mean calprotectin level, oral candidiasis, and vitamin D deficiency. *Vitamin D deficiency associated with higher mean calprotectinemia [ANOVA F = 5.08 (d.f. = 81), p = 0.027]; oral candidiasis not associated with higher mean calprotectinemia [ANOVA F = 1.10 (d.f. = 81), p = 0.240]; interaction of vitamin D deficiency and oral candidiasis not significant (p > 0.05). Error bars ± 2 mean standard errors (SE)
A multivariate analysis of factors previously described as potentially associated with OC, including CD4 cell count, HIV viral load, cigarette smoking, heroin/methadone use, vitamin D deficiency, and antiretroviral therapy, was conducted with or without calprotectin as an independent variable. In model 1, without calprotectin, vitamin D deficiency was found to be a statistically significant predictor of OC with an adjusted odds ratio (AOR) of 5.66 [95% confidence interval (CI) 1.01-31.71] (p < 0.05) (Table 2). The only other statistically significant (p < 0.05) predictor of OC was CD4 under or equal to 200 (AOR = 4.79; CI 1.04-21.98), with tobacco smoking approaching significance (p = 0.073) with an AOR of 4.16 (CI 0.88, 19.77). The introduction of calprotectin to the multivariate model (Model 2) maintained the significance of CD4 under or equal to 200 (P < 0.05) as a predictor of OC; CD4 at or below 200 (AOR 4.74; CI 1.02, 21.11). Calprotectin itself was not a significant predictor of OC. Notably, the predicting value of OC by vitamin D deficiency was not statistically significant when calprotectin was introduced in the model.
Table 2.
Multivariable Logistic Regression Models of the Presence of OC on Vitamin D Deficiency with or without the Adjustment of Calprotectinemia
| Model 1 Vitamin D Deficiency Predicting OC |
Model 2 Vitamin D Deficiency Predicting OC, Adjustment for Calprotectinemia |
|||
|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
| Vitamin D deficiency | 5.66 (1.01, 31.71) | 0.049 | 4.87 (0.84, 28.23) | 0.077 |
| CD4 <= 200 | 4.79 (1.04, 21.98) | 0.044 | 4.74 (1.02, 21.11) | 0.048 |
| Viral load > 50 k | 1.30 (0.27, 6.35) | 0.743 | 1.45 (0.29, 7.19) | 0.646 |
| Any ART use | 1.83 (0.34, 9.94) | 0.485 | 1.74 (0.31, 9.79) | 0.529 |
| Tobacco smoking | 4.16 (0.88, 19.77) | 0.073 | 5.93 (0.95, 37.14) | 0.057 |
| Heroin/methadone use | 1.67 (0.23, 12.23) | 0.614 | 1.69 (0.22, 12.77) | 0.614 |
| Calprotectinemia | — | 1.00 (1.00, 1.00) | 0.338 | |
| Model fit | ||||
| Correct classification | 82.3% | 84.6% | ||
| Nagelkerke Pseudo R-square | 0.313 | 0.326 | ||
| Model chi-square | 16.15, p = 0.013 | 16.81, p = 0.019 | ||
Discussion
Significant progress has been made in therapeutics targeting the HIV life cycle. Additional improvement in the management of HIV-infected patients may require addressing health concerns in addition to viral suppression and the preservation or recovery of immune competence. In this report, we found an association between vitamin D deficiency and oral candidiasis. Whether vitamin D deficiency is contributing etiologically to incidence of OC is unknown but should be evaluated in future prospective studies aimed at normalizing vitamin D levels.
Although our sample size was modest (n = 84), the study was retrospective, and the sample selection was a convenience sample based on the existence of concurrent vitamin D and oral examination data in the Chicago WIHS cohort, it nevertheless offers support for an association between vitamin D deficiency and OC. In fact, vitamin D deficiency was a stronger predictor of OC than other established factors such as CD4 count, tobacco usage, or HIV targeted treatment.
The absence of multicollinearity among predictor variables indicates that individual model predictors are not redundant, and their estimates relative to each other are reliable. The wide confidence intervals in the multivariate models predicting OC are due to the small number of cases in certain cells. While the multivariate models we present are robust, interpretation of the odds ratios and CIs should be done with caution.
Vitamin D deficiency is a prevalent and under-diagnosed condition in the general population and in specific risk groups (Adeyemi et al., 2011). Prevalence rates of vitamin D deficiency among HIV-infected populations may be 60% or more, depending on levels used to define deficiency (Wasserman and Rubin, 2010). In our sample, 55% of the HIV-infected women were vitamin D–deficient at a cutoff of 20 ng/mL. There is no consensus about what level defines normal physiologic vitamin D levels, and this may differ by health outcome. Analysis of our data suggests that vitamin D levels below 20 ng/mL may be relevant for oral conditions including OC, similar to reports for periodontal healing (Bashutski et al., 2011).
Vitamin D deficiency has been linked to a wide range of diseases and health outcomes beyond that of calcium homeostasis, including periodontal healing (Bashutski et al., 2011), mucosal or systemic infection including sepsis (Ginde et al., 2011), food allergies (Bozzetto et al., 2011), and asthma (Bener et al., 2011). The health benefits of sufficient vitamin D levels, specifically as they relate to oral health (Stein and Tipton, 2011), have been attributed to the anti-inflammatory effect (Krishnan and Feldman, 2011) and the ability of vitamin D to stimulate the production of antimicrobial peptides such as cathelicidin (Liu et al., 2007). Here we present evidence supporting an inverse relationship between vitamin D levels and calprotectinemia, an antimicrobial peptide with immune regulatory functions. This inverse relationship is in accordance with the down-regulating of calprotectin expression by vitamin D in oral keratinocytes (Haussler et al., 2010). The source of circulating calprotectin is however unknown, and the cells on which vitamin D would exercise its calprotectin reducing effect are also unidentified.
This study is limited by its modest size, its cross-sectional and retrospective nature, and the fact that it relies on stored samples which could have degraded. Moreover, the sample was selected by convenience from a previous study. This could have introduced a selection bias which could have affected the analysis and for which we have not controlled. Moreover, whereas it is tempting to speculate that vitamin D may influence the risk of OC, it is also possible that OC and vitamin D deficiency share common risk factors but have no direct or indirect connection. Additional larger prospective studies aimed at confirming the findings presented herein should address directly the possible benefits of vitamin D supplementation in vitamin D–deficient patients. This approach would be aimed at decreasing the occurrence rate of OC and at examining the possible mechanism(s) for this health benefit. We suggest that calprotectinemia and neutrophil functions should be among the mechanisms investigated.
Finally, the effect of vitamin D on OC may be modulated by numerous factors, including its previously reported ability to modulate the transcription of several inflammatory genes (Krishnan and Feldman, 2011). Vitamin D deficiency reduces the effectiveness of antiretroviral therapy to improve CD4 levels in HIV (Ross et al., 2011). Consequently, vitamin D may indirectly affect OC by hampering therapeutic immune reconstitution. The multivariate analysis we present indicated that vitamin D was associated with OC independently of CD4 counts, suggesting that the effect of vitamin D status on OC was not limited to an indirect effect modulated by CD4 counts. The cross- sectional nature of our study did not allow us specifically to investigate the effect of vitamin D on the reconstitution of CD4 counts.
In our previous work, we showed that calprotectin inhibits the recruitment of neutrophils and their oxidative metabolism (Sroussi et al., 2006, 2010). It is tempting to speculate that vitamin D deficiency may therefore produce an exaggerated calprotectinemia which would then depress neutrophil responsiveness, contributing to a higher risk of OC. Future studies testing this hypothetical model are warranted, since this model is supported by this preliminary analysis, and the clinical significance of the question it addresses is potentially of wide-ranging importance not limited to HIV, OC, and the oral cavity.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group, with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI- 31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). Data in this manuscript were collected by the Oral Substudy of the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium; The Connie Wofsy Study Consortium of Northern California (Deborah Greenspan, John S. Greenspan); Los Angeles County/Southern California Consortium (Roseann Mulligan, Mahvash Navazesh); Chicago Consortium (Mario Alves); Data Coordinating Center (Stephen Gange). The WIHS Oral Substudy was funded by the National Institute of Dental and Craniofacial Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adeyemi OM, Agniel D, French AL, Tien PC, Weber K, Glesby MJ, et al. (2011). Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr 57:197-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre JM, Echebarria MA, Ocina E, Ribacoba L, Montejo M. (1999). Reduction of HIV-associated oral lesions after highly active antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:114-115 [DOI] [PubMed] [Google Scholar]
- Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. (1998). The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9:117-125 [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. (2011). The impact of vitamin D status on periodontal surgery outcomes. J Dent Res 90:1007-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bener A, Ehlayel MS, Tulic MK, Hamid Q. (2011). Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol 157:168-175 [DOI] [PubMed] [Google Scholar]
- Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. (2011). Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy 67:10-17 [DOI] [PubMed] [Google Scholar]
- De Bernardis F, Tacconelli E, Mondello F, Cataldo A, Arancia S, Cauda R, et al. (2004). Anti-retroviral therapy with protease inhibitors decreases virulence enzyme expression in vivo by Candida albicans without selection of avirulent fungus strains or decreasing their anti-mycotic susceptibility. FEMS Immunol Med Microbiol 41:27-34 [DOI] [PubMed] [Google Scholar]
- French AL, Adeyemi OM, Agniel DM, Evans CT, Yin MT, Anastos K, et al. (2011). The association of HIV status with bacterial vaginosis and vitamin D in the United States. J Women’ s Health (Larchmt) 20: 1497-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitan-Cepeda LA, Dominguez-Sanchez A, Pavia-Ruz N, Munoz-Hernandez R, Verdugo-Diaz R, Valles-Medina AM, et al. (2010). Oral lesions in HIV+/AIDS adolescents perinatally infected undergoing HAART. Med Oral Patol Oral Cir Bucal 15:e545-e550 [PubMed] [Google Scholar]
- Ginde AA, Camargo CA, Jr, Shapiro NI. (2011). Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad Emerg Med 18:551-554 [DOI] [PubMed] [Google Scholar]
- Greenspan D, Komaroff E, Redford M, Phelan JA, Navazesh M, Alves ME, et al. (2000). Oral mucosal lesions and HIV viral load in the Women’s Interagency HIV Study (WIHS). J Acquir Immune Defic Syndr 25:44-50 [DOI] [PubMed] [Google Scholar]
- Greenspan JS, Barr CE, Sciubba JJ, Winkler JR. (1992). Oral manifestations of HIV infection. Definitions, diagnostic criteria, and principles of therapy. The U.S.A. Oral AIDS Collaborative Group. Oral Surg Oral Med Oral Pathol 73:142-144 [DOI] [PubMed] [Google Scholar]
- Haussler MR, Haussler CA, Whitfield GK, Hsieh JC, Thompson PD, Barthel TK, et al. (2010). The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol 121:88-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. (1984). Oral candidiasis in high-risk patients as the initial manifestation of the Acquired Immunodeficiency Syndrome. N Engl J Med 311:354-358 [DOI] [PubMed] [Google Scholar]
- Kleinegger CL, Stoeckel DC, Kurago ZB. (2001). A comparison of salivary calprotectin levels in subjects with and without oral candidiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:62-67 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Feldman D. (2011). Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 51:311-336 [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. (2007). Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179:2060-2063 [DOI] [PubMed] [Google Scholar]
- Perera C, McNeil HP, Geczy CL. (2010). S100 Calgranulins in inflammatory arthritis. Immunol Cell Biol 88:41-49 [DOI] [PubMed] [Google Scholar]
- Powderly WG, Landay A, Lederman MM. (1998). Recovery of the immune system with antiretroviral therapy: the end of opportunism? J Am Med Assoc 280:72-77 [DOI] [PubMed] [Google Scholar]
- Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. (2011). Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther 16:555-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KF, Herzberg MC. (2001). Calprotectin expression by gingival epithelial cells. Infect Immun 69:3248-3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson B, Fagerhol MK, Sunderkotter C, Golden BE, Richmond P, Klein N, et al. (2002). Hyperzincaemia and hypercalprotectinaemia: a new disorder of zinc metabolism. Lancet 360:1742-1745 [DOI] [PubMed] [Google Scholar]
- Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. (2006). S100A8 triggers oxidation-sensitive repulsion of neutrophils. J Dent Res 85 :829-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroussi HY, Villines D, Epstein J, Alves MC, Alves ME. (2007). Oral lesions in HIV-positive dental patients—one more argument for tobacco smoking cessation. Oral Dis 13:324-328 [DOI] [PubMed] [Google Scholar]
- Sroussi HY, Lu Y, Zhang QL, Villines D, Marucha PT. (2010). S100A8 and S100A9 inhibit neutrophil oxidative metabolism in-vitro: involvement of adenosine metabolites. Free Radic Res 44:389-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SH, Tipton DA. (2011). Vitamin D and its impact on oral health—an update. J Tenn Dent Assoc 91:30-33 [PubMed] [Google Scholar]
- Strasser F, Gowland PL, Ruef C. (1997). Elevated serum macrophage inhibitory factor-related protein (MRP) 8/14 levels in advanced HIV infection and during disease exacerbation. J Acquir Immune Defic Syndr Hum Retrovirol 16:230-238 [DOI] [PubMed] [Google Scholar]
- Sun J. (2010). Vitamin D and mucosal immune function. Curr Opin Gastroenterol 26:591-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet SP, Denbury AN, Challacombe SJ. (2001). Salivary calprotectin levels are raised in patients with oral candidiasis or Sjogren’s syndrome but decreased by HIV infection. Oral Microbiol Immunol 16:119-123 [DOI] [PubMed] [Google Scholar]
- Wasserman P, Rubin DS. (2010). Highly prevalent vitamin D deficiency and insufficiency in an urban cohort of HIV-infected men under care. AIDS Patient Care STDs 24:223-227 [DOI] [PubMed] [Google Scholar]