Abstract
Transplants of culture-expanded bone marrow stromal cells (BMSCs) combined with hydroxy-apatite tricalcium phosphate (HA/TCP) scaffolds successfully form cortico-cancellous bone to reconstruct the dog craniofacial skeleton. Yet, these transplants’ long-term stability in large animal models has not been evaluated. Theis study’s purpose was the evaluation of long-term BMSC transplant stability when used to augment the mandible. Here, autologous BMSC-HA/TCP transplants were introduced onto the unilateral dog mandible as onlay grafts, while contralateral control mandibles received HA/TCP onlays alone. Quantitative CT (qCT) scans were obtained both early and late after transplantation. Transplants were harvested up to 19 months later for histologic and mechanical analyses. In all dogs, BMSC transplants formed significantly greater amounts of bone over their control counterparts. The new bone formed an extensive union with the underlying mandible. BMSC transplants retained the majority of their initial volume, while control (HA/TCP only) transplants were nearly completely resorbed. By qCT, the extent of newly formed bone could be determined non-invasively. In summary, HA/TCP particles alone undergo a high degree of resorption, while autologous cultured BMSC- HA/TCP transplants provide long-term bony augmentation of the mandible.
Keywords: AFM, Autologous cell, Bone tissue engineering, Hydroxyapatite composite, Stem cell, Transplantation
Introduction
Mandibular atrophy is a disease of multiple etiologies, of which tooth loss is a leading factor. It remains a significant health problem that is accentuated by our aging population. An atrophic mandible presents patients with several problems, including abnormalities of facial contour, poor fitting of dentures, and mandibular fragility. The atrophic mandible is also less likely to tolerate placement of osseointegrated implants for dental reconstruction because of its reduced width. These problems can be ameliorated by mandibular augmentation with autogenous bone graft, but this major surgical procedure is complicated by the need for substantial graft material, the creation of deformities in the donor site, uncertain rates of graft resorption, and the need to wait months before the implant is able to be fitted and used.[1]
Bone marrow stromal cells (BMSCs) include cells with the ability to differentiate into several mesodermal tissue lines in vitro. These cells can also form mature mesodermal tissue types in vivo following transplantation with appropriate matrices. Because osteoblasts number among their potential progeny, BMSCs are believed to play an essential role in bone formation and remodeling. Since they are easy to extract from the bone marrow and expand in tissue culture, they remain an attractive candidate for cell-based therapies to reconstruct bone deficits. When combined with an appropriate matrix, cultured BMSCs have been used to repair critical-sized calvarial bone defects in small and large animal models, and they have successfully augmented the normal mouse mandible.[2–4] Use of BMSCs to clinically augment the atrophic mandible represents a logical elaboration of this technology. Until now, however, no large animal study has described the use of autologous BMSCs to augment the normal mandible.
Thus, in this study, BMSCs were harvested from healthy dogs, expanded in tissue culture, attached to HA/TCP particles, and autotransplanted onto the normal mandible. We then evaluated the histologic, radiographic, and mechanical properties of the tissues and demonstrated that BMSC autotransplantation is a feasible therapy for mandibular augmentation.
Methods
Transplant preparation and placement
Bone marrow was harvested from the distal femur of three nine month old male mongrel dogs, in accordance with an approved NIH animal protocol (97-031). Multi-colony derived strains of BMSCs were obtained from the bone marrow in a manner previously described.[3, 5] Briefly, bone marrow cells were cultured in growth medium consisting of αMEM (Invitrogen, Grand Island, NY), 2 mM L-glutamine, 100 U/mL penicillin, 100 ug/mL streptomycin sulfate (Biofluids, Rockville, MD), 10−8 M dexamethasone (Sigma, St. Louis, MO), 10−4 M L-ascorbic acid phosphate magnesium salt n-hydrate (Wako, Osaka, Japan), and 20 percent fetal bovine serum of a pre-selected lot (Equitech-Bio, Kerrville, TX). Cells were cultured at 37°C in an atmosphere of 100% humidity and 5% CO2 and passaged upon approaching confluence.
Trypsin-released cells from passages 2 to 4 were pipetted into 50 mL polypropylene Falcon tubes (Becton, Dickinson and Company; Franklin Lakes, NJ). Separately, HA/TCP particles (Zimmer; Warsaw, Indiana) of size range 0.5 – 1.0 mm were isolated using a sieve shaker (CSC Scientific; Fairfax, Virginia). Between 100×106 and 150×106 BMSCs were allowed to attach to 2 grams of HA/TCP particles. Control transplants consisted of HA/TCP particles which were moistened with growth medium, but received no cells. The mixtures were incubated for 90 minutes at 37°C on a slowly rotating platform. Following centrifuging of the mixtures at 1200 rpm for 120 seconds, the supernatant was discarded.
Under general anesthesia, each of the three dogs underwent creation of a sub-periosteal pocket on the inner surface of the mandible, bilaterally. Each pocket measured approximately 1.5 cm diameter, and each was large enough to easily encompass a transplant without allowing for migration of the transplant mixture. In each dog, one pocket was filled the BMSC + HA/TCP mixture, while the contralateral pocket was filled with control HA/TCP particles with no cells attached. Each side received an equivalent amount of transplant material. The periosteal incisions were then carefully repaired with absorbable suture, and the skin was closed in layers.
To insure consistency between BMSC and control sides, sub-periosteal pockets of similar dimension were created on each side through blunt dissection. The transplants were placed and the periosteum closed over them and secured with suture. The periosteum remained securely attached to the bone where it had not been lifted. The pocket shapes and volumes were thus constrained by size of the bone which had been exposed and the overlying periosteum which was not expansile.
Each of 3 dogs underwent an initial CT scan at 2–5 months following transplantation, and a second CT scan at 15–16 months following transplantation. The interval between consecutive scans for each animal was 11–13 months. The dogs underwent sacrifice and full harvest of the mandibles and transplants at 17–19 months following transplantation. Specimens underwent demineralized and undemineralized processing for histologic analysis.
Histomorphometry of bone sections
H&E stained histology slides of transplants were examined to quantify the areas which had either formed or not formed bone. Because each of the transplants was so large, they were first divided into 4 pieces, and histologic sections obtained of each piece. Each section was analyzed. Throughout the sections, areas of bone formation (B) were easily distinguished from areas devoid of bone formation (NB). B, NB, and the total transplant area (T) were then measured for each section. As expected, B + NB = T. All histologic images were magnified using a Zeiss Axioplan microscope (Zeiss, Germany), and they were captured using the microscope-imaging program, Spot Advanced (Diagnostic Instruments, Inc; Sterling Heights, Michigan). Area measurements were obtained with ImageJ version 1.36 (National Institutes of Health; Bethesda, Maryland). The B, NB, and T values of the 4 sections for each transplant were averaged to compute an overall B, T, and B/T value for each transplant.
Non-invasive monitoring of transplants using qCT
CT scans were acquired in a GE CTI (GE Medical Systems; Milwaukee, WI) at an energy of 80 kVp/200 mA and slice thickness of 1.0 mm. Each scan included a Mindways Model 2 phantom (Mindways Software, South San Francisco, CA). The BMD of each image slice of each transplant was obtained using QCT Pro v. 2.0.3 (Mindways Software; South San Francisco, CA) on a Dell XPS R450 (Dell Computer Corporation; Round Rock, Texas). BMD values were expressed in terms of mg/cc of K2HPO4 in distilled water, where a BMD of 0 corresponded to the density of distilled water alone (no additional K2HPO4) and a BMD with value >0 corresponded to non-aerated biologic tissue.
Each transplant was represented by 4 to 33 slices, each 1.0 mm thick and bounded by an oval-shaped region of interest (ROI). The software provided the ROIs, whose size and shape could be determined by the investigator. In each slice of a transplant, the ROI was adjusted to match the size and shape of the transplant, which was typically oval in cross-section. The BMD of all slices of the transplant were pooled; the mean of these individual BMD values was used as the overall BMD value for the transplant. All BMD values were calculated by the primary investigator (MHM), who was blinded to the identity of the transplants.
The BMD of the mandibular bone underlying the transplants was determined by defining an ROI of the mandible in six slices from a CT scan of each of the dogs. The BMD of all mandibular slices were pooled to arrive at a mean mandibular BMD.
Determination of transplant volume
Total transplant volume at transplantation was measured non-invasively. At each CT scan, the total volume of each transplant was acquired by analyzing a series of qCT images (slices) that encompassed the entire transplant for each dog, using ImageJ. A color contrast level was identified and applied to all scanned images to optimize the grayscale balance, allowing us to distinguish the transplant from the underlying dog mandible. The pixel/distance ratio was calculated by measuring the number of pixels along a line segment covering a known distance of 164 mm. In each slice, circumscription of the transplant area was done by hand due to the variability of the morphology of each transplant. Care was taken to make sure the outline of the transplant closely followed the outer edge of the transplant and the border between transplant and normal dog mandible. For each circumscribed area, ImageJ was used to calculate the enclosed area. Since each slice measured 1.0 mm in thickness, transplant volume could be computed. The sum of the individual slice volumes totaled the entire transplant volume.
Statistical analyses
Statistical analyses between groups of transplants utilized a non-parametric unpaired t-test, performed using InStat version 3.06 (GraphPad Software; San Diego, CA).
Results
Three dogs underwent bone marrow harvest, BMSC expansion, and BMSC autografting to the mandible without complication.
Transplant morphology
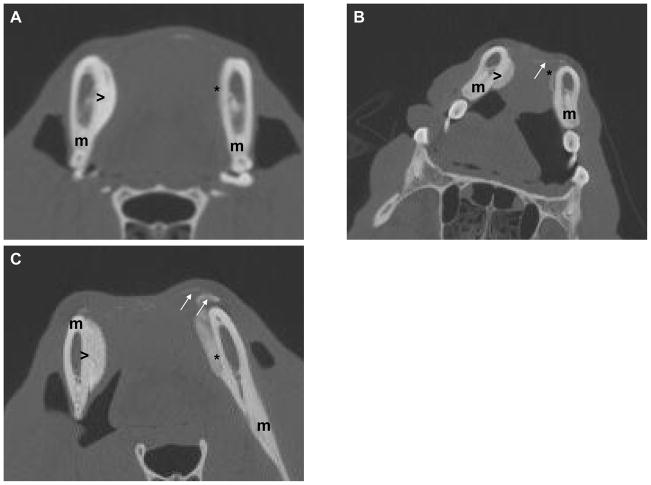
Examination of the BMSC-containing transplants demonstrated a cohesive structure, with HA/TCP particles bound to each other throughout the transplant; no loose HA/TCP particles were noted in these transplants. Particles were found within the confines of the transplant, with no gross evidence of particle migration. BMSC transplants were rigid on gross palpation, and were firmly attached to the underlying mandible. The transplant shapes and volumes were roughly comparable to their condition at placement. In contrast, BMSC-free transplants had a much smaller volume, and in 2 dogs, they did not project beyond the silhouette of the underlying mandible (Figures 1A, 1B). In all 3 BMSC-free transplants, loose HA/TCP particles were found to have migrated away from the main transplant, and lay in the overlying soft tissues (Figure 1C).
Figure 1.
Figure 1A: CT image of dog #1 at 17 months following transplantation. BMSC transplant (>) exhibits substantial volume and density, and is tightly adherent to underlying mandible (m). BMSC-free transplant (*), in contrast, has undergone nearly complete resorption.
Figure 1B: CT image of dog #2 at 15 months following transplantation. Loose HA/TCP particles remain at site of BMSC-free transplant site (arrows). The BMSC transplant retains its volume and density (>).
Figure 1C: CT image of dog #3 at 3 months following transplantation. BMSC-free transplant (*) already demonstrates substantial resorption. Loose HA/TCP particles (arrows) have already begun migrating away from the BMSC-free transplant (*). The BMSC transplant (>) exhibits greater density and volume than BMSC-free transplant.
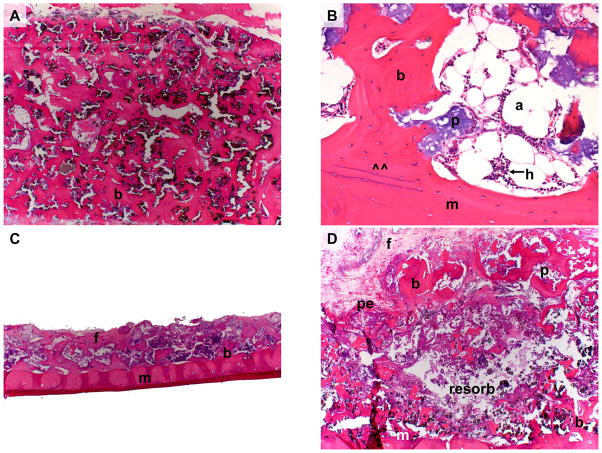
Histologically, all BMSC transplants contained abundant and extensive meshwork of new lamellar cortical bone firmly attached to both particles and the adjacent mandible (Figure 2A). The transplant formed a substantial bone union with the underlying mandible. The bone encircled hematopoietic tissue and occasional adipocytes, all of which were spatially associated with the new bone and the HA/TCP particles (Figure 2B). Little fibrovascular tissue could be observed. The overlying periosteum was intact. Bone was present across the thickness of the transplant, starting at the superficial edge and extending down to the mandible. In contrast, BMSC-free transplants were severely limited in size, and they were profoundly smaller and thinner than their BMSC counterparts (Figure 2C). The majority of sections exhibited no new bone, with the remaining sections having very little bone; sections were instead populated predominantly by fibrovascular tissue surrounding the HA/TCP particles. In a control transplant from the third dog, a unique histologic picture presented itself- a thin layer of new bone was present just deep to the periosteum and another bone-containing layer was superficial to the mandible (Figure 2D). Between these 2 layers, an extensive zone of bone-free fibrovascular tissue remained. No such bone-free zone was seen in any BMSC transplant.
Figure 2.
Figure 2A: BMSC-containing transplant (Animal 2) 17 months post-operatively. Note extensive cortico-cancellous bone (b) formed throughout the entire thickness of the transplant.
Figure 2B: BMSC-containing transplant (Animal 3) 19 months post-operatively. Note extensive cortico-cancellous bone encircling hematopoiesis (h) and adipocytes (a). Newly formed bone has formed a union (at ^^) with the underlying mandible (m).
Figure 2C: BMSC-free transplant (Animal 2) 17 months post-operatively. Note that residual transplant has minimal volume and that much of the original HA/TCP scaffold has been resorbed. Minimal bone (b) and extensive fibrous tissue (f) lay on the mandible (m).
Figure 2D: BMSC-free transplant (Animal 3) 19 months post-operatively. Note resorption of transplant interior leaving behind minimal tissue (resorb), and minimal residual bone (b) along mandible (m) and periosteum (pe).
(b = bone, f = fibrous connective tissue, p = particle, m = mandible, pe = periosteum, a = adipocyte, h = hematopoiesis), resorb = (resorbed tissue within BMSC-free transplant)
Magnification: 2.5x (A, C, D). Magnification: 20x (B).
Stain: Hematoxylin and eosin; paraffin embedding following demineralization (A–D)
Extent of bone formation
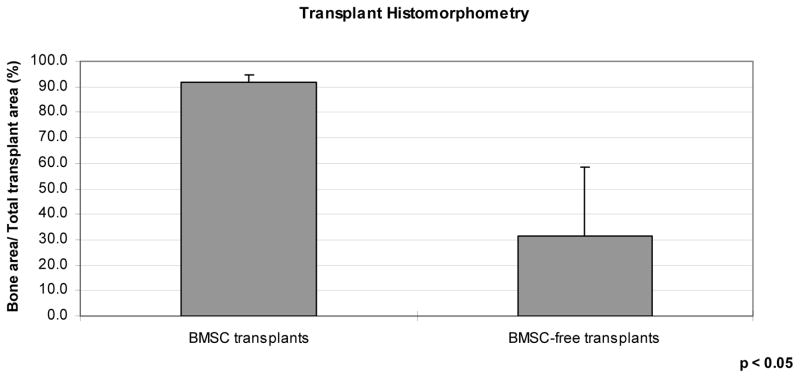
The extent of bone and of non-bone tissue, as well as the size of the transplants, was evaluated by histomorphometry of 4 histologic tissue sections from each transplant. The cross-sectional area of new bone in the middle of BMSC-containing transplants in Dogs 1 through 3 was 36.8, 40.9, and 46.0 cm2, respectively, while the non-bone area of these transplants was 4.3, 2.1, and 4.9 cm2, respectively (Figure 3). The percentage of the new bone areas in the 3 BMSC transplants was 89.5, 95.2, and 90.4, respectively. In contrast, the cross-sectional area of new bone in BMSC-free transplants in Dogs 1 through 3 was 6.6, 0.2, and 9.3 cm2, while the non-bone area of these transplants was 5.0, 9.3, and 16.6 cm2, respectively. The percentage of each non-BMSC transplant which contained bone was 57.0, 2.1, and 35.8, respectively. Thus, the BMSC-containing transplants included predominantly bone, while the BMSC-free transplants had minimal to moderate bone fractions. The cross-sectional area of the BMSC transplants was profoundly greater (41.1, 43.3, and 50.9 cm2, respectively) than the area of the BMSC-free transplants (11.7, 9.5, and 25.9 cm2, respectively).
Figure 3.
Extent of bone on histologic tissue sections, as a function of transplant type, assessed via histomorphometry, as a percentage of total transplant cross-sectional area.
Transplant volume and density
CT images of the each dog mandible were obtained at early and late time points. Early imaging occurred from 2 to 5 months following transplantation, and late imaging occurred from 15 to 17 months following transplantation.
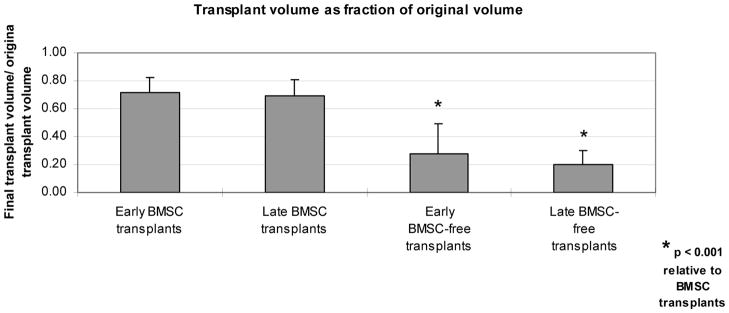
Compared to baseline volume measurements, all transplants demonstrated a decrease in overall volume by the first CT examination. Among BMSC transplants, this decrease was slight (Figure 4). In contrast, BMSC-free transplants had lost a substantial fraction of their volume. Between the early and late CT examinations, transplants exhibited only slight additional decreases in volume, whether or not they included BMSCs.
Figure 4.
Transplant volume on CT examinations, as a function of transplant type and time of imaging, as a percentage of transplant volume at time of transplantation.
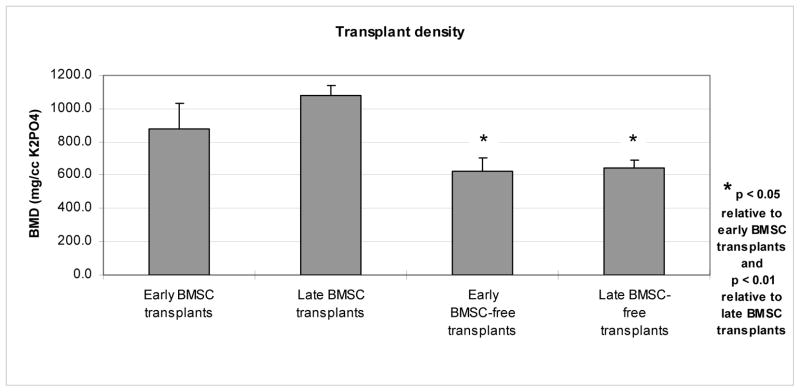
BMD was substantially higher among BMSC transplants than among BMSC-free transplants at both early and late CT examinations (Figure 5). BMD increased among all BMSC transplants, while it decreased among 2 of the 3 BMSC-free transplants.
Figure 5.
Bone mineral density (BMD) on CT examinations, as a function of transplant type and time of imaging.
Discussion
BMSCs have aroused interest as a potential source for cell-based implants to reconstruct hard tissue defects. In both small and large animals, they have been shown to repair calvarial and femoral deficits.[2–4, 8] These models have represented challenging clinical situations in which autogenous bone graft is sometimes inadequate for achieving bone healing. The model presented in this study also represents a clinical challenge, one in which onlayed autogenous bone graft can undergo substantial resorption in the weeks and months following placement. Bone onlays are most typically used to augment the atrophic mandible prior to the placement of osseointegrated implants or dentures, but are also used to treat hypoplastic bone in conditions such as hemifacial microsomia.[9–11] Given the uncertain longevity of autologous bone grafts, the purpose of this study was the evaluation of the potential for BMSC HA/TCP transplants to form stable, long-lasting bone constructs which could successfully augment the mandible in a large animal model. Secondary goals of the study were the measurement of bone density by non-invasive studies and the assessment of changes in transplant volume over the course of the study.
In this study, we transplanted culture-expanded autologous dog BMSCs onto the hemimandible. The cells had been combined with an osteoconductive ceramic, HA/TCP, immediately prior to transplantation. Each dog also received a control HA/TCP transplant devoid of BMSCs on the contralateral hemimandible. In each dog, transplant size and bone formation were tracked with 2 CT scans which were separated in time by one year. The BMSC transplants exhibited a slight diminishment in volume, a consistent increase in BMD, and profound amounts of bone formation over the course of the study, while the control transplants underwent a substantial loss of volume, a slight increases or decreases in BMD, and minimal amounts of bone formation. In brief, BMSCs helped reduce transplant volume loss while increasing bone formation. Histologic assessment of BMSC transplants also showed a substantial bone union to the underlying mandible. BMSC transplants maintained their cohesiveness, in that particles did not migrate from the transplant into the surrounding tissues, while BMSC-free transplants exhibited substantial particle migration. Many of these findings, including long-term transplant stability, substantial bone formation and transplant density, and minimal HA/TCP particle migration, have been observed in long-term autologous BMSC + HA/TCP transplants used to reconstruct canine critical-sized calvarial defects.[3]
The selection of these timepoint ranges reflects our increasing familiarity in working with dogs and with expanding large numbers of BMSCs. We chose to complete the cell harvest, tissue culture, and transplantation of the first dog before commencing with the second, and completing the second before commencing with the third. Once all dogs were transplanted, they were then managed simultaneously, in that they underwent CT examinations at the same time, and tissue harvest at the same time. In our experience with smaller human BMSC transplants in the mouse, we have found that transplants evolve (remodel) significantly during the first year but very slowly in the second year. Thus, results from a harvest time range of 17 to 19 months should not significantly differ from a set harvest at, for instance, 18 months.
While 3 recent studies have examined the role of BMSCs in mandibular defect repair, none have addressed 3 points which are unique to this study.[12–14] First, BMSCs in our study have augmented existing bone, an environment which places them at greater risk of resorption than segmental defects. Second, our transplants have been followed with sequential CT scans for 17–19 months, offering unique information on their evolution over time. Third, a slowly degradable, particulate HA/TCP scaffold was used, one which closely matches FDA-approved, commercially-available bone scaffolds. Contrary to our study, autologous dog BMSCs combined with a rapidly degradable TCP block were used to reconstruct mandibular segmental defects but were followed for only 3 months.[12] At the end of 3 months, BMSC transplants maintained a greater volume than BMSC-free transplants, although no comparison was made to the initial volume of the constructs. In a similar study, autologous dog BMSCs and TCP block scaffolds used to reconstruct mandibular segmental defects were followed for 7.5 months, although a volumetric analysis was not completed to assess final transplant volume.[13] In a third study, Ito and co-workers used autologous dog BMSCs combined with fibrin and platelet-rich plasma to assist in bone healing around dental implants placed into dental defects and followed for 8 weeks; BMSC-seated implants demonstrated greater bone-implant contact.[14] Here, likewise, a volumetric assessment of the newly formed bone was not reported, perhaps due to interference by the dental implants.
A logical extension of the techniques presented in this study would be the use of BMSC transplants to restore facial appearance and increase bone thickness and strength in patients with mandibular atrophy, especially where it results from tooth absence. The augmented mandible could serve as a base for the eventual placement of osseointegrated implants and then dentures, allowing for the restoration of normal mastication. Non-invasive qCT analysis of the transplants during bone formation, as was conducted during this study, would help predict when implant fixation has occurred and thus when normal chewing could resume.
In summary, we have demonstrated the feasibility of using large volume autologous BMSC transplants to augment the mandible in a large animal model, we have confirmed that such transplants can maintain their volume even in the absence of significant loading, and we have again demonstrated that newly-formed bone can be detected non-invasively. The success of such large transplants increases the potential therapeutic applications of BMSC technology.
Acknowledgments
The authors are indebted to Zimmer (Warsaw, Indiana) for its gift of HA/TCP, to Mindways Software (San Francisco, California) and Dr. Chris Cann for technical assistance, and to Dr. Nilo Avila, Mr. Dennis Johnson, Mr. David Williams, and Mr. Ronald Norman (Department of Radiology, Clinical Center, National Institutes of Health, Bethesda, Maryland) for assistance with the imaging. Mr. Kent Yamaguchi Jr. provided additional technical assistance. This research was supported in part by the University of California- San Francisco Research Evaluation and Allocation Committee and in part by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, NIH, DHHS.
References
- 1.Dado DV, Izquierdo R. Absorption of onlay bone grafts in immature rabbits: membranous versus enchondral bone and bone struts versus paste. Ann Plast Surg. 1989;23:39–48. doi: 10.1097/00000637-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Mankani MH, Kuznetsov SA, Wolfe RM, Marshall GW, Robey PG. In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells. 2006;24:2140–9. doi: 10.1634/stemcells.2005-0567. Epub 2006 Jun 8. [DOI] [PubMed] [Google Scholar]
- 3.Mankani MH, Kuznetsov SA, Shannon B, Nalla RK, Ritchie RO, Qin Y, Robey PG. Canine cranial reconstruction using autologous bone marrow stromal cells. Am J Pathol. 2006;168:542–50. doi: 10.2353/ajpath.2006.050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–8. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey PG. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 6.Marshall GW, Jr, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Doerner MF, Nix WD. A method for interpreting the data from depth-sensing indentation instruments. J Mater Res. 1986;1:601–609. [Google Scholar]
- 8.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–62. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 9.Sanfilippo F, Bianchi AE. Osteoporosis: the effect on maxillary bone resorption and therapeutic possibilities by means of implant prostheses--a literature review and clinical considerations. Int J Periodontics Restorative Dent. 2003;23:447–57. [PubMed] [Google Scholar]
- 10.Ousterhout DK, Vargervik K. Surgical treatment of the jaw deformities in hemifacial microsomia. Aust N Z J Surg. 1987;57:77–87. doi: 10.1111/j.1445-2197.1987.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 11.Munro IR. Rigid fixation and facial asymmetry. Clin Plast Surg. 1989;16:187–94. [PubMed] [Google Scholar]
- 12.He Y, Zhang ZY, Zhu HG, Qiu W, Jiang X, Guo W. Experimental study on reconstruction of segmental mandible defects using tissue engineered bone combined bone marrow stromal cells with three-dimensional tricalcium phosphate. J Craniofac Surg. 2007;18:800–5. doi: 10.1097/scs.0b013e31806901f5. [DOI] [PubMed] [Google Scholar]
- 13.Yuan J, Cui L, Zhang WJ, Liu W, Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials. 2007;28:1005–13. doi: 10.1016/j.biomaterials.2006.10.015. Epub 2006 Nov 7. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Yamada Y, Naiki T, Ueda M. Simultaneous implant placement and bone regeneration around dental implants using tissue-engineered bone with fibrin glue, mesenchymal stem cells and platelet-rich plasma. Clin Oral Implants Res. 2006;17:579–86. doi: 10.1111/j.1600-0501.2006.01246.x. [DOI] [PubMed] [Google Scholar]