Abstract
Several Bmp genes are expressed in the developing mouse tooth germ from the initiation to the late-differentiation stages, and play pivotal roles in multiple steps of tooth development. In this study, we investigated the requirement of BMP activity in early tooth development by transgenic overexpression of the extracellular BMP antagonist Noggin. We show that overexpression of Noggin in the dental epithelium at the tooth initiation stage arrests tooth development at the lamina/early-bud stage. This phenotype is coupled with a significantly reduced level of cell proliferation rate and a down-regulation of Cyclin-D1 expression, specifically in the dental epithelium. Despite unaltered expression of genes known to be implicated in early tooth development in the dental mesenchyme and dental epithelium of transgenic embryos, the expression of Pitx2, a molecular marker for the dental epithelium, became down-regulated, suggesting the loss of odontogenic fate in the transgenic dental epithelium. Our results reveal a novel role for BMP signaling in the progression of tooth development from the lamina stage to the bud stage by regulating cell proliferation and by maintaining odontogenic fate of the dental epithelium.
Keywords: BMP signaling, BMP antagonist, Noggin overexpression, tooth progression, Cyclin-D, transgenic mice
Introduction
In mice, tooth development begins at embryonic day 10.5 (E10.5), when the tooth-forming site and tooth type are determined in the oral cavity (Neubüser et al., 1997; Tucker et al., 1998). At E11.5, a local thickening of the dental epithelium becomes evident as the presumptive dental epithelial cells elongate along the apical-basal axis and change cell shape from cuboidal to columnar, known as the lamina stage. At E12.5, the thickened dental epithelium proliferates and invaginates into the subjacent dental mesenchyme. This process continues until E13.5, forming a definite tooth bud (the bud stage). Subsequently, the epithelial bud undergoes specific folding, forming a cap structure at E14.5 (the cap stage), and a bell structure at E16.5 (the bell stage), when the epithelium-derived ameloblasts and mesenchyme-derived odontoblasts begin to differentiate (Zhang et al., 2005).
Multiple families of signaling molecules have been demonstrated to play crucial roles in mammalian tooth development (Tummers and Thesleff, 2009). Among them is the family of bone morphogenetic protein (BMP) that belongs to the TGF-β superfamily. Like other members of the TGF-β superfamily, BMP signaling is transduced into cells via the heteromeric receptor complex of one type II transmembrane serine-threonine kinase receptor with each of 3 type I receptors (BMPR-IA, BMPR-IB, and Alk2). The ligand-bound heteromeric receptor phosphorylates the receptor-regulated Smads, mainly Smads-1. -5, and -8, in the cytoplasm. These phosphorylated Smads (pSmads) further bind to the common Smad (Smad4) and enter the nucleus to regulate gene expression. In addition to this Smad-dependent canonical BMP signaling pathway, BMP signaling can also be mediated through Smad-independent mitogen-activated protein kinase (MAPK) pathways, known as non-canonical pathways (Sieber et al., 2009). In the developing mouse tooth, several Bmp genes, including Bmp-2, -3, -4, and -7, are expressed in either epithelial or mesenchymal components (Nie et al., 2006), and BMP activities have been implicated in the determination of tooth-forming site and tooth type (Neubüser et al., 1997; Tucker et al., 1998), progression of tooth development from the bud stage to the cap stage (Chen et al., 1996; Zhang et al., 2000; Zhao et al., 2000), and terminal differentiation and tooth eruption (Gluhak-Heinrich et al., 2010; Yao et al., 2010). BmprIa and BmprIb have been shown to be expressed, and to function redundantly to a certain extent, in the developing tooth (Li et al., 2011). While BmprIb null mice developed normal teeth, and a tooth phenotype was not reported in mice carrying tissue-specific inactivation of Alk2 in the neural-crest-derived dental mesenchyme (Yi et al., 2000; Dudas et al., 2004), inactivation of BmprIa in either dental epithelium or dental mesenchyme or both led to an arrest of tooth development at the bud/cap stages (Andl et al., 2004; Liu et al., 2005; Li et al., 2011), indicating the importance of BMPR-IA-mediated BMP signaling in the progression of the bud to the cap stage. Interestingly, Bmp2, Bmp4, and Bmp7 are all expressed in the developing mouse tooth at the lamina stage and during the transition from the lamina to the bud stage (Åberg et al., 1997). Due to potential functional redundancy among these BMPs and among multiple BMP receptors, genetic inactivation studies have not yet provided evidence as to whether BMP signaling is required in this earlier tooth developmental progression. In this study, we investigated the consequence of inhibition of BMP activity at the tooth initiation stage by conditional transgenic overexpression of the BMP antagonist Noggin. Our results demonstrate an absolute requirement for BMP signaling in tooth development to reach the definite bud stage.
Materials & Methods
Animals
The generation and genotyping of the K14-Cre and pMes-Nog transgenic animals used in this study have been described previously (Andl et al., 2004; Xiong et al., 2009). Embryos were collected from timed pregnant mice, and embryos carrying double transgenic alleles (K14Cre;pMes-Nog) were determined under a Leica fluorescent stereoscope (Leica Microsystems Inc., Buffalo Grove, IL, USA) by the expression of Egfp that was linked to the Noggin transgene through the IRES sequences (Xiong et al., 2009). Use of animals in this study was approved by the Tulane University IACUC.
Histology, in situ Hybridization, and Immunohistochemistry
For histological and in situ section hybridization analyses, 4% paraformaldehyde (PFA) fixed embryonic heads were embedded in paraffin and sectioned at 10 µm, followed by standard hematoxylin/eosin staining or non-radioactive in situ hybridization as described previously (St Amand et al., 2000). The same process was also applied to immunostaining for Cyclin-D1 (antibodies from Santa Cruz Biotechnology, Santa Cruz, CA, USA). For pSmad1/5/8 immunohistochemical staining (antibodies from Cell Signaling Technology, Beverly, MA, USA, and Abcam, Cambridge, MA, USA, respectively), 4% PFA fixed samples were washed in 30% sucrose/PBS, embedded in O.C.T. compound, and cryo-sectioned as described previously (He et al., 2010). Whole-mount in situ hybridization was performed following a standard protocol established in the lab (St Amand et al., 2000). Each experiment was repeated at least twice.
BrdU Labeling and TUNEL Assays
Cell proliferation rate was measured by BrdU labeling with a BrdU labeling and detection kit (Roche Diagnostic Corporation, Indianapolis, IN, USA), as described previously (Xiong et al., 2009). We counted BrdU-positive cells and total numbers of cells in fixed arbitrary areas from 3 adjacent sections of each embryo (N = 3 for each genotype), and subjected the data to Student’s t test to determine the significance of differences. A TUNEL assay was conducted on paraffin sections as described previously (Alappat et al., 2005).
Results
Noggin Overexpression in the Early Tooth Germ Arrests Tooth Development at the Lamina/Early-bud Stage
To achieve tissue-specific expression of Noggin, a naturally secreted BMP antagonist that binds preferentially to BMP2, BMP4, and BMP7 to prevent their signaling (Zimmerman et al., 1996; Groppe et al., 2002), we utilized a conditional transgenic Noggin allele pMes-Nog (Xiong et al., 2009) and the K14-Cre transgenic line that expresses Cre recombinase in the oral epithelium at E11.5 (Andl et al., 2004; He et al., 2010; Appendix Fig.). The pMes-Nog transgenic allele harbors the chicken β-actin/CMV enhancer promoter, the LoxP flanked STOP cassette, and the mouse Noggin coding sequence, followed by the IRES2-Egfp sequence and a rabbit β-globin polyA tail (Xiong et al., 2009). Upon Cre-mediated DNA recombination, deletion of the STOP cassette allows for simultaneous tissue-specific expression of Noggin and Egfp (Figs. 1A-1D). Histological analyses revealed severe tooth developmental defects in K14Cre;pMes-Nog (Fig. 1). At E11.5, the dental placodes formed normally in transgenic embryo, as evidenced by the formation of thickened dental epithelium in both incisor and molar germs (N = 5) (Fig. 1F; and data not shown). At E12.5, when the controls developed to the early-bud stage, the transgenic tooth exhibited retarded development, remaining at the lamina/early-bud stage (N = 5) (Fig. 1H). At E13.5 and E14.5, the molars in the transgenic embryos had regressed, but the incisors remained at the early-bud stage (N = 3 at E13.5; N = 2 at E14.5) (Figs. 1I-1L; data not shown). At E16.5, while residual upper incisor epithelia were still present, residual lower incisor and molar epithelia were never found in K14Cre;pMes-Nog mice (N = 2) (Figs. 1M-1P; and data not shown).
Figure 1.
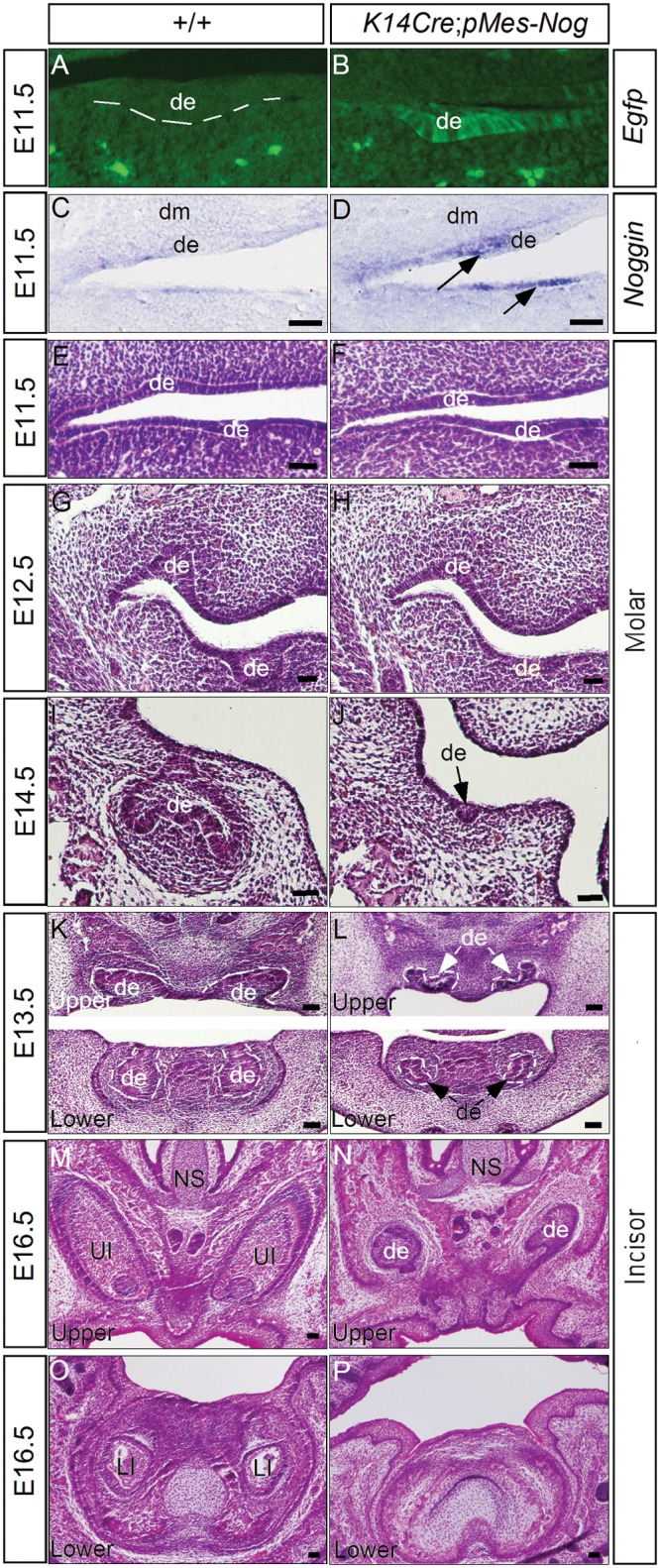
Overexpression of Noggin in either dental epithelium or dental mesenchyme arrests tooth development at the lamina/early-bud stage. (A-D) Ectopic expression of Noggin in the dental epithelium of transgenic animals but not in the controls at E11.5, as determined by detection of Egfp expression (A,B) and by in situ hybridization (C,D). (E, F) Molar development in transgenic embryos appeared normal at the E11.5 lamina stage. (G-J) Arrest of molar development at the laminar/early-bud stage began at E12.5 (G, H) and had regressed at E14.5 (I, J). (K, L) The development of both upper and lower incisors in transgenic embryos was arrested at the early-bud stage at E13.5. (M-P) At E16.5, residual dental epithelium was present in the upper incisors but not in the lower incisors of transgenic embryos. de, dental epithelium; dm, dental mesenchyme; NS, nasal septum; LI, lower incisor; UI, upper incisor. Scale bar = 100 µm.
Overexpression of Noggin Inhibits Cell Proliferation in the Dental Epithelium
We chose the K14Cre;pMes-Nog molar for further cellular and molecular analyses. Immunohistochemical studies showed, in the dental mesenchyme of the transgenic molar, a slightly reduced level of phosphorylated Smad1/5/8 proteins (pSmad1/5/8), an indicator of BMP activity, at E11.5 and E12.5 (Figs. 2A-2D). While TUNEL assays showed no increased apoptotic cells (N = 2; data not shown), BrdU labeling experiments demonstrated a dramatically reduced rate of cell proliferation, specifically in the dental epithelium but not in the mesenchyme of the transgenic molars at E12.0 (N = 5), a stage that was selected before obvious phenotypes appeared (Figs. 2E, 2F, 2M). Thus, this defective cell proliferation in the dental epithelium provides a cellular base for the arrest of tooth development at the lamina/early-bud stage. To investigate the molecular defects responsible for altered cell proliferation, we examined the expression of several Cyclin-D genes by in situ hybridization. Despite unaltered expression of Cyclin-D2 and -D3, Cyclin-D1 expression became dramatically down-regulated in the dental epithelium at E12.5 (Figs. 2G, 2H; and data not shown). Interestingly, Cyclin-D1 mRNA expression in the mesenchyme of the maxilla-mandibular junction appeared unaffected, suggesting a tissue-specific effect (Figs. 2G, 2H). We further confirmed this inhibition of Cyclin-D1 expression in the dental epithelium at the protein level by immunohistochemical staining at the same stage (Figs. 2I, 2J). However, Cyclin-D1 expression was not affected in the dental epithelium of the transgenic molar at E11.5 (Figs. 2K, 2L), consistent with its normal morphology at this stage (Fig. 1F).
Figure 2.
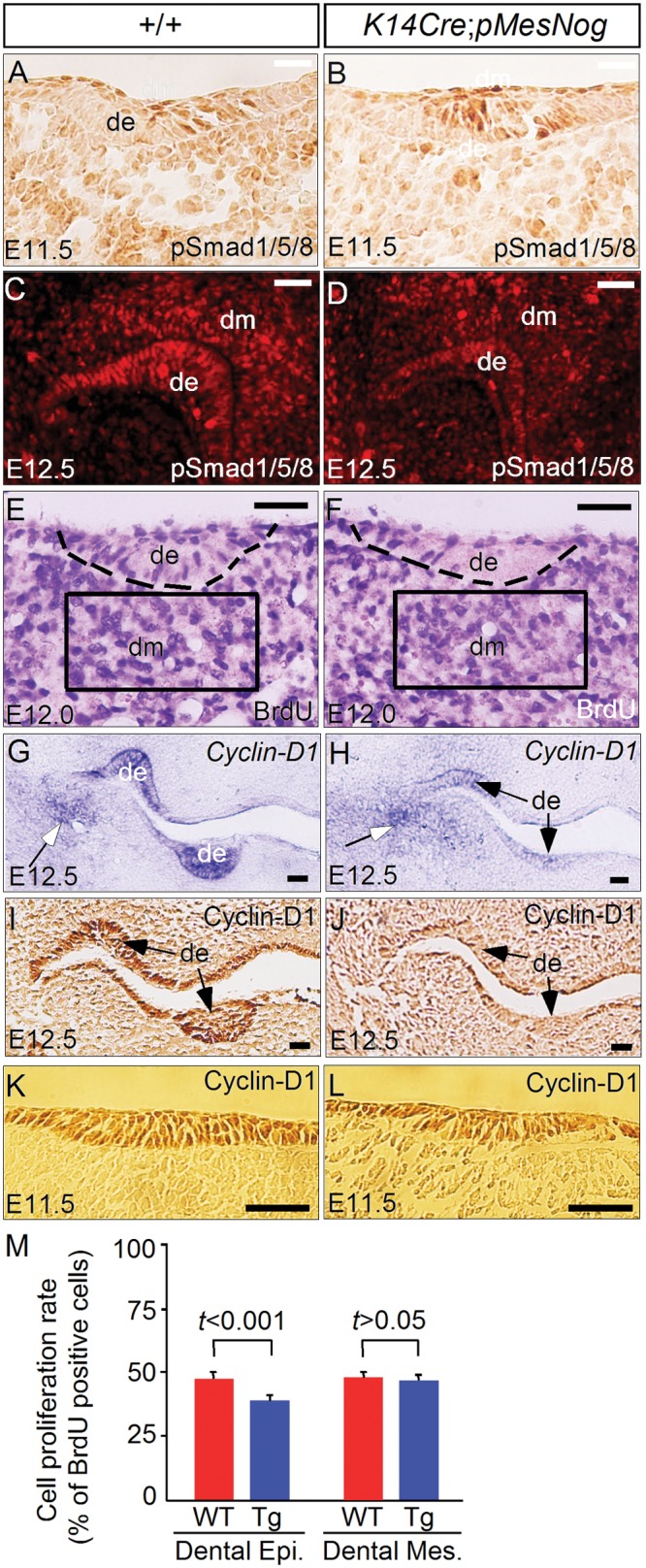
Inhibition of cell proliferation and down-regulation of Cyclin-D1 in the transgenic dental epithelium. (A-D) Immunohistochemical staining showed slightly reduced levels of pSmad1/5/8 in molar teeth of a Noggin-overexpressing embryo at E11.5 and E12.5. (E, F) BrdU labeling experiment showed a significantly reduced rate of cell proliferation in the dental epithelium but not in the mesenchyme of a Noggin-overexpressing embryo at E12.0. (G, H) In situ hybridization showed significant down-regulation of Cyclin-D1 expression in the dental epithelium (arrows) but not in the mesenchyme surrounding the maxilla-mandibular junction (open arrows) in transgenic embryo at E12.5. (I, J) Immunohistochemical staining further confirmed down-regulation of Cyclin-D1 in the dental epithelium of a transgenic embryo at E12.5. (K, L) Immunohistochemical staining showed unaltered expression of Cyclin-D1 in the dental epithelium of a transgenic embryo at E11.5. (M) Comparison of BrdU-labeled cells in the dental epithelium and dental mesenchyme in control and transgenic embryos. Standard deviation values are presented as error bars. The significance of differences was determined by Student’s t test, and the values are presented. de, dental epithelium; dm, dental mesenchyme. Scale bar = 100 µm.
Noggin Overexpression Inhibits Pitx2 Expression in the Dental Epithelium
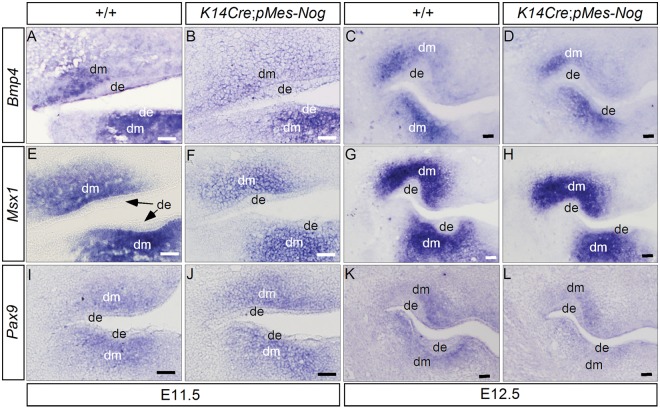
We next examined, by in situ hybridization, the expression of genes known to play critical roles in early tooth development in K14Cre;pMes-Nog mice at E11.5 and E12.5. For the genes that are expressed in the dental mesenchyme, we observed slight down-regulation of Bmp4 and Msx1 at E11.5 (N = 3 for each gene; Figs. 3A, 3B, 3E, 3F), consistent with a slight reduced level of pSmad1/5/8 in the dental mesenchyme. However, Pax9 expression appeared unaltered (N = 3; Figs. 3I, 3J). Interestingly, the expression of all these genes exhibited levels comparable with those in the controls at E12.5 (N = 2 for each gene; Fig. 3). For those genes expressed in the dental epithelium, including Shh, Bmp2, Pitx2, and Fgf8, almost identical expression patterns were observed in the incisor and the first molar-forming regions in both wild-type and transgenic embryos at E11.5 (N = 3 for each gene; Figs. 4A-4H). However, at E12.5, while Shh and Bmp2 exhibited similar expression levels and patterns in the developing teeth of controls and transgenic mice (N = 3 for each gene; Figs. 4I-4L), Pitx2 expression was down-regulated significantly in K14Cre;pMes-Nog teeth, as evidenced by both whole-mount and section in situ hybridization assays (N = 2 for either whole-mount or section in situ hybridization; Figs. 4M-4P).
Figure 3.
Expression of Bmp4, Msx1, and Pax9 in the dental mesenchyme of Noggin-overexpressing embryos. (A-D) Bmp4 expression was down-regulated to a certain extent in the dental mesenchyme of the transgenic embryo at E11.5 (B), but not at E12.5 (D). (E-H) Msx1 expression was also slightly down-regulated in the dental mesenchyme of the transgenic embryo at E11.5 (F), but returned to a level comparable with that of the control at E12.5 (H). (I-L) Similar levels of Pax9 expression were observed in the molar mesenchyme of transgenic and control embryos at E11.5 and E12.5. de, dental epithelium; dm, dental mesenchyme. Scale bar = 100 µm.
Figure 4.
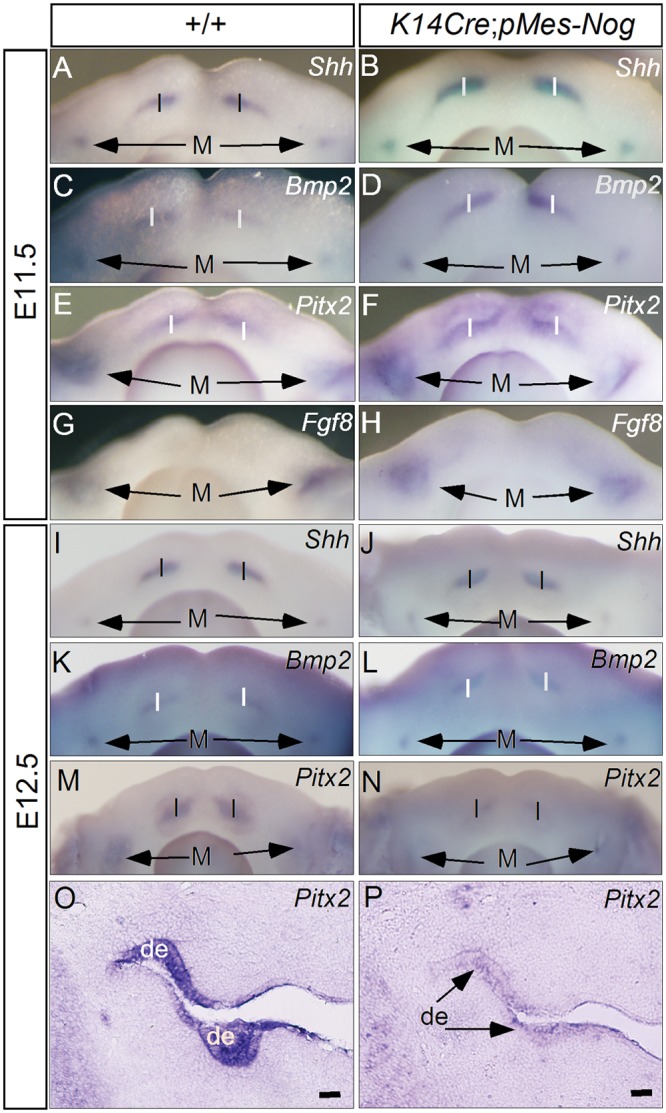
Analyses of gene expression in the dental epithelium of Noggin-overexpressing embryo. (A-H) Whole-mount in situ hybridization showed unaltered expression of Shh, Bmp2, Pitx2, and Fgf8 in the lower incisors and molars of Noggin-overexpressing embryos at E11.5. (I-L) Whole-mount in situ hybridization showed unaltered expression of Shh and Bmp2 in the lower incisors and molars of transgenic embryos at E12.5. (M-P) Whole-mount and section in situ hybridization demonstrated significant down-regulation of Pitx2 in the dental epithelium of transgenic embryos at E12.5. I, incisor-forming region; M, the first molar-forming region; de, dental epithelium. Scale bar = 100 µm.
Discussion
During early tooth development, BMP signaling has been shown to be required for progression of the tooth germ from the bud to the cap stage (Chen et al., 1996; Zhang et al., 2000; Zhao et al., 2000; Andl et al., 2004; Liu et al., 2005; Li et al., 2011). However, whether BMP signaling plays a role in the transition of the tooth germ from the lamina to the bud stage remained unknown, likely due to potential functional redundancy among the multiple Bmps and their receptors that are co-expressed in the early developing tooth (Nie et al., 2006). While inactivation of Smad4 in the cranial neural crest cells leads to an arrest of tooth development at the lamina stage (Ko et al., 2007), it cannot be counted as the consequence of loss of BMP activity alone, since Smad4 functions as the central mediator for canonical signaling of the entire TGF-β superfamily. In the current study, we used a transgenic approach to ‘tune down’ BMP activity at the beginning of tooth development. Our results demonstrate an absolute requirement for BMP signaling in the progression of tooth development to the bud stage. However, in a similar study, Plikus et al. (2005) reported different tooth phenotypes in mice carrying the K14-Noggin transgenic allele. These mice exhibited selective loss of mandibular molars, but formed maxillary molars and incisors with abnormal patterning and defective differentiation of ameloblasts and odontoblasts (Plikus et al., 2005). The different tooth phenotypes reported in the current study and in the study by Plikus and colleagues could be attributed to different expression timing of the K14 promoter and also possibly different levels of transgenically expressed Noggin. Indeed, the importance of the timing of BMP activity in tooth development has been highlighted in several previous studies. For example, inhibition of BMP activity by Noggin in the incisor-forming region at E10 transforms the incisor germ to a molar-like structure, but causes formation of multiple smaller incisors if Noggin is applied at E12 (Tucker et al., 1998; Munne et al., 2010). In addition, exogenously applied BMP protein is able to inhibit Pitx2 expression in the dental epithelium at E10.5 but not at E11.5 (St Amand et al., 2000). In the current study, the activity of the K14-Cre transgenic allele was initially detected in the oral epithelium, including the dental epithelium, at E11.5 (Appendix Fig.), when the dental placode has formed. This explains the initial formation of the dental placode but subsequent repression of tooth development in K14Cre;pMes-Nog embryo.
Since we did not observe an altered level of cell apoptosis in the tooth germs of the K14Cre;pMes-Nog embryo, the selective inhibition of cell proliferation in the dental epithelium of the transgenic embryo provides a cellular base contributing to the earlier arrested tooth development. This suppression of cell roliferation apparently resulted from the down-regulated expression of Cyclin-D1 that is known to participate in the regulation of cell proliferation in the odontogenic epithelium (Kumamoto et al., 2001). Several signaling pathways, including BMP signaling, have been implicated in the regulation of Cyclin-D1 expression (Ovchinnikov et al., 2006; Nemoto et al., 2009). The down-regulation of Cyclin-D1 in the dental epithelium, but not in the mesenchyme around the maxilla-mandibular junction, of K14Cre;pMes-Nog mice suggests a tissue-specific requirement of BMP activity for Cyclin-D1 expression. Our results also showed a repression of Pitx2 expression, a molecular marker for the dental epithelial lineage throughout tooth morphogenesis (St Amand et al., 2000), suggesting a change in odontogenic fate of the dental epithelium in the transgenic mice. BMP signaling is thus also required for the maintenance of odontogenic fate in the dental epithelium.
One surprising observation in the K14Cre;pMes-Nog tooth germ was the almost unaltered expression of BMP downstream target genes, including Bmp4, Msx1, and Pax9, in the dental mesenchyme. This also holds true in developing teeth of the K14-Noggin transgenic mice, reported previously (Plikus et al., 2005). These observations could be explained by the potential functional redundancy between/among members of the TGF-β superfamily and between TGF-β canonical and non-canonical pathways. For example, Activin is known to be expressed in the mesenchyme of the developing tooth and to signal through Alk2, which also serves as a type I receptor for BMP signaling (Tummers and Thesleff, 2009). This could also explain why pSmad1/5/8 level is only slightly reduced in the dental mesenchyme. Furthermore, it was reported previously that Smad4-mediated canonical and p38 MAPK-mediated non-canonical pathways are functionally redundant in mediating TGFβ/BMP signaling during tooth development (Xu et al., 2008). It appears that the primary defect that leads to the early arrest of tooth development in Noggin-overexpressing mice resides in the dental epithelium.
In sum, our studies reveal a novel function of BMP signaling in early tooth development. We provide evidence demonstrating that BMP signaling regulates the progression of tooth development from the lamina to the bud stage by controlling Cyclin-D1 expression and thereby cell proliferation in the dental epithelium, and by maintaining the odontogenic fate of dental epithelium.
Acknowledgments
The authors thank members of the Chen lab for technical support and for sharing mouse lines.
Footnotes
Y.W. was supported by a fellowship from the College of Stomatology, The Fourth Military Medical University, P.R. China. Y.Z. was supported by a fellowship from the Department of Health, Fujian Province, P.R. China. This study was supported by the NIH (R01 DE12329 and R01 DE15123).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Åberg T, Wozney J, Thesleff I. (1997). Expression patterns of bone morphogenetic proteins (bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn 210:383-396 [DOI] [PubMed] [Google Scholar]
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, et al. (2005). The cellular and molecular etiology of the cleft palate in Fgf10 mutant mice. Dev Biol 277:102-113 [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. (2004). Epithelial BmprIa regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131:2257-2268 [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. (1996). Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122:3035-3044 [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. (2004). Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev 121:173-182 [DOI] [PubMed] [Google Scholar]
- Gluhak-Heinrich J, Guo D, Yang W, Harris MA, Lichtler A, Kream B, et al. (2010). New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone 46:1533-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, et al. (2002). Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420:636-642 [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, et al. (2010). Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol 347:109-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SO, Chung IH, Xu X, Oka S, Zhao H, Cho ES, et al. (2007). Smad4 is required to regulate the fate of cranial neural crest cells. Dev Biol 312:435-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto H, Kimi K, Ooya K. (2001). Detection of cell cycle-related factors in ameloblastomas. J Oral Pathol Med 30:309-315 [DOI] [PubMed] [Google Scholar]
- Li L, Lin M, Wang Y, Cserjesi P, Chen Z, Chen YP. (2011). BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development. Dev Biol 340:451-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, et al. (2005). Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132:1453-1461 [DOI] [PubMed] [Google Scholar]
- Munne PM, Felszeghy S, Jussila M, Suomalainen M, Thesleff I, Jernvall J. (2010). Splitting placodes: effects of bone morphogenetic protein and Activin on the patterning and identity of mouse incisors. Evol Dev 12:383-392 [DOI] [PubMed] [Google Scholar]
- Nemoto E, Koshikawa Y, Kanaya S, Tsuchiya M, Tamura M, Somerman MJ, et al. (2009). Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone 44:805-812 [DOI] [PubMed] [Google Scholar]
- Neubüser A, Peters H, Ballings R, Martin GR. (1997). Antagonistic interactions between FGF and BMP4 signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 90:247-255 [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kettunen P. (2006). BMP signaling in craniofacial development. Int J Dev Biol 50:511-521 [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Selever J, Wang Y, Chen YT, Mishina Y, Martin JF, et al. (2006). BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol 295:103-115 [DOI] [PubMed] [Google Scholar]
- Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JG, et al. (2005). Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol Dev 7:440-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber C, Kopf J, Hiepen C, Knaus P. (2009). Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20:343-355 [DOI] [PubMed] [Google Scholar]
- St Amand TR, Zhang YD, Semina EV, Zhao X, Hu YP, Nguyen L, et al. (2000). Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol 217:323-332 [DOI] [PubMed] [Google Scholar]
- Tucker AS, Matthews KL, Sharpe PT. (1998). Transformation of tooth type induced by inhibition of BMP signaling. Science 282:1136-1138 [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. (2009). The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool (Mol Dev Evol) 312B:309-319 [DOI] [PubMed] [Google Scholar]
- Xiong W, He F, Morikawa Y, Yu X, Zhang Z, Lan Y, et al. (2009). Hand2 is required in the epithelium for palatogenesis in mice. Dev Biol 330:131-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr, Deng C, Chai Y. (2008). Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev Cell 15:322-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Prpic V, Pan F, Wise GE. (2010). TNF-alpha upregulates expression of BMP-2 and BMP-3 genes in the rat dental follicle—implications for tooth eruption. Connect Tissue Res 51:59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. (2000). The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127:621-630 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, et al. (2000). A new function of BMP4: dual role of BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127:1431-1443 [DOI] [PubMed] [Google Scholar]
- Zhang YD, Chen Z, Song Y, Liu C, Chen YP. (2005). Making a tooth: growth factors, transcription factors, and stem cells. Cell Res 15:301-316 [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Z, Song Y, Zhang X, Zhang Y, Hu Y, et al. (2000). Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech Dev 99:29-38 [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. (1996). The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86:599-606 [DOI] [PubMed] [Google Scholar]