Abstract
The onset of stroke in children rarely owes to traditional stroke risk factors such as hypertension or diabetes. Rather, stroke in this patient group typically results from the simultaneous occurrence of multiple stroke risk factors, the presence of which necessitates a thorough evaluation. Several challenges exist in the care of children with stroke. Of note, recognition of pediatric stroke onset by parents and caregivers is often delayed, highlighting the need for increased awareness of and education regarding this condition. In terms of diagnostic challenges, various neurological conditions resemble stroke in pediatric patients and definite diagnosis of stroke will require MRI; adding to the challenge, young children may need to be sedated to undergo acute MRI. Perhaps the most significant challenge is the need for clinical research studies focusing on pediatric stroke treatment, so as to allow evidence-based treatment decision-making. A final challenge is standardizing outcome assessment after stroke across a wide range of ages and developmental levels. In this Review, we examine recent findings and diagnostic issues pertaining to both arterial ischemic stroke and hemorrhagic stroke in children.
Introduction
Pediatric stroke is an important cause of long-term disability, with children often living for many years with significant neurological deficits. This condition is also associated with notable acute and chronic health-care costs (Box 1). An often quoted fact is that in children, stroke occurs as frequently as brain tumor.1 Data suggest, however, that stroke is slightly more common than brain tumor in this patient group, with an incidence of 2–13 per 100,000 person-years.2–5 Despite efforts to raise awareness regarding stroke in children, this condition is often overlooked as a cause of symptoms by health-care providers and families.6, 7 Delay in diagnosis is just one of the issues relating to the care of children with stroke. This Review will focus on challenges in the diagnosis and treatment of arterial ischemic stroke (AIS) and hemorrhagic stroke in children, highlighting recent advances in the field and areas where further research is critically needed. AIS is defined as cerebral ischemia conforming to an arterial vascular distribution, while hemorrhagic stroke is defined as spontaneous intraparenchymal hemorrhage, intraventricular hemorrhage or subarachnoid hemorrhage. Arterial ischemic perinatal stroke and hemorrhagic perinatal stroke were excluded from this article, as these entities have different presentations, risk factors and recurrence risk to stroke in older children. Cerebral sinovenous thrombosis was excluded primarily to limit the scope of this work and because an excellent review covering this condition was recently published.8
Box 1. The cost of pediatric stroke.
Using the 2003 data from the Kid’s Inpatient Database, researchers estimated the mean cost of acute hospital care for patients aged 3 months to 20 years with any new stroke to be US$20,927 per discharge.93 When these data were separated by stroke type, the mean cost of acute care for patients with ischemic stroke was $15,003 and for patients with intracerebral hemorrhage was $24,117. Another study examined the 5 year direct costs of stroke in a Northern Californian health-maintenance organization from1996–2003, encompassing inpatient and outpatient health-service costs (including costs from out-of-plan facilities).94 The investigation found that the average adjusted 5 year cost for childhood stroke was $135,161, and that the average cost of a pediatric stroke admission was $81,869. Moreover, the heath-care costs of children with stroke were 15-fold greater than the health-care costs of children without stroke. Althoughpost-hospital costs declined after the first year, they continued to exceed the costsof the age-matched stroke-free control children, even 5 yearspoststroke.94
The health-care costs discussed above are only applicable to children in North America; however, these data emphasize that pediatric stroke has both economic and personal costs, as children with stroke often receive treatment for and will live with the resulting functional deficits for many years.
Etiology and risk factors
Arterial ischemic stroke
The most common etiologies for pediatric AIS include cerebral arteriopathies (Figure 1),9 which are believed to be present in 50–80% of childhood AIS cases,9–11 congenital or acquired cardiac disease, and sickle cell disease.12 Thrombophilias are also important causes of AIS.13, 14 Significant infections such as varicella, sepsis or meningitis have been recognized for many years to be causes of pediatric stroke as well.12, 15 By contrast, the discovery that minor infection is a potential risk factor for childhood AIS constitutes a more recent addition to the literature. A nested case–control study from the Kaiser Pediatric Stroke Study compared non-neonatal cases of pediatric AIS (n = 97) with controls (three controls per case) matched for birth year and primary care facility.16 Exposure was defined as an outpatient visit for a minor infection up to 4 weeks before an ischemic stroke or a visit over the same time period to the care facility by the controls. Encounters for minor infection were documented in 26.6% of AIS cases and 13% of controls (odds ratio (OR) 2.93, 95% CI 1.51–5.68; P>0.001). An additional nine AIS cases but no controls had a serious infection causing meningitis or sepsis. The OR for the association of any infection (major or minor) with AIS was 5.01 (95% CI 2.64–9.54; P<0.0001).16 The possibility that minor infection is a potential risk factor for stroke in children represents a challenge to physicians, given the high incidence of such infections in this patient group. Children who present with AIS often have multiple risk factors for this condition, and the nature of these risk factors shows high variability between individuals.17 Examples of apparently healthy children presenting with acute AIS who are then found to have multiple, unexpected stroke risk factors have been reported, such as a healthy, atheletic school-aged boy found to have atrial fibrillation and a clotting disorder after an embolic AIS in the setting of a febile illness.18 One challenge for clinicians is to determine when all the appropriate risk factors for AIS have been identified in an individual child, so as to initiate appropriate treatment.
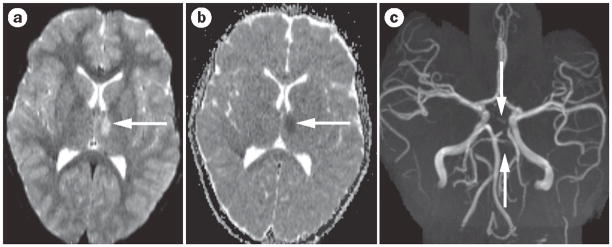
Figure 1.
Arteriopathy in pediatric stroke. a| Diffusion-weighted MRI shows an acute thalamic stroke (bright area; arrow) in a previously healthy 8 year-old girl. b | The apparent diffusion coefficient map is dark and confirms the acute stroke (arrow). c | Time-of-flight, noncontrast magnetic resonance angiography shows narrowing (near occlusion) of the posterior cerebral artery and superior cerebellar arteries on the same side (arrows). Extensive evaluation showed no cause for this arteriopathy.
Hemorrhagic stroke
Hemorrhagic stroke typically includes spontaneous intracerebral hemorrhage (ICH)—parenchymal and intraventricular hemorrhage—and nontraumatic subarachnoid hemorrhage. The most common causes of pediatric hemorrhagic stroke are cerebral vascular abnormalities ranging from 40 to 90% of children with hemorrhagic stroke;11, 19 these abnormalities include arteriovenous malformations (AVMs), cavernous malformations, and aneurysms. Other causes of hemorrhagic stroke such as a bleeding disorder, thrombocytopenia and brain tumor (hemorrhagic stroke may constitute the presenting symptom of brain tumor) are less common.11, 19, 20 Hypertension has also been identified to be a risk factor for ICH in children, but occurs less frequently (and, hence, is a less important risk factor) in this patient group than in adults.21, 22 Recent cohort studies have revealed that, even after thorough patient evaluations, 9–23% of childhood ICH is still considered idiopathic.11, 21
Diagnostic challenges
Delay in diagnosis and stroke mimics
The average time from symptom onset to presentation to the hospital for children with AIS has been reported in older literature to be 24 h.23 More-recent work suggests that much of the delay to diagnosis in children with this condition now occurs within hospitals.6, 7 Rafay et al. found that while the median prehospital delay from symptom onset to presentation in cases of pediatric AIS was 1.7 h, the median in-hospital delay from presentation to diagnosis in such cases was 12.7 h.6
Symptom onset is often rapid and dramatic in children with hemorrhagic stroke, occurring over minutes to hours. At times, however, symptoms of this condition can be insidious, developing over several hours to days.22 In a cohort of children with intraparenchymal hemorrhage, the median time to presentation in hospital was 70 min, although 23% of children presented after 24 h.11
Pediatric stroke is often under-recognized by health-care providers. In the study by Rafay and colleagues, for example, AIS was only suspected on initial assessment in 38% of children.6 One reason healthcare providers may overlook stroke in children is that there is a broad differential diagnosis for many of the non-specific presenting symptoms of childhood stroke such as seizures, headache, and hemiparesis. In fact, a pediatric stroke team examined their acute stroke calls and found that only 21% of children had stroke mimics.24 Among these children, one-third had benign conditions, while two-thirds had serious diagnoses unrelated to stroke, including reversible posterior encephalopathy syndrome, intracranial infection, inflammatory disease and tumor. Note that, in children, hemorrhagic stroke can have a similar presentation to AIS, so it is likely that the information on stroke mimics of AIS apply to hemorrhagic stroke as well.
Neuroimaging challenges
Arterial ischemic stroke
Diffusion-weighted MRI of the brain is the most sensitive method to diagnose acute AIS; however, in children aged <8 years old, sedation or anesthesia may be required to undergo such imaging, as individuals must hold still for an extended period of time. One challenge in imaging pediatric stroke is, therefore, having an appropriate team available at all times for urgent sedation of patients. Delays in neuroimaging may result from the need for sedation.
In general, head CT generally does not require patient sedation, but the sensitivity of this method to detect acute AIS is low. In fact, a study from a large tertiary children’s medical center in Australia found that ischemic stroke was not visualized on head CT in 62 of 74 (84%) children with this condition; all of these children had their stroke confirmed by MRI of the brain.7
Neuroimaging is always tailored to the individual patient with AIS or hemorrhagic stroke, although general guidelines are available. Brain imaging should occur as soon as possible after the stroke. Investigations should include MRI (the gold standard) of the brain, incorporating diffusion-weighted imaging (Figure 1). Vascular imaging of the brain and neck vessels should also be undertaken. In most cases of pediatric AIS, such imaging will involve magnetic resonance (MRA) angiography as the first-line examination, with the addition of magnetic resonance venography (MRV) if venous infarction or cerebral venous sinus thrombosis (CVST) is suspected. CT angiography (CTA) and CT venography (CTV) may also be used to identify vascular abnormalities.
The disadvantages of CTA and CTV, in comparison with MRAand MRV, include exposure to ionizing radiation and iodinated contrast agents. New CTA protocols can reduce the amount of radiation to which a child is exposed. Nevertheless, CTA presents another difficulty; the delivery of intravenous contrast has to be accurately timed to achieve a high quality image. In a child with a small intravenous line, it may not be possible to inject contrast quickly enough to allow imaging.25 Another issue associated with CTA is that some nonsedated children move when the contrast agent is injected, and this movement can reduce the quality of the scan. Advantages of CTA over MRA are that that former is often more widely available and, at many centers, offers excellent and possibly superior visualization of vascular structures.26 Conventional, digital subtraction angiography (DSA) remains the gold standard for vascular imaging27 and may be required for a definitive diagnosis of small-vessel vasculitis, moyamoya disease, arterial stenosis or cervicocephalic arterial dissection.
Hemorrhagic stroke
In a child presenting with stroke-like symptoms, AIS and hemorrhagic stroke are both possible differential diagnoses. If a head CT is not obtained before brain MRI, ‘blood-sensitive’ MRI sequences (that is, gradient echo or susceptibility-weighted imaging [SWI]) can be added to standard MRI protocols to assess for brain hemorrhage. These MRI sequences have not been studied in children with stroke, but in adults with this disorder, these sequences are at least as sensitive as head CT for the detection of blood28 and are often helpful in demonstrating cerebral cavernous malformations (Figure 2). Given the importance of vascular abnormalities as causes of hemorrhagic stroke in children, detailed vascular imaging in this patient group is particularly important. When possible, a complete MRI sequence (including MRI, MRA and MRV) should be performed to look for unusual causes of hemorrhagic stroke, such as high-grade brain tumor or CSVT with hemorrhagic venous infarction. If noninvasive imaging fails to detect the cause of the hemorrhage or a vascular malformation is suspected, DSA should be performed.29
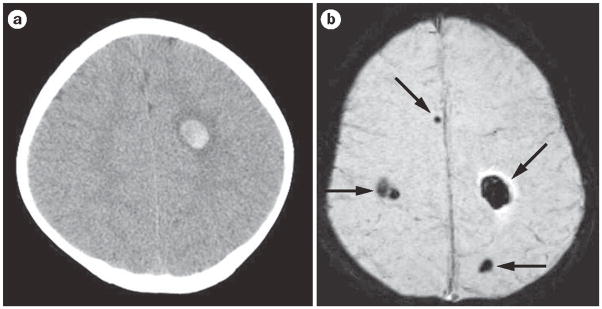
Figure 2.
Acute hemorrhagic stroke and susceptibility-weighted imaging. a | The head CT shows a single, small right frontal acute parenchymal hemorrhage. b | The MRI susceptibility-weighted image shows the right frontal hemorrhage as well as multiple additional hemorrhages not visualized on head CT. This child has multiple cerebral cavernous malformations.
Sometimes brain AVMs are not evident on MRI, MRA or even DSA immediately after an acute hemorrhage. Thus, when vascular imaging is normal or inconclusive in the acute setting, studies should be repeated once the parenchymal hematoma has been reabsorbed; the reabsorption process often takes 2–8 weeks.
Laboratory and cardiac evaluation
Arterial ischemic stroke
As cardioembolic stroke is a major cause of AIS in children, use of echocardiography would seem to be a logical step to identify undetected congenital heart disease. However, the role of patent foramen ovale (PFO) in childhood stroke and the proper echocardiographic technique to detect a PFO or other intracardiac shunt that might predispose to stroke in children is controversial.10, 30 An electrocardiogram (ECG) should be obtained to document the cardiac rhythm in all children with AIS. If arrhythmia is suspected on the basis of the patient’s history or ECG, then longer, Holter or telemetry monitoring is indicated.
The laboratory evaluation of childhood AIS should assess markers of inflammation, hyperlipidemia, rheumatologic disease and thrombosis. Thus, tests for complete blood count, erythrocyte sedimentation rate, C-reactive protein levels, the fasting lipid profile and antinuclear antibody levels should be conducted. A basic thrombophilia evaluation should also be performed.31
A meta-analysis has estimated the impact of thrombophilia on the risk of first-ever childhood stroke, and the resultant findings may prove helpful to clinicians as they decide what tests should be conducted.31 Analysis of data from 22 studies meeting strict methodologic inclusion criteria revealed that several thrombophilia risk factors had a significant association with first-ever stroke in children. An antithrombin deficiency, a protein C deficiency, a protein S deficiency, the factor V (F5) gene mutation 1691G>A (Leiden allele), the factor II (F2) gene mutation 20210G>A and high plasma lipoprotein(a) levels were identified as risk factors for both AIS and CSVT. By contrast, the presence of antiphospholipid antibodies was strongly associated with AIS only (OR 6.95, 95% CI 3.67–13.14). The methylene tetrahydrofolate reductase (MTHFR) gene mutation 677C>T was also associated with AIS only, albeit weakly (OR 1.58, 95% CI 1.20–2.08). Combined thrombophilias had the strongest association with pediatric stroke and CSVT (OR 11.86, 95% CI 5.93–23.73).32
The meta-analysis reveals the strong associations between particular thrombophilia risk factors and pediatric AIS and the importance of multiple concurrent clotting risk factors in determining stroke risk. This analysis possessed, however, several important limitations, as acknowledged by the study’s researchers. The study mostly examinedwhite children; thus, the detected associations may not be applicable to other patient groups. Indeed, F5 1691G>A and F2 20210G>A are rare in black and Asian populations.33, 34 In adult thrombophilia, MTHFR mutations only confer a high risk of AIS in the presence of high homocysteine levels. Homocysteine data were not available in the pediatric cohort examined in the meta-analysis, but the authors of the study advised clinicians to test for homocysteinemia rather than MTHFR mutations, based on adult data.35 Finally, only limited data were available on the risk of recurrent stroke in children with thrombophilia in the cohort of patients examined.
In addition to a careful laboratory evaluation for thrombophilia in suspected cases of pediatric AIS, consideration should be given to performance of both a toxicology screen, particularly for cocaine, and a pregnancy test in girls and young women of childbearing age as these are additional AIS risk factors.
Hemorrhagic stroke
A meta-analysis similar to the one in AIS has not been conducted for hemorrhagic stroke to identify risk factors for bleeding. Thus, recommendations for the laboratory evaluation of childhood hemorrhagic stroke are rudimentary. In a child with this condition, tests should include a platelet count, basic clotting studies (such as determination of the prothrombin time or international normalized ratio) and determination of the activated partial thromboplastin time, with further testing being guided by the family’s history of bleeding disorders and the clinical picture.29 Additional studies, such as metabolic testing and more-detailed testing for inflammatory or rheumatological markers, should be decided on an individual basis.
Treatment options and challenges
Three sets of pediatric stroke guidelines have been published: the Royal College of Physicians guidelines,36 the American College of Chest Physicians guidelines,37 and the American Heart Association (AHA) guidelines.29
Among these guidelines, only those from the AHA discuss hemorrhagic stroke. Several articles have reviewed and compared these guidelines.38, 39 The three sets of guidelines are all limited by the lack of treatment studies that have been conducted for pediatric stroke (with the notable exception of studies focusing on sickle cell disease). Overall, further research into the management of stroke in children is needed.
Supportive care
Supportive measures that have shown benefit in adults with stroke are recommended for children who develop this condition. These measures include maintenance of normal body temperature (via acetaminophen administration and use of cooling blankets), provision of sufficient intravenous fluids to maintain euvolemia,40 and avoidance of hyperglycemia.41
Blood pressure management
The AHA guidelines suggest “control of systemic hypertension” in children with AIS and hemorrhagic stroke.29 This recommendation is based on expert opinion and is reasonable, but specific guidelines for blood pressure values are absent.
Anticonvulsants and EEG monitoring
Seizures are a common complication of pediatric stroke, affecting ≤25% of children withAIS and ≤20% of children with ICH.42 When they occur, seizures should be treated aggressively.29
Prophylactic anticonvulsants are often used in the setting of intraparenchymal or subarachnoid hemorrhage in adults, although this approach is not evidence-based practice. The AHA pediatric stroke guidelines recommend against prophylactic anticonvulsant use in ischemic stroke but do not make recommendations in the setting of hemorrhagic stroke.29 Note that no studies to support treatment or non-treatment with anticonvulsants have been conducted in children.
A study by Messé et al. analyzed data from CHANT for neuroprotection in adults with ICH.43 The researchers found that prophylactic anticonvulsant use in adults with acute intraparenchymal hemorrhage was associated with poor outcomes: however, only 8% of the study participants (n = 23) were placed on prophylactic medication. Similar findings were seen in a prospective observational study of prophylactic anticonvulsant use in 98 adults with ICH.44 In this study, five of seven patients with a clinical seizure had their seizure on the day of their intraparenchymal hemorrhage. Use of phenytoin but not leveteracitam was associated with a longer hospital stay and a poorer score on the modified Rankin Scale (mRS) at 14 days, 28 days, and 3 months compared to patients who were not treated with anticonvulsants. Note that selection bias existed in these studies, as patients who had the largest hemorrhages or who were critically ill were most likely to receive prophylactic anticonvulsants.
Continuous EEG monitoring is often utilized in intensive care units, although the benefit of this technique remains unproven. One study examined 100 children who had continuous EEG monitoring for a diverse array of clinical indications, not specifically ischemic stroke or ICH. Seizure detection via EEG monitoring led to the initiation or escalation of antiseizure medications in 43 patients.45 In many of these children, the indication for EEG monitoring was prolonged unresponsiveness after a clinical seizure. The application of these data to children with acute stroke and no history of seizure is unclear. Nevertheless, continuous EEG monitoring should be considered in children who exhibit a persistently altered mental status that is not clearly explained by their stroke (AIS or hemorrhagic stroke) or demonstrate movements or vital sign changes that are suggestive of seizure but cannot be captured on a routine EEG.
Management of intracranial pressure
A decline in mental status in a child with AIS or hemorrhagic stroke is a worrisome sign, which may indicate a rise in intracranial pressure (ICP). Other symptoms and signs of increased ICP include positional headache (a headache that intensifies when a patient is in a supine position, but improves when they are upright), vomiting, irritability or combativeness, sixth nerve palsies, and papilledema. Cushing’s triad of signs for elevated ICP, comprising hypertension, bradycardia and irregular respirations, is usually a late finding. With AIS, increased ICP may develop several days after stroke onset as infarcted brain tissue becomes edematous. In hemorrhagic stroke, increased ICP may occur acutely, owing to mass effect from a hemorrhage. Increased ICP may also occur in the acute phase or subacutely if an intraventricular hemorrhage is accompanied by communicating hydrocephalus. An intraventricular catheter (IVC) may prove advantageous in some cases of pediatric hemorrhage stroke, providing both a means to measure ICP and, via drainage of cerebrospinal fluid, to manage ICP. As this sort of monitoring requires ventricular enlargement for placement, the insertion of an IVC is not an option for all children with hemorrhagic stroke. A subdural bolt is available for children who cannot have an IVC but require ICP monitoring. In a recent series of children with hemorrhagic stroke, 27% required a ventriculostomy.11
Nonsurgical methods for acutely lowering elevated ICP include keeping the head of a patient’s bed at 30°, so as to promote good cerebral venous drainage, hyperventilation to a pCO2 of 25–30 mmHg to constrict cerebral blood vessels slightly as a means to reduce intracranial blood volume, and hyperosmolar therapy—with either mannitol or hypertonic saline—to promote osmotic diuresis. Plasma osmoles and electrolytes must be monitored frequently when osmotic agents are used to avoid hypovolemia, hypo or hypernatremia and renal failure. In some cases, sedation may be required to help manage elevated ICP. Hyperosmolar therapy and particularly hyperventilation are generally. Corticosteroids should be avoided, since their efficacy for lowering increased ICP has not been demonstrated in adult studies,46, 47 and hyperglycemia resulting from corticosteroid treatment has been associated with poor outcomes in adults with ICH and AIS.41, 48
AIS-specific treatments
Antithrombotic therapy
Antithrombotic therapy includes both antiplatelet (typically aspirin) and anticoagulant (unfractionated heparin, low molecular weight heparin, and warfarin) medications. Of note, the only treatments that limit brain injury after stroke are therapies that promote reperfusion, for example, tPA and mechanical clot retrieval, or reduce metabolic demands (avoiding hyperpyrexia or hyperglycemia); all other interventions are designed for secondary stroke prevention. In non-neonates, treatment with antithrombotic therapy is recommended for secondary stroke prevention.29 Neonates with first-ever AIS but showing no evidence of an ongoing cardioembolic source are not, however, typically treated with antiplatelets or anticoagulants.37
Children with sickle cell disease (SCD) are the exception amongst older children with AIS; aspirin and anticoagulation aren’t typically recommended. Instead, transfusions are recommended to lower the percentage of sickle hemoglobin to <30%; this evidence-based guideline encompasses both secondary stroke prevention and primary stroke prevention.49–51 Evidence for acute transfusion in the setting of first-ever AIS is not as strong, although acute transfusions are commonly performed in clinical practice.52
For non-SCD-related childhood stroke, the American College of Chest Physicians guidelines recommend initial anticoagulation or aspirin therapy at 1–5 mg/kg/day until cervicocephalic arterial dissection or cardioembolic causes have been excluded, followed by long-term aspirin therapy for a minimum of 2 years.37 All major guidelines recommend consideration of anticoagulation (3–6 months treatment with low molecular weight heparin or warfarin) for confirmed cervicalcephalic artery dissection or cardioembolic stroke.29 Anticoagulation is not typically recommended for children with moyamoya disease (Figure 3) or moyamoya syndrome, given the small risk of spontaneous intracerebral hemorrhage.53
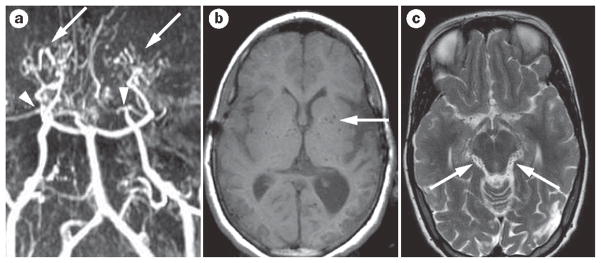
Figure 3.
Idiopathic moyamoya disease. a | Time-of-flight, noncontrast, magnetic resonance angiography shows moyamoya vasculopathy with bilateral occlusion of the internal carotid arteries (arrowheads). Robust lenticulostriate collaterals can also be observed (arrows). b | The lenticulostriate collaterals in moyamoya disease are also seen on the MRI scan, with multiple flow voids piercing the basal ganglia (arrow). c| These moyamoya collateral vessels are also seen as multiple small-flow voids around the brainstem (arrows).
Overall, the choice of anticoagulation or antiplatelet therapy for children with AIS varies geographically, with centers in the US using anticoagulation less often than centers in Australia, Europe and Canada.54 Some investigators argue that these geographic differences in care comprise an equipoise that would allow a randomized clinical trial of aspirin versus anticoagulation for pediatric AIS to be conducted.54, 55
Thrombolytic therapy
Much effort has been devoted to the education of physicians and the public regarding thrombolytic therapy in adult stroke since the landmark National Institute of Neurological Disorders and Stroke trial of intravenous tissue plasminogen activator (tPA);56 however, use of thrombolytic therapy for patients aged <18 years is much more controversial. The current AHA guidelines recommend that tPA use in young children is limited to a clinical trial, and no consensus has been reached regarding the use of this thrombolytic agent in older adolescents who meet standard adult criteria for tPA therapy.29 Evidence for the safety and efficacy of thrombolysis in children with stroke is extremely limited, and existing studies of this treatment approach for systemic clots suggest a high risk of hemorrhagic complications.57 Amlie-LeFond et al. compared published case reports of tPA use in children with tPA use reported as part of the International Pediatric Stroke Study (IPSS).58 The researchers found that children receiving tPA in the IPSS were younger, had a longer time to treatment, and had poorer outcomes than individual cases reported in the medical literature.58 The bias towards publishing cases with the best outcomes is well known. Thus, while some children with AIS may benefit from tPA, good reason exists for a safety and dosing-finding tPA study in children (Figure 4). Such a study is now planned and will require radiological confirmation of acute ischemia or vessel occlusion before tPA is administered.59
Figure 4.
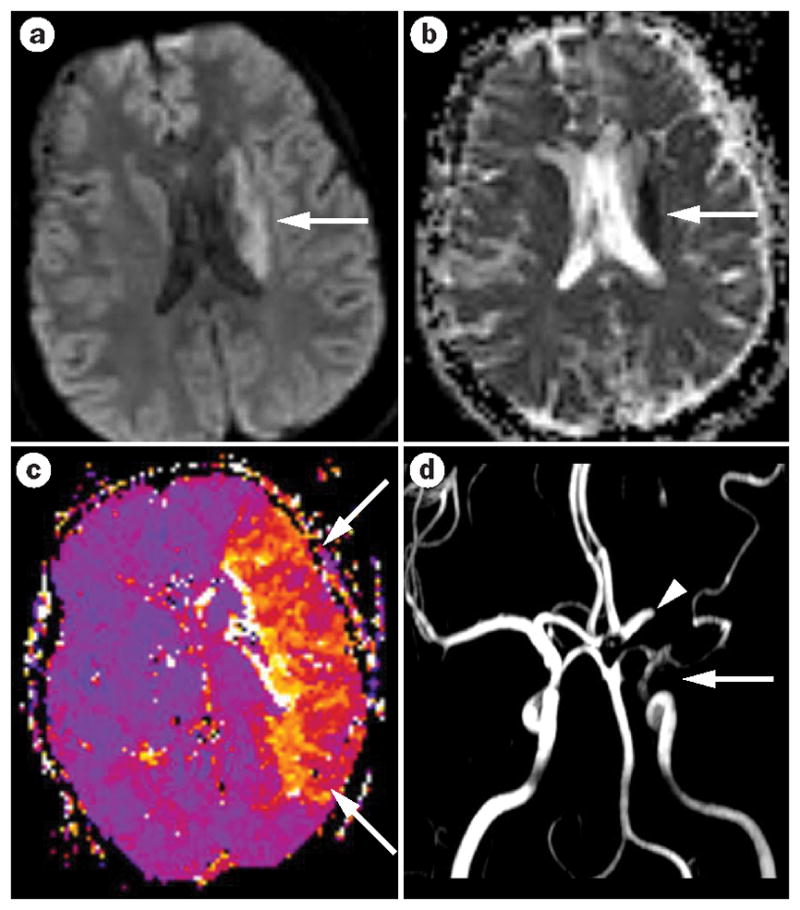
Acute arterial ischemic stroke with diffusion–perfusion mismatch. a| The MRI reveals a small acute stroke that shows restriction on diffusion-weighted imaging in the periventricular region (arrow). b| The corresponding apparent diffusion coefficient map confirms the occurrence of acute ischemia (arrow). c| The perfusion-weighted image shows a large area of hypoperfusion—basically the entire left middle cerebral artery territory (arrow)—representing diffusion–perfusion mismatch. d| This stroke was caused by embolization of a cardiac thrombus that led to partial occlusion of the internal carotid artery—little flow is seen on magnetic resonance angiography (arrow)—and complete occlusion of the middle cerebral artery (arrowhead).
Surgical therapy
Hemicraniectomy may be both life-saving and function-sparing in adults with a large AIS who display a rapid deterioration in level of consciousness or progress to signs and symptoms of impending herniation.60–62 In children, no formal studies of hemicraniectomy have been performed. In a recent case series of 10 children with malignant middle cerebral artery infarction,63 seven underwent hemicraniectomy, all of whom survived and had moderately good recovery (all seven had hemiparesis but were able to walk and had fluent speech despite left-sided infarcts). In this study, time to surgery ranged from 23–291 h in survivors. The lowest Glasgow Coma Scale scores ranged from 4 to 9 in children who survived. The three children who died in this case series did not undergo hemicraniectomy and died of increased ICP. The recommendation was to consider hemicraniectomy in children with malignant middle cerebral artery infarction even in the presence of deep coma.
Hemorrhagic stroke-specific treatment
The role of surgical evacuation of hemorrhage in children and young adults, <45 years of age with ICH is yet another controversial area of stroke therapy. STICH demonstrated that in adult patients with spontaneous supratentorial ICH, emergent surgical evacuation of hematoma within 72 h of bleeding onset did not improve patient outcomes over best medical management.64 STICH required that the local investigator have clinical equipoise for surgical versus medical management. Younger patients (<45 years) with lobar hemorrhages may not have been enrolled in STICH because a small retrospective study had already provided some evidence that early surgery was beneficial in young adults with ICH.65 For these reasons, it is unlikely that the results of the STICH trial are applicable to children. Further, children with ICH may require more urgent intervention than adults with hemorrhage to reduce elevated ICP and to prevent brain herniation (expansion of a hematoma or cerebral edema may rapidly cause such herniation) because they do not have cerebral atrophy that is present in many older adults with ICH.
Recurrence risk and neurological outcomes
Arterial ischemic stroke
Recurrence risk
Among children who suffer a first-ever AIS outside the neonatal period and go untreated, the risk of recurrence is ≈10–25%,12, 66, 67 although this value may be as low as 6% in those with ≤1 risk factors17 or as high as 90% in those with SCD.68
The underlying stroke AIS mechanism seems to be particularly influential in regard to recurrence risk and timing of recurrence. In the Great Ormond Street series, genetic thrombophilia was significantly more common in children with cryptogenic stroke who recurred than in those who did not recur.66 Along the same lines, children with cardiac disease, moyamoya disease or a systemic disease that predisposes to stroke may have recurrence many years after the original stroke, whereas children with transient cerebral arteriopathy or dissection are most likely to have an early recurrence of stroke.69 In a study of children with AIS (n = 97) in CA, USA, the 5 year cumulative risk of AIS recurrence in older children was 19% (95% CI 12–30%), with 12 of the 15 cases of recurrent stroke occurring in children with arteriopathy.12 Thus, a comprehensive evaluation of children with AIS for potential stroke risk factors may allow stratification of children into high-risk and low-risk groups for recurrent stroke, and may afford information about the timing of stroke recurrence.
Neurological outcomes
Neurological sequelae of stroke are present in more than half of children after AIS.70, 71 Motor deficits are the most recognizable deficits after childhood AIS, with cognitive impairments being more subtle. Up to 25% of children with acute stroke will have seizures; however, clear data on the persistence of seizures and development of epilepsy are lacking. Cortical lesions and persistence of seizures beyond 2 weeks of the acute insult have previously been identified as risk factors for secondary epilepsy.72 Young age, male sex and bihemispheric infarction predict poor outcome after AIS.73
The idea is often touted that young brains exhibit more plasticity than adult brains and, therefore, have more capacity for recovery after injury. In studies of childhood AIS however, the younger the age at stroke onset, the poorer the cognitive and behavioral outcomes that have been observed.67, 74, 75 Note that these studies are limited by sample size, their cross-sectional design and the use of age-matched normal controls rather than matched sibling controls.
Hemorrhagic stroke
Recurrence risk
Few studies have commented on the incidence of rebleeding in cases of pediatric hemorrhagic stroke. In one retrospective cohort from Switzerland, comprising of 34 children who presented with hemorrhagic stroke between 1990 and 2000, recurrent ICH occurred in three children (9%).22 All three recurrences occurred within the first year of the incident ICH and were in children with unrepaired vascular anomalies (two individuals) and unknown cause of hemorrhage (one individual). In a population-based cohort of 116 children in Northern California who presented between January 1993 and December 2004, 11 individuals (9.5%) had recurrent ICH.76 The recurrences occurred at a median of 3.1 months from the incident hemorrhage (among all the recurrences, >60% occurred within 6 months of the initial hemorrhage and >90% occurred within 3 years of the incident ICH). Of the children with recurrent ICH, none had idiopathic ICH and nine had vascular anomalies, six of which were never treated. The two children with medical etiologies of ICH both recurred within 1 week of the incident hemorrhage. One child with recurrent ICH had endstage renal disease leading to hypertensive ICH, the other child was a term neonate with idiopathic thrombocytopenia.
Neurological outcomes
Outcome studies in hemorrhagic stroke have been sparse. One study pooled data from non-population-based studies and reported an average mortality rate of 25% in children with hemorrhagic stroke.73 Estimates of the mortality rate from individual studies range from 7–54%.21, 72 A more-modern, population-based study found a hemorrhagic stroke case fatality rate of 5.2%.77
Predictors of poor outcome in hemorrhagic stroke include infratentorial hemorrhage location, Glasgow Coma Score (GCS) ≤7 at admission, aneurysm, age <3 years, and underlying hematological disorder.22 A retrospective study showed that in children with this form of stroke, a large ICH volume predicted a poor outcome.21 A prospective series confirmed the importance of ICH volume for predicting patient outcomes and demonstrated that altered mental status within 6 h of hospital presentation was an added risk factor for poor short-term neurological outcome.11 Bedside methods to estimate hemorrhage volume in children and, thus, to aid clinical outcome prediction have recently been developed.77, 78
One important point to consider is that although children often ‘walk and talk’ after AIS or hemorrhagic stroke, the functional impact of their impairments on daily life may be significant. Similarly, lesions in the developing brain may result in impairments (such as cognitive deficits) that only emerge at the appropriate developmental stage.
Rehabilitation
Like traditional physical, occupational and speech therapies, neuropsychological testing with educational support does not have a strong evidence base, but should still be included in the recommended treatment plan for children with stroke. The AHA and RCP guidelines both support this view as these evaluations are useful in planning educational programs after a child’s stroke.29, 36 Newer, less traditional therapies for pediatric stroke are also available. Constraint-induced movement therapy (CIMT), which involves restraining a patient’s good arm and hand and intensive therapy for the weaker arm and hand, has been shown to be beneficial in adults undergoing rehabilitation for stroke.79, 80 To date, each pediatric CIMT study has involved no more than 55 partcipants. Nevertheless, together these small studies showed that CIMT was moderately effective in improving use of the stroke-affected upper extremity.81–84
Botulinum toxin A (BoNT-A) injections for spasticity have not been studied specifically for children with stroke, but good evidence exists that this agent, when administered as adjunctive therapy to standard physical and occupational therapy, can improve upper extremity function in children with hemiplegic cerebral palsy.84 Predictors of good response to BoNT-A are good grip strength before commencement of treatment and, potentially, young age.85
A small randomized controlled trial (n = 10 in each treatment arm) of repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS in children with subcortical AIS suggested that inhibition of the contralesional cortex could improve hand function.86 A larger study of this technique for the treatment pediatric AIS is ongoing. Transcranial direct-current stimulation has the advantage of not carrying a risk of seizure.87 This technique has shown promise in adult stroke for both motor and language recovery, but has not yet been studied in children.88
Unfortunately, few studies have been undertaken to guide the use of thevarious techniques discussed above in children. Families of children with stroke understandably desire rehabilitation techniques that will improve function, but the lack of data means that health-care providers have little to base recommendations on. Additionally, health-care benefits from third-party payers are often limited or nonexistent for unproven therapies. Further study of rehabilitation techniques for childhood stroke is critically needed.
Future directions
Clinical trials are a priority in pediatric AIS, including studies of antithrombotic medications (aspirin versus anticoagulation), acute antithrombolytic or antifibrinolytic therapy, and rehabilitation methods. Clinical trials of treatments for hemorrhagic stroke are perhaps more distant but are equally necessary.
The NIH stroke scale (NIHSS) has been used successfully to quantify initial stroke severity in adults. The NIHSS has advanced clinical trials for stroke in this patient group because the score provides a baseline measure against which stroke outcomes may be compared and treatment effects may be assessed. A pediatric version of this scale (PedNIHSS) will be available soon (a study to validate the PedNIHSS is nearing completion).89 Hopefully, the PedNIHSS will have similar impact in childhood stroke to the impact NIHSS has had in adult stroke.
Also of great importance is the ability to accurately measure outcome after pediatric stroke across a wide range of ages and developmental levels. The pediatric stroke outcome measure (PSOM) is a validated measure suitable for children aged 2–18 years and comprises a standardized neurological examination performed by a neurologist.90 Nevertheless, a simpler functional scale, similar to the mRS, which is used in adults is also needed.91 The strength of the mRS is that it is a checklist that can be completed by a nurse or other health-care provider.92 A validated pediatric version of this scale or a similar outcome scale would be particularly helpful in clinical trials. More detailed outcome measures to adequately assess for cognitive deficits and behavioral problems will be necessary for more comprehensive outcome assessment.
Conclusions
Pediatric stroke is an important cause of childhood disability. The factors responsible for stroke in children are quite different from those that cause stroke in adults. Arteriopathy, cardiac disease and SCD represent just a few of the potential causes of stroke in children. Many challenges exist in the evaluation and treatment of these children, including nonrecognition of stroke by families and health-care providers, the frequent need for anesthesia support for diagnostic MRI in children, and the absence of treatment studies outside of SCD. The PedNIHSS validation study results will be reported this year. This scale will quantify initial stroke severity and will provide a baseline measure against which stroke outcomes may be compared. Treatment trials are needed and will include a dose-finding and safety study of acute AIS treatment with tPA, starting this year, as well as ideally, a trial comparing aspirin versus warfarin or low molecular weight heparin for secondary stroke prevention in the future. To assess properly whether various treatments truly improve outcome, well-validated, easily applied outcome measures will need to be developed.
Key points.
Common risk factors for pediatric arterial ischemic stroke include cerebral arteriopathies, congenital or acquired cardiac disease, and sickle cell disease
Cerebral vascular abnormalities are the most common causes of hemorrhagic stroke in children
A combination of multiple, seemingly minor risk factors may lead to stroke in childhood Delayed recognition of stroke in children by families and health-care providers is a significant issue for both medical care and clinical trials
Clinical trials examining treatment strategies—particularly antithrombotic medications (aspirin versus anticoagulation) and rehabilitation methods—are needed for pediatric stroke
Acknowledgments
L. C. Jordan (grant K23NS062110) and A. E. Hillis (grants RO1 NS047691 and RO1 DC05375) receive funding from the NIH.
Footnotes
Competing interests L. C. Jordan declares an association with the following company: Berlin Heart. See the article online for full details of the relationship. A. E. Hillis declares no competing interests.
L. C. Jordan has acted as a consultant for Berlin Heart. A. E. Hillis declares no competing interests.
Author contributions
L. C. Jordan researched the data for the article, provided a substantial contribution to discussions of the content, wrote the article and contributed to review and/or editing of the manuscript before submission. A. E. Hillis provided a substantial contribution to discussions of the content and contributed to review and/or editing of the manuscript before submission.
References
- 1.Kaatsch P, Rickert CH, Kuhl J, Schuz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001;92:3155–64. doi: 10.1002/1097-0142(20011215)92:12<3155::aid-cncr10158>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 3.Giroud M, et al. Stroke in children under 16 years of age. Clinical and etiological difference with adults. Acta Neurologica Scandinavica. 1997;96:401–406. doi: 10.1111/j.1600-0404.1997.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 4.Chung B, Wong V. Pediatric stroke among Hong Kong Chinese subjects. Pediatrics. 2004;114:e206–212. doi: 10.1542/peds.114.2.e206. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke; a journal of cerebral circulation. 2009;40:3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafay MF, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40:58–64. doi: 10.1161/STROKEAHA.108.519066. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124:e227–234. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 8.Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurgery clinics of North America. 2010;21:511–527. doi: 10.1016/j.nec.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amlie-Lefond C, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Annals of Neurology. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 11.Beslow LA, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke; a journal of cerebral circulation. 2010;41:313–318. doi: 10.1161/STROKEAHA.109.568071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 13.Strater R, et al. Genetic risk factors of thrombophilia in ischaemic childhood stroke of cardiac origin. A prospective ESPED survey. European journal of pediatrics. 1999;158 (Suppl 3):S122–125. doi: 10.1007/pl00014336. [DOI] [PubMed] [Google Scholar]
- 14.Nowak-Gottl U, et al. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 1999;94:3678–3682. [PubMed] [Google Scholar]
- 15.Sebire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: a case-control study. Annals of Neurology. 1999;45:679–680. doi: 10.1002/1531-8249(199905)45:5<679::aid-ana22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Hills NK, Johnston SC, Sidney S, Fullerton HJ. Minor infection predisposes to arterial ischemic stroke in children. Stroke. 2009;40:e124. [Google Scholar]
- 17.Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: the coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54:371–378. doi: 10.1212/wnl.54.2.371. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman JT, Gailloud P, Jordan LC. Recovery from spatial neglect and hemiplegia in a child despite a large anterior circulation stroke and Wallerian degeneration. Journal of child neurology. 2010;25:500–503. doi: 10.1177/0883073809339060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: etiology and presentation. Journal of child neurology. 2000;15:284–289. doi: 10.1177/088307380001500503. [DOI] [PubMed] [Google Scholar]
- 20.Jordan LC, Johnston SC, Wu YW, Sidney S, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke. 2009;40:400–5. doi: 10.1161/STROKEAHA.108.518761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke; a journal of cerebral circulation. 2009;40:1666–1671. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain & development. 2003;25:416–421. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 23.Gabis LV, Yangala R, Lenn NJ. Time lag to diagnosis of stroke in children. Pediatrics. 2002;110:924–928. doi: 10.1542/peds.110.5.924. [DOI] [PubMed] [Google Scholar]
- 24.Shellhaas RA, Smith SE, O’Tool E, Licht DJ, Ichord RN. Mimics of childhood stroke: characteristics of a prospective cohort. Pediatrics. 2006;118:704–709. doi: 10.1542/peds.2005-2676. [DOI] [PubMed] [Google Scholar]
- 25.Bowen BC. MR angiography versus CT angiography in the evaluation of neurovascular disease. Radiology. 2007;245:357–60. doi: 10.1148/radiol.2452061706. discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 26.Truwit CL. CT angiography versus MR angiography in the evaluation of acute neurovascular disease. Radiology. 2007;245:362–6. doi: 10.1148/radiol.2452061670. discussion 366. [DOI] [PubMed] [Google Scholar]
- 27.Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003;52:624–31. doi: 10.1227/01.neu.0000047895.82857.eb. discussion 630–1. [DOI] [PubMed] [Google Scholar]
- 28.Kidwell CS, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA: the journal of the American Medical Association. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 29.Roach ES, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke; a journal of cerebral circulation. 2008;39:2644–2691. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 30.Dowling MM, Ikemba CM. Intracardiac shunting and stroke in children: a systematic review. J Child Neurol. 2011;26:72–82. doi: 10.1177/0883073810383913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beslow LA, Jordan LC. Pediatric stroke: the importance of cerebral arteriopathy and vascular malformations. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2010;26:1263–1273. doi: 10.1007/s00381-010-1208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenet G, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121:1838–1847. doi: 10.1161/CIRCULATIONAHA.109.913673. [DOI] [PubMed] [Google Scholar]
- 33.Pepe G, et al. Prevalence of factor V Leiden mutation in non-European populations. Thromb Haemost. 1997;77:329–31. [PubMed] [Google Scholar]
- 34.Hessner MJ, et al. Prevalence of prothrombin G20210A, factor V G1691A (Leiden), and methylenetetrahydrofolate reductase (MTHFR) C677T in seven different populations determined by multiplex allele-specific PCR. Thromb Haemost. 1999;81:733–8. [PubMed] [Google Scholar]
- 35.Varga EA, Sturm AC, Misita CP, Moll S. Cardiology patient pages. Homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation. 2005;111:e289–93. doi: 10.1161/01.CIR.0000165142.37711.E7. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed February 8, 2011]; http://www.gosh.nhs.uk/clinical_information/clinical_guidelines/cmg_guideline_00058.
- 37.Monagle P, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]
- 38.DeVeber G, Kirkham F. Guidelines for the treatment and prevention of stroke in children. Lancet neurology. 2008;7:983–985. doi: 10.1016/S1474-4422(08)70231-X. [DOI] [PubMed] [Google Scholar]
- 39.Eleftheriou D, Ganesan V. Controversies in childhood arterial ischemic stroke and cerebral venous sinus thrombosis. Expert review of cardiovascular therapy. 2009;7:853–861. doi: 10.1586/erc.09.41. [DOI] [PubMed] [Google Scholar]
- 40.Broderick JP, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–15. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 41.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ (Clinical research ed) 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan LC, Hillis AE. Hemorrhagic stroke in children. Pediatric neurology. 2007;36:73–80. doi: 10.1016/j.pediatrneurol.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messe SR, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocritical care. 2009;11:38–44. doi: 10.1007/s12028-009-9207-y. [DOI] [PubMed] [Google Scholar]
- 44.Naidech AM, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2009;40:3810–3815. doi: 10.1161/STROKEAHA.109.559948. [DOI] [PubMed] [Google Scholar]
- 45.Abend NS, et al. Impact of Continuous EEG Monitoring on Clinical Management in Critically Ill Children. Neurocritical care. 2010 doi: 10.1007/s12028-010-9380-z. epub May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poungvarin N, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. The New England Journal of Medicine. 1987;316:1229–1233. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- 47.Tellez H, Bauer RB. Dexamethasone as treatment in cerebrovascular disease. 1. A controlled study in intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 1973;4:541–546. doi: 10.1161/01.str.4.4.541. [DOI] [PubMed] [Google Scholar]
- 48.Passero S, Ciacci G, Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;61:1351–1356. doi: 10.1212/01.wnl.0000094326.30791.2d. [DOI] [PubMed] [Google Scholar]
- 49.Adams RJ, Brambilla D. Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I., Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. The New England Journal of Medicine. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 50.Adams RJ, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. The New England Journal of Medicine. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 51.Enninful-Eghan H, Moore RH, Ichord R, Smith-Whitley K, Kwiatkowski JL. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. The Journal of pediatrics. 2010;157:479–484. doi: 10.1016/j.jpeds.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hulbert ML, et al. Exchange blood transfusion compared with simple transfusion for first overt stroke is associated with a lower risk of subsequent stroke: a retrospective cohort study of 137 children with sickle cell anemia. The Journal of pediatrics. 2006;149:710–712. doi: 10.1016/j.jpeds.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 53.Scott RM, et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. Journal of neurosurgery. 2004;100:142–149. doi: 10.3171/ped.2004.100.2.0142. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg NA, et al. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet neurology. 2009;8:1120–1127. doi: 10.1016/S1474-4422(09)70241-8. [DOI] [PubMed] [Google Scholar]
- 55.DeVeber G. In pursuit of evidence-based treatments for paediatric stroke: the UK and Chest guidelines. Lancet neurology. 2005;4:432–436. doi: 10.1016/S1474-4422(05)70120-4. [DOI] [PubMed] [Google Scholar]
- 56.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. The New England Journal of Medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 57.Monagle P, Chan A, Massicotte P, Chalmers E, Michelson AD. Antithrombotic therapy in children: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:645S–687S. doi: 10.1378/chest.126.3_suppl.645S. [DOI] [PubMed] [Google Scholar]
- 58.Amlie-Lefond C, et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet neurology. 2009;8:530–536. doi: 10.1016/S1474-4422(09)70106-1. [DOI] [PubMed] [Google Scholar]
- 59.Amlie-Lefond C, et al. Thrombolysis in acute childhood stroke: design and challenges of the thrombolysis in pediatric stroke clinical trial. Neuroepidemiology. 2009;32:279–286. doi: 10.1159/000203076. [DOI] [PubMed] [Google Scholar]
- 60.Ruf B, et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: results of a pilot study. Critical care (London, England) 2003;7:R133–8. doi: 10.1186/cc2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004;55:55–61. doi: 10.1227/01.neu.0000126875.02630.36. discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 62.Vahedi K, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet neurology. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 63.Smith SE, et al. Outcome following decompressive craniectomy for malignant middle cerebral artery infarction in children. Developmental medicine and child neurology. 2011;53:29–33. doi: 10.1111/j.1469-8749.2010.03775.x. [DOI] [PubMed] [Google Scholar]
- 64.Mendelow AD, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 65.Rabinstein AA, Atkinson JL, Wijdicks EF. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: to what purpose? Neurology. 2002;58:1367–1372. doi: 10.1212/wnl.58.9.1367. [DOI] [PubMed] [Google Scholar]
- 66.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 67.De Schryver EL, Kappelle LJ, Jennekens-Schinkel A, Boudewyn Peters AC. Prognosis of ischemic stroke in childhood: a long-term follow-up study. Developmental medicine and child neurology. 2000;42:313–318. doi: 10.1017/s0012162200000554. [DOI] [PubMed] [Google Scholar]
- 68.Russell MO, et al. Effect of transfusion therapy on arteriographic abnormalities and on recurrence of stroke in sickle cell disease. Blood. 1984;63:162–169. [PubMed] [Google Scholar]
- 69.Chabrier S, Husson B, Lasjaunias P, Landrieu P, Tardieu M. Stroke in childhood: outcome and recurrence risk by mechanism in 59 patients. Journal of child neurology. 2000;15:290–294. doi: 10.1177/088307380001500504. [DOI] [PubMed] [Google Scholar]
- 70.Hogan AM, Kirkham FJ, Isaacs EB. Intelligence after stroke in childhood: review of the literature and suggestions for future research. Journal of child neurology. 2000;15:325–332. doi: 10.1177/088307380001500509. [DOI] [PubMed] [Google Scholar]
- 71.Ganesan V, et al. Outcome after ischaemic stroke in childhood. Developmental medicine and child neurology. 2000;42:455–461. doi: 10.1017/s0012162200000852. [DOI] [PubMed] [Google Scholar]
- 72.Yang JS, Park YD, Hartlage PL. Seizures associated with stroke in childhood. Pediatric neurology. 1995;12:136–138. doi: 10.1016/0887-8994(94)00152-r. [DOI] [PubMed] [Google Scholar]
- 73.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. Journal of child neurology. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 74.Everts R, et al. Cognitive functioning, behavior, and quality of life after stroke in childhood. Child neuropsychology: a journal on normal and abnormal development in childhood and adolescence. 2008;14:323–338. doi: 10.1080/09297040701792383. [DOI] [PubMed] [Google Scholar]
- 75.Westmacott R, Askalan R, MacGregor D, Anderson P, Deveber G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Developmental medicine and child neurology. 2010;52:386–393. doi: 10.1111/j.1469-8749.2009.03403.x. [DOI] [PubMed] [Google Scholar]
- 76.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: a population-based cohort study. Stroke; a journal of cerebral circulation. 2007;38:2658–2662. doi: 10.1161/STROKEAHA.107.481895. [DOI] [PubMed] [Google Scholar]
- 77.Beslow LA, et al. ABC/XYZ estimates intracerebral hemorrhage volume as a percent of total brain volume in children. Stroke; a journal of cerebral circulation. 2010;41:691–694. doi: 10.1161/STROKEAHA.109.566430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kleinman JT, Hillis AE, Jordan LC. ABC/2: estimating intracerebral haemorrhage volume and total brain volume, and predicting outcome in children. Dev Med Child Neurol. 2011;53:281–4. doi: 10.1111/j.1469-8749.2010.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf SL, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA: the journal of the American Medical Association. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 80.Wolf SL, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet neurology. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyd R, et al. INCITE: A randomised trial comparing constraint induced movement therapy and bimanual training in children with congenital hemiplegia. BMC neurology. 2010;10:4. doi: 10.1186/1471-2377-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aarts PB, Jongerius PH, Geerdink YA, van Limbeek J, Geurts AC. Effectiveness of modified constraint-induced movement therapy in children with unilateral spastic cerebral palsy: a randomized controlled trial. Neurorehabilitation and neural repair. 2010;24:509–518. doi: 10.1177/1545968309359767. [DOI] [PubMed] [Google Scholar]
- 83.Deluca SC, Echols K, Law CR, Ramey SL. Intensive pediatric constraint-induced therapy for children with cerebral palsy: randomized, controlled, crossover trial. Journal of child neurology. 2006;21:931–938. doi: 10.1177/08830738060210110401. [DOI] [PubMed] [Google Scholar]
- 84.Hoare BJ, et al. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE) Cochrane database of systematic reviews (Online) 2010;(1):CD003469. doi: 10.1002/14651858.CD003469.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fehlings D, Rang M, Glazier J, Steele C. Botulinum toxin type A injections in the spastic upper extremity of children with hemiplegia: child characteristics that predict a positive outcome. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2001;8 (Suppl 5):145–149. doi: 10.1046/j.1468-1331.2001.00047.x. [DOI] [PubMed] [Google Scholar]
- 86.Kirton A, et al. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet neurology. 2008;7:507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- 87.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of Neurology. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke; a journal of cerebral circulation. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ichord RN, et al. Interrater Reliability of the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) in a Multicenter Study. Stroke; a journal of cerebral circulation. 2011 doi: 10.1161/STROKEAHA.110.607192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitchen L, et al. A validation study of the paediatric stroke outcome measure. Stroke. 2003;34:Abstract #P331–316. [Google Scholar]
- 91.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke; a journal of cerebral circulation. 2009;40:3393–3395. doi: 10.1161/STROKEAHA.109.557256. [DOI] [PubMed] [Google Scholar]
- 92.Saver JL, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA) Stroke; a journal of cerebral circulation. 2010;41:992–995. doi: 10.1161/STROKEAHA.109.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perkins E, Stephens J, Xiang H, Lo W. The cost of pediatric stroke acute care in the United States. Stroke. 2009;40:2820–7. doi: 10.1161/STROKEAHA.109.548156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gardner MA, Hills NK, Sidney S, Johnston SC, Fullerton HJ. The 5-year direct medical cost of neonatal and childhood stroke in a population-based cohort. Neurology. 2010;74:372–378. doi: 10.1212/WNL.0b013e3181cbcd48. [DOI] [PMC free article] [PubMed] [Google Scholar]