Abstract
BACKGROUND
c-Cbl is an E3 ubiquitin ligase of many tyrosine kinase receptors. We previously detected c-Cbl mutation and low protein expression in non-small cell lung cancer (NSCLC). Therefore, we hypothesized that the overexpression of c-Cbl wild type (WT) may exhibit tumor inhibition.
METHODS
Wound healing and transwell assays were conducted to examine cell motility after c-Cbl WT transfection in NSCLC cell lines. Cell cycle was investigated by flow cytometry. A549 and H1299-luc c-Cbl WT xenograft and experimental metastasis model were performed to investigate tumor growth and metastasis inhibition in vivo.
RESULTS
Wound healing and transwell assays showed inhibition of migration in A549 and H226br cells 4-24 hr post-transfection. Ectopic c-Cbl WT expression reduced cell proliferation at 48 hr in A549 cells. Importantly, A549 and H1299-luc cells with ectopic c-Cbl WT expression showed inhibition of tumor growth in vivo. A549 cells overexpressing c-Cbl WT inhibited tumor metastasis in animal models.
CONCLUSIONS
Our study demonstrates for the first time that c-Cbl WT protein overexpression inhibits tumor metastasis and tumor growth in lung cancer xenograft models. Our results provide evidence that ectopic expression of c-Cbl WT protein can be potentially applied as targeted therapy for lung cancer treatment.
Keywords: c-Cbl, proliferation, migration, metastasis, gene therapy
INTRODUCTION
Overexpression of tyrosine kinase receptors (RTKs) has been detected in lung cancer (1,2). Therefore, promotion of degradation system of RTKs is a new approach for tumor growth inhibition (3). Many studies indicate that Casitas B-lineage lymphoma (Cbl) plays an important role in the down-regulation of RTKs by its E3 ubquitin ligase activity (4,5). Cbl family, especially c-Cbl protein also associates with the endocytosis mechanism and thus has a crucial role in the termination of signaling of RTKs such as c-Met and epidermal growth factor receptor (EGFR) (6).
c-Cbl mutations were first reported in human acute myeloid leukemia (AML) and other types of leukemia (7,8). Our previous study was the first to report that c-Cbl mutation also occurred in a solid tumor. Our data indicated that overexpression of c-Cbl mutants in non-small cell lung cancer (NSCLC) cell lines led to increased cell proliferation and motility (9). A recent study showed that mutation or knockdown of c-Cbl induced cell migration in breast cancer (10). Therefore, we hypothesized that the overexpression of c-Cbl wild type (WT) may exhibit tumor inhibition.
MATERIALS AND METHODS
Cell culture and c-Cbl transfection
Human NSCLC cell lines were obtained from the American Type Culture Collection (Manassas, VA). Luciferase expressing NSCLC cell line, H1299-Luc, was kindly provided by Dr. P-J. Lu (Institute of Clinical Medicine, National Cheng Kung University). Human NSCLC cell line, AS2, was obtained from Dr. W.-C. Su (Department of Internal Medicine, National Cheng Kung University) . Human bronchial cell line, BEAS-2B, was obtained from Dr. P.-C. Yang (Department of Internal Medicine, National Taiwan University).
Vector constructs were previously described by Tan et al (9). The cells were transfected with c-Cbl WT vector or empty vector control using ExGen500 transfection reagent (Fermentas, Glen Burnie, MD). After 48 hours, cells were collected for Western blot, cell proliferation, migration assays, and animal studies.
Western blot, tissue Western blot and Immunofluorescence assay
Immunoblotting was performed for various proteins using the conditions described below: c-Cbl, 1:200; p-EGFR(Tyr-1173), 1:200; STAT3, 1:1000 (Santa Cruz, Santa Cruz, CA); p-AKT(Ser-473), 1:1000; AKT, 1:1000; p-Met(Tyr-1234/Tyr-1235), 1 : 500 ; Met , 1 : 1000 ; EGFR , 1 : 500 ; p-STAT3(Tyr-705), 1:2000; p-ERK(Thr-202/Tyr-204), 1:1000 (Cell signaling, Danvers, MA); ERK, 1:1000; RAS, 1:1000 (Upstate, Billerica, MA); p-Src(Tyr-416), 1:2000 (Invitrogen, Carlsbad, CA); Src, 1:1000 (obtained from Dr. T.-H. Leu, Department of Pharmacology, National Cheng Kung University); p-FAK(Tyr-397), 1:200; FAK, 1:1000; β-actin, 1:5000 (Abcam, Cambridge, England). For tissue Western, xenografts were collected after mice sacrifice and were homogenized with CelLyticTMMT lysis buffer (Sigma-Aldrich St. Louis, MO) and used for immunoblotting. Pericellular poly-fibronectin assemblies were detected with anti-fibronectin (1:600, Sigma-Aldrich) (11) using bright field and fluorescent microscopy.
Transient expression of c-Cbl, cell proliferation analysis, flow cytometry, and wound healing and transwell migration assays
These assays were performed as previously described (9).
Tumor growth and Metastasis analyses in vivo
Female BALB/c nude mice, 5-6 weeks of age, were acquired from the National Laboratory Animal Center (Taipei, Taiwan) after obtaining appropriate institutional review board permission and raised in pathogen-free environment. Transfected A549 cells (1×106) in 200 μl were injected by tail vein for in vivo experimental metastasis analysis. The mice were euthanized at the indicated times and lung tumors were photographed with a digital camera. For tumor growth inhibition assay, transfected A549 or H1299-luc cells (5 × 106) in 100 μl were implanted subcutaneously into mice. The size of the tumor mass was measured and the tumor volume was calculated as 1/2×length×width2 in mm3 for A549 xenograft. The growth of the H1299-luc xenograft was observed under IVIS50 in vivo imaging system (XENOGEN) after endotoxin-free luciferase substrate (VivoGlo™, Promega, Madison, WI) injection. Mice body weight was measured. Tumors were fixed and stained with hematoxylin and eosin (H&E) for further pathological confirmation.
Statistical analysis
For continuous variables, group comparisons were performed using ANOVA followed by Sidak's adjustment for multiple comparisons. Experiments involving measurements over time were analyzed using repeated measures ANOVA with the Greenhouse-Geisser adjustment.
RESULTS
Ectopic expression of c-Cbl WT inhibits cell proliferation and motility
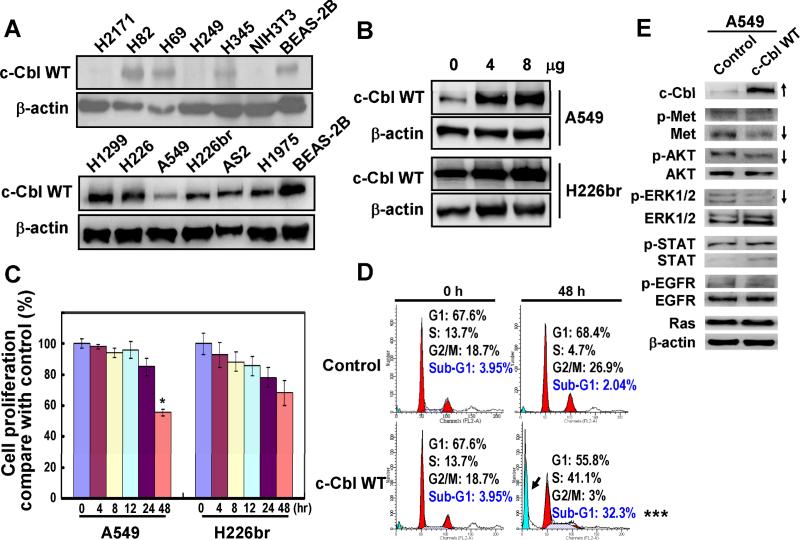
Western blot analysis was performed to examine the expression of c-Cbl protein in various lung cancer cell lines. H2171, H249, A549, H226br, AS2 and H1975 cells showed lower c-CBL expression than the BEAS-2B bronchial epithelial cell (Fig. 1A). Therefore, A549 and H226br cells were selected as cell models for further investigations. Transient transfection with 8 μg c-Cbl WT was used for all assays because it showed the highest expression of ectopic c-Cbl protein (Fig. 1B). The cell growth results showed that c-Cbl WT inhibited cell proliferation (Fig. 1C) and induced sub-G1 population (Fig. 1D), possibly via reduction of total c-Met protein level and phospho-AKT/ERK survival signaling (Fig. 1E) in A549 cells at 48 hr post-transfection.
Fig. 1. Cell proliferation and signaling experiments of c-Cbl WT transfection in A549 and H226br cells.
(A) Western blot analysis for c-Cbl expression in NSCLC cells and normal BEAS-2B cells. (B) A549 and H226br cells were transfected with 0, 4 and 8μg c-Cbl WT expression vector for 48 hr. (C) Cell proliferation assay and (D) flow cytometry indicated that c-Cbl WT expression inhibited cancer cell growth and induced sub-G1 phase in A459 cells. *, P<0.05; ***, P<0.001. (E) Western blot showing the effects of c-Cbl WT expression in A549 cells on cellular signaling.
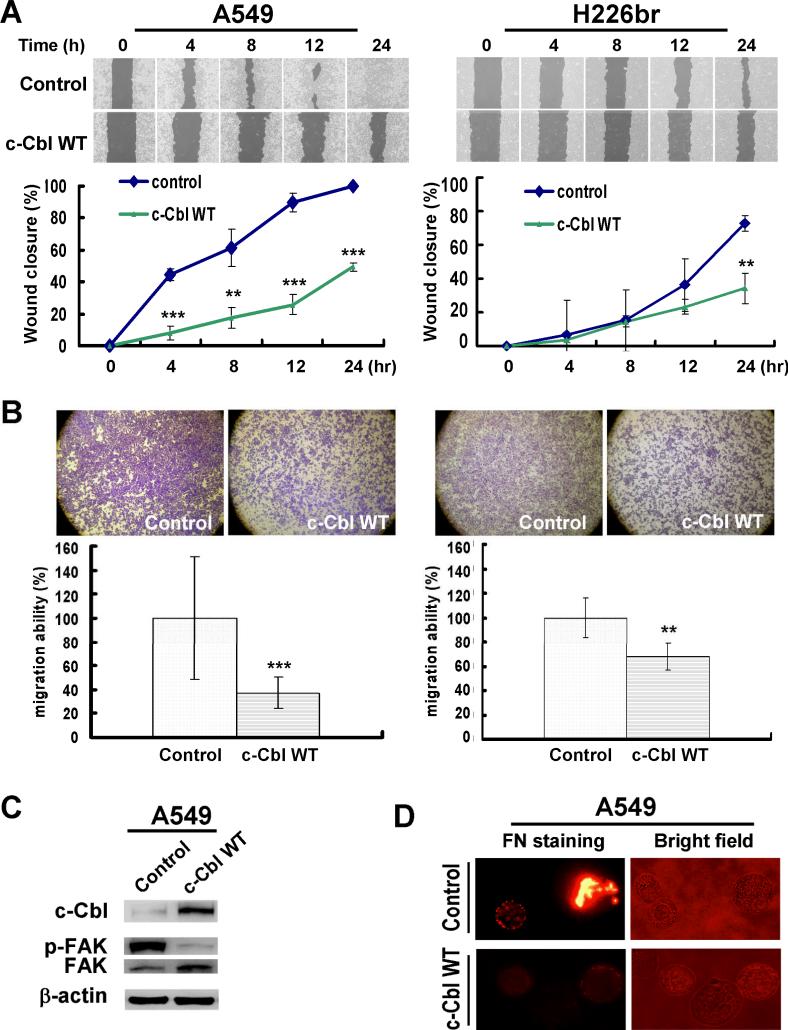
We performed wound healing and transwell migration assays on A549 and H226br cells transiently transfected with c-Cbl WT expression vector. The wound gaps of c-Cbl WT transfection in both A549 and H226br were all significantly larger than control cells transfected with empty vector at 24 hr post-transfection (Fig. 2A). Transwell migration assay confirmed that c-Cbl WT expression inhibited migration ability of both cells (Fig. 2B). Ectopic expression of c-Cbl WT in A549 cells inhibited phospho-FAK signaling (Fig. 2C) and pericellular poly-fibronectin assemblies on cell surface (Fig. 2D) at 48 hr post-transfection compared to the control.
Fig. 2. Migration assays of c-Cbl WT transfection in A549 and H226br cells.
(A) Wound closure was monitored at the indicated times in cells transfected with control vector and c-Cbl WT vector (upper). The wound closure was quantified and normalized to 0 hr (lower). (B) The cells on the transwell membranes were monitored at 24 h after c-Cbl WT transfection (upper). The migration ability was quantified and normalized to control group (lower). **, P<0.01; *** P<0.001. (C) Western blot and (D) Immunoflurescence assay of pericellular fibronectin (FN) in A549 cells expressing c-Cbl WT for 48 hr.
c-Cbl WT transfection effectively inhibits tumor growth in animal model
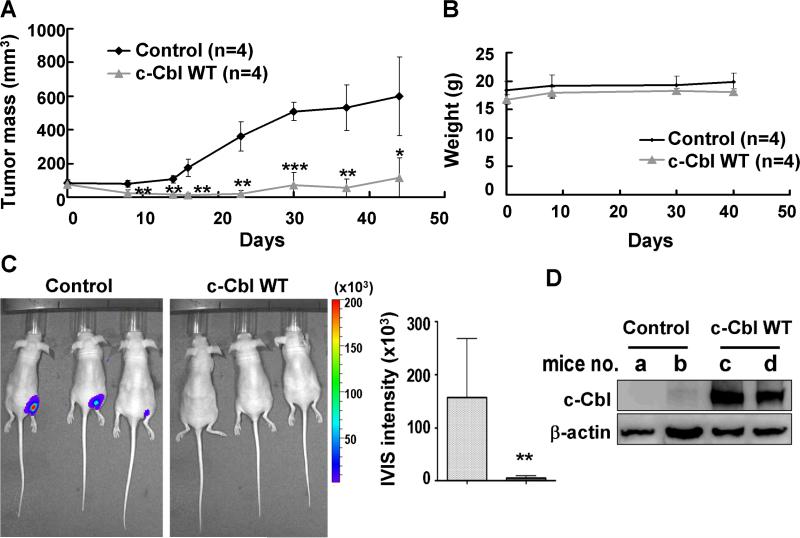
To examine whether c-Cbl WT could inhibit in vivo tumor growth, we first transfected c-Cbl WT into A549 or H1299-luc cells and implanted them subcutaneously into nude mice. Animals with A549 xenograft expressed c-Cbl WT resulted in significant reduction of tumor mass compared with control group without changes in body weight (Figs 3A and 3B). Tissue Western blots demonstrated that ectopic expressed c-Cbl WT remained overexpression at 44 days after xenograft implantation (Fig. 3D). c-Cbl WT transfection-induced anti-tumor growth was confirmed by the reduction of luciferase intensity of H1299-luc xenograft (Fig. 3C).
Fig. 3. c-Cbl WT transfection inhibits the growth of A549 and H1299-luc xenografts.
(A) Tumor mass and (B) body weight of mice injected subcutaneously with A549 cells transfected with c-Cbl WT or empty vector (Control). Tumor nodules were small in c-Cbl WT group compared with control but body weight remained unchanged. (C) The treated H1299-luc cells were injected into mice and observed for the luciferase signals and photographed using IVIS50 for 13 days after cell injection (left). Quantitation results showed that c-Cbl WT significantly inhibited tumor growth (right). (D) Tissue Western blot showed higher c-Cbl expression in A549 xenograft of c-Cbl WT group than control group after mice were sacrificed at the 44th day. *, P<0.05; *, P< 0.01; *** P< 0.001.
c-Cbl WT transfection effectively inhibits tumor metastasis in animal model
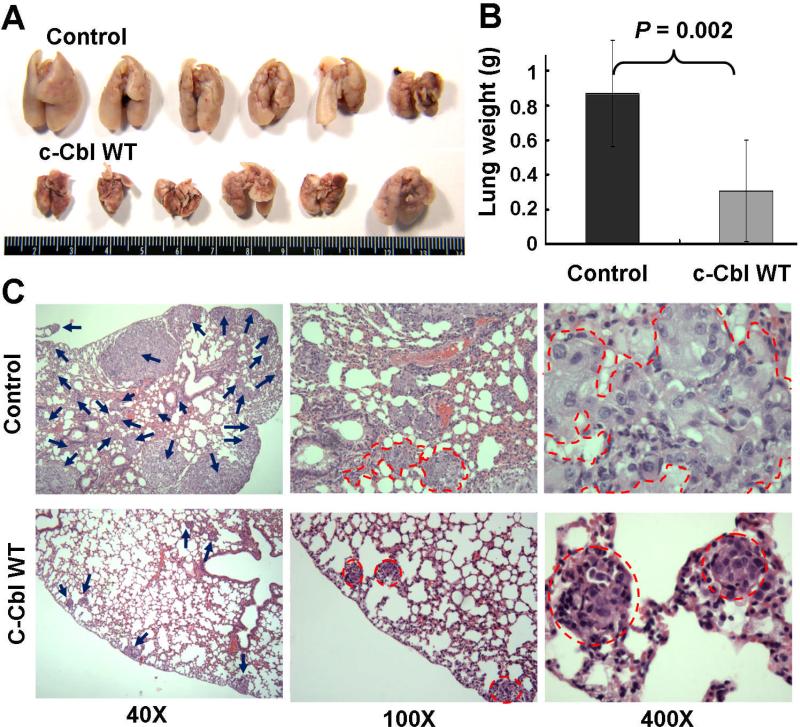
To examine whether c-Cbl WT could inhibit tumor metastatic in vivo, A549 cells with or without ectopic expression of c-Cbl WT were tail-vein injected into nude mice. After six weeks, animals were sacrificed for lung tissue examination. c-Cbl WT transfection group showed a significant decrease in metastatic lung tissue weight compared to control group (Figs 4A and 4B). H&E staining results demonstrated that the number and sizes of metastatic tumor colonies in the lungs of nude mice receiving control A549 cells were significantly higher than those receiving c-Cbl WT A549 cells (Fig. 4C).
Fig. 4. Study of metastasis in animal model and H&E staining of A549 xenograft.
(A) Tissue images and (B) lung weight measurements of mice receiving tail-vein injected control or c-Cbl WT A549 cells. (C) H&E staining of metastatic tumor colonies (arrows) in the lungs (40X). Tumor boundaries of selected colonies (red lines) are shown (100X and 400X).
DISCUSSION
c-Cbl E3 ubquitin ligase induces internalization and ubiquitination of RTK such as c-Met and EGFR (7). The signaling experiment using Western blots showed that overexpression of c-Cbl WT decreased total c-Met protein level but not EGFR. This might be because c-Cbl only represents one aspect of EGFR post-translational regulation (12). Further studies to examine whether transduction of c-Cbl WT is also effective in NSCLC cells with c-Met overexpression or EGFR mutation are warranted.
c-Cbl has been shown to target other kinases including PDGF, CSF-1, and Src (13). c-Cbl also functions as a signal transduction molecule affecting pathways such as RAS, PI3K/AKT, and STAT (13). Although we did not see changes in RAS and phospho-STAT status, we can not rule out other potential effects as contributing factors.
In this study, we demonstrate for the first time in lung cancer that ectopic expression of c-Cbl WT inhibits tumor growth and metastasis in vivo. c-Cbl WT-induced inhibition of tumor growth may be mediated by down-regulation of phospho-AKT (survival) and phospho-ERK1/2 (proliferation-differentiation) signaling (13). In addition, anti-metastasis effect may by through inhibition of phospho-FAK (motility control) and pericellular poly-fibronectin assemblies (tumor colonization) (11). Loss of c-Cbl function in AML and myelodysplastic syndrome supports the potential clinical therapeutic value of c-Cbl WT gene therapy in hematologic malignancies other than lung cancer.
Acknowledgments
Grant supports: This work was supported in part by grant NSC 99-2628-B-006 -004 -MY3 and grant DOH98-TD-G-111-024 from (YCW) and NCI 5R01CA125541-04 (RS).
Footnotes
The first 2 authors contributed equally to this article.
REFERENCES
- 1.Fu YN, Yeh CL, Cheng HH, et al. EGFR mutants found in non-small cell lung cancer show different levels of sensitivity to suppression of Src: implications in targeting therapy. Oncogene. 2008;27:957–965. doi: 10.1038/sj.onc.1210684. [DOI] [PubMed] [Google Scholar]
- 2.Shtiegman K, Kochupurakkal BS, Zwang Y, et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–6978. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 3.Peschard P, Fournier TM, Lamorte L, et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell. 2001;8:995–1004. doi: 10.1016/s1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 4.Pennock S, Wang Z. A tale of two Cbls: interplay of c-Cbl and Cbl-b in epidermal growth factor receptor downregulation. Mol Cell Biol. 2008;28:3020–3037. doi: 10.1128/MCB.01809-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 6.Bacher U, Haferlach C, Schnittger S, Kohlmann A, Kern W, Haferlach T. Mutations of the TET2 and CBL genes: novel molecular markers in myeloid malignancies. Ann Hematol. 2010;89:643–652. doi: 10.1007/s00277-010-0920-6. [DOI] [PubMed] [Google Scholar]
- 7.Sargin B, Choudhary C, Crosetto N, et al. Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood. 2007;110:1004–1012. doi: 10.1182/blood-2007-01-066076. [DOI] [PubMed] [Google Scholar]
- 8.Sanada M, Suzuki T, Shih LY, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460:904–908. doi: 10.1038/nature08240. [DOI] [PubMed] [Google Scholar]
- 9.Tan YH, Krishnaswamy S, Nandi S, et al. CBL is frequently altered in lung cancers: its relationship to mutations in MET and EGFR tyrosine kinases. PLoS One. 2010;5:e8972. doi: 10.1371/journal.pone.0008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truitt L, Freywald T, DeCoteau J, Sharfe N, Freywald A. The EphB6 receptor cooperates with c-Cbl to regulate the behavior of breast cancer cells. Cancer Res. 2010;70:1141–1153. doi: 10.1158/0008-5472.CAN-09-1710. [DOI] [PubMed] [Google Scholar]
- 11.Cheng HC, Abdel-Ghany M, Pauli BU. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem. 2003;278:24600–24607. doi: 10.1074/jbc.M303424200. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z, Du R, Jiang S, et al. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. Br J Cancer. 2008;99:911–922. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]