Abstract
Background and Purpose
Cyclooxygenase-2 (COX-2) and Microsomal Prostaglandin E2 Synthase-1 (mPGES-1) catalyze isomerization of the cyclooxygenase product PGH2 into PGE2. Deletion of COX-2/mPGES-1 suppresses carotid artery atherogenesis, angiotensin II-induced aortic aneurysms formation, and attenuates neointimal hyperplasia after vascular injury in mice. The upregulation of COX-2/mPGES-1 in the wall of ruptured human cerebral aneurysms is not known.
Methods
Ten patients with intracranial aneurysms (five ruptured and five non-ruptured) underwent microsurgical clipping. During the procedure, a segment of the aneurysm dome was resected and immunostained with monoclonal antibodies for COX-1, COX2 and mPGES-1. A segment of the superficial temporal artery (STA) was also removed and immunostained with monoclonal antibodies for COX-1, COX2 and mPGES-1.
Results
All ten aneurysm tissues stained positive for mPGES-1 monoclonal antibody. Expression of mPGES-1 was more abundant in ruptured aneurysm tissue than non-ruptured aneurysms, based on a semiquantitative grading. None of the STA specimens expressed mPGES-1. COX-2 was upregulated in the same distribution as mPGES-1. COX-1 was present constitutively in all tissues.
Conclusion
COX-2/mPGES-1 are expressed in the wall of human cerebral aneurysms and more abundantly in ruptured aneurysms than non-ruptured. We speculate that the protective effect of aspirin against rupture of cerebral aneurysms may be mediated in part by inhibition of COX-2/mPGES-1
Keywords: Aneurysm, mPGES-1, inflammation, COX-2, COX-1
Introduction
The etiology of saccular intracranial artery aneurysm (IA) is not clear. Several studies in humans and experimental animal on intracranial aneurysms support the hypothesis that chronic inflammation contributes to degeneration of intracranial aneurysms and potentially may increase the risk of rupture 1-3. We recently reported that daily intake of aspirin reduces the incidence of human cerebral aneurysm rupture by 60% 4. The mechanism by which aspirin exerts this surprising effect is not clear.
Arachidonic acid is metabolized by cyclooxygenases to prostaglandin (PG) H2, which is converted to specific PGs. COX-1, COX-2 and mPGES-1 catalyze the isomerization of PGH2 into PGE2 and PGI2. COX-1 is responsible for baseline levels of prostaglandins and inflammation induces expression of COX-2 (5). Both COX-1 and COX-2 are inhibited by aspirin 5.
Aoki et al showed the presence of COX-2, mPGES-1, and prostaglandin E receptor 2 (EP2) in endothelial cells in the walls of unruptured cerebral aneurysms 6. They also demonstrated that inhibition or loss of COX-2 or EP2 attenuated inflammation and reduced the incidence of aneurysm formation in rats and mice with cerebral aneurysm 6. Recent studies also indicate that deletion of mPGES-1, which is one step downstream from COX-2, suppresses carotid artery atherogenesis, angiotensin II-induced aortic aneurysm formation, and attenuates neointimal hyperplasia after vascular injury in mice 7-11.
The purpose of this study was to extend these findings to test the hypothesis that expression of COX-1, COX-2 and mPGES-1 are upregulated in ruptured human intracranial aneurysms.
Methods
The study was approved by University of Iowa Institutional Review Board (IRB). Ten consecutive patients with intracranial aneurysms who underwent microsurgical clipping were identified during a six months interval. No patients were excluded, except patients who had coiling of their aneurysm. Five patients with non-ruptured aneurysms and five patients with ruptured aneurysms were included in the study. Mean age was 55 (range: 44-67 years old) (Table 1). Informed consent was obtained and the patients underwent microsurgical clipping. A segment of the aneurysm dome (≥ 1mm) was removed and placed in formalin. A ≥ 2mm specimen from the STA was removed and placed in formalin. These specimens were collected from the same 10 patients. All 20 specimens (ten aneurysms and ten STA) were immunostained with monoclonal antibodies to COX-1 (Epitomics, Burlington CA), COX-2 (Epitomics, Burlington CA), and mPGES-1 (Cayman Chemical, Ann Arbor, MI).
Table 1.
F=female; M=Male; L=left; R=right; ICA=internal carotid artery; MCA=middle cerebral Artery; A-Comm=anterior communicating artery; Pcomm=posterior communicating artery; PICA=Posterior communicating artery; A/STA= aneurysm/superficial temporal artery; Grade 0= 0 cells per HPF, grade 1 = 0-10 cells per HPF, grade 2 = 10-20 cells per HPF and grade 3 = >20 cells per HPF
| Patient # | Age | Sex | Aneurysm Location | Aneurysm Size (mm) | Rupture (SAH) | COX-1 A/STA | COX-2 A/STA | mPGES-1 A/STA |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | F | L-ICA | 9 × 6 | no | 3/3 | 1/0 | 1/0 |
| 2 | 56 | M | R-MCA | 5 × 4 | no | 2/3 | 1/0 | 1/0 |
| 3 | 52 | F | L-MCA | 10 × 10 | no | 3/3 | 1/0 | 1/0 |
| 4 | 67 | F | R-MCA | 7 × 8 | no | 2/3 | 2/0 | 2/0 |
| 5 | 47 | M | R-Pcomm | 14 × 11 | no | 2/2 | 1/0 | 1/0 |
| 6 | 68 | M | L-MCA | 12 × 9 | yes | 3/3 | 3/0 | 3/0 |
| 7 | 44 | M | R-MCA | 6 × 5 | yes | 3/3 | 2/0 | 2/0 |
| 8 | 38 | F | A-comm | 8 × 8 | yes | 2/2 | 2/0 | 2/0 |
| 9 | 55 | F | L-MCA | 9 × 10 | yes | 2/3 | 3/0 | 3/0 |
| 10 | 74 | M | R-PICA | 14 × 15 | yes | 3/3 | 2/0 | 3/0 |
Semiquantitative analysis of the slides was performed based on cell count (immunostained positive cells) per high-power field (HPF) (40×): grade 0= 0 cells per HPF, grade 1 = 0-10 cells per HPF, grade 2 = 10-20 cells per HPF and grade 3 = >;20 cells per HPF. Assessment of slides stained only with COX-1, COX-2 and mPGES-1 was made by an observer who was not aware of the source of tissues. Statistical analysis was performed using Kruskal-Wallis test, a nonparametric ANOVA test.
Results
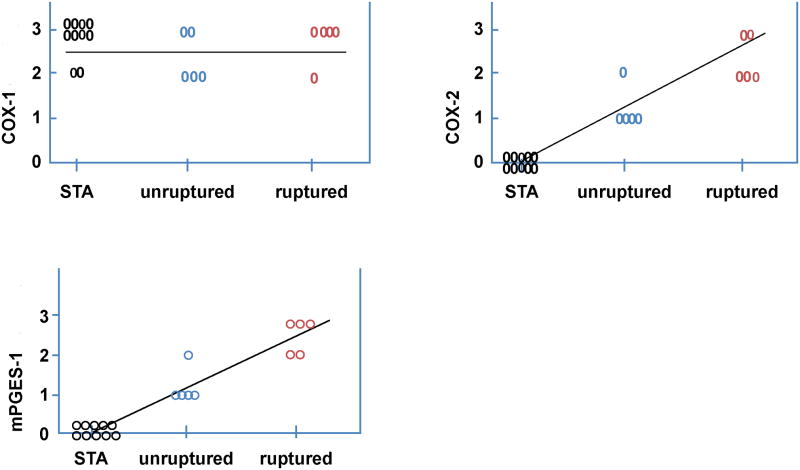
Ten patients with 10 aneurysms were included in this study. All ten aneurysms stained positive for expression of COX-2 and mPGES-1, using COX-2 and mPGES-1 monoclonal antibodies (figure 1 and 2). Ruptured cerebral aneurysm stained more abundantly with COX-2 and mPGES-1 monoclonal antibodies than non-ruptured aneurysms (figure 2). Staining of COX-2 and mPGES-1 was noted in all layers of the aneurysm tissue, but was more prominent in adventitia (figure 1). Superficial temporal artery (STA) tissue did not immunostain for COX-2 or mPGES-1 in any of the ten samples (figure 1 and 2).
Figure 1.
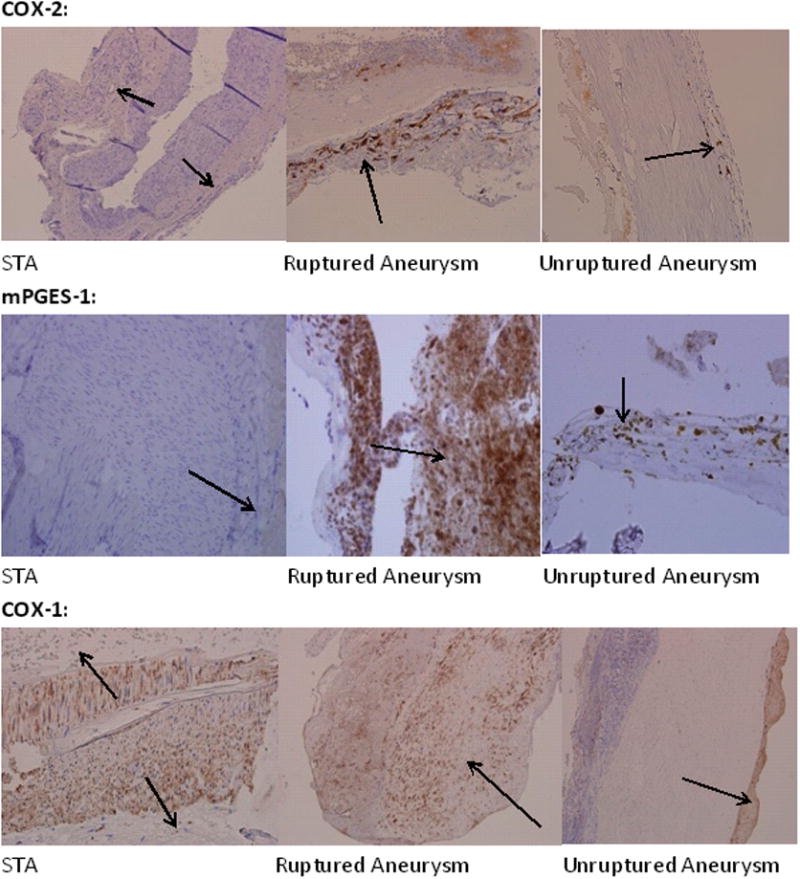
Expression of cyclooxygenases in tissue obtained from STA (A), ruptured (B) and unruptured cerebral aneurysm (C). Tissues were stained with monoclonal antibodies to COX-2, mPGES-1, and COX-1. Expression of COX-2 and mPGES-1, but not COX-1 was greater in cerebral aneurysms than STA, and greater in ruptured than non-ruptured aneurysms. Black arrows point to adventitia.
Figure 2.
Distribution of immunostaining in STA, ruptured, and unruptured aneurysms stained with monoclonal antibodies for COX-1, COX-2, and mPGES-1. Expression of COX-2 and mPGES-1, but not COX-1, was greater in cerebral aneurysms than STA, and greater in ruptured than non-ruptured aneurysms.
All tissue samples from STA and aneurysms (ruptured and non-ruptured) stained positively for COX-1 (figure 1 and 2).
There was no difference in expression of COX-1 among STA, ruptured and unruptured cerebral aneurysm (p=0.574). Expression of COX-2 tended to be greater (p=0.095) in ruptured aneurysms and was greater (p=0.001) in ruptured and un-ruptured aneurysms vs STA. Expression of mPGES-1, also tended to be greater in ruptured aneurysms vs unruptured aneurysms (p=0.071), and was greater in ruptured and unruptured aneurysms vs STA (p=0.001).
Discussion
Inflammation in Cerebral Aneurysm
Several stresses, including hemodynamic stress, infiltration of inflammatory cells, and release of inflammatory molecules and cytokines appear to play an integral role in progression of cerebral aneurysm to being rupture-prone and ultimately to rupture with devastating sequelae of subarachnoid hemorrhage. This concept is based on several studies in humans and animals which suggest that hemodynamic stress on endothelium leads to molecular signaling and formation of proinflammatory and proliferative pathways 12.
Endothelial stress leads to activation of transcription factor nuclear factor kappa B (NF-kB) 13, increased expression of monocyte chemotactic protein-1 (MCP-1) 14-17, and VCAM-1 1,17, which are highly chemotactic to inflammatory cells, macrophages, T cells, Natural Killer (NK) cells and basophils. Kanematsu et al 18 reported that depletion of macrophages in mice significantly reduced incidence of cerebral aneurysm formation. They also demonstrated a reduced incidence of cerebral aneurysms in mice with deletion of MCP-118. This finding supports the concept that inflammation plays a major role in aneurysm formation and rupture.
Recently we reported a paradoxical effect of aspirin, at least in relation to effects of aspirin on platelets, that daily aspirin use in humans decreases the incidence of aneurysm rupture by 60 % 4. Because aspirin is antiinflammatory, we suggest that this indirect evidence supports a role of inflammation in formation and rupture of aneurysms.
COX-2, mPGES-1, and COX-1 in Cerebral Aneurysms
Aoki et al demonstrated the presence of COX-2, mPGEs-1 and EP2 in endothelial cells in five unruptured human cerebral aneurysms and compared their findings to cadaver specimens6. They also showed that inhibition or loss of COX-2 or EP2 in vivo attenuated expression of the other, suppressed nuclear factor k-beta mediated chronic inflammation and reduced the incidence of cerebral aneurysm formation in rats and mice with cerebral aneurysm6. They did not examine the presence of these enzymes in ruptured aneurysm. In the present study, we confirm the presence COX-2/mPGES-1 in unruptured cerebral aneurysms, and demonstrate that expression leads to increase in ruptured aneurysms, and these enzymes are not expressed in extracranial arteries. The role of expression of these enzymes in the pathophysiology of aneurysm rupture is not clear. Aneurysms in both humans and rodents, however, exhibit the hallmarks of inflammation, as described above. Thus we hypothesize that this molecular complex (COX-2/mPGES-1) and prostaglandins could play a major role in rupture of aneurysms. In our study, these enzymes (COX-2/mPGES-1) localized mainly in the adventitia, in contrast to the findings of Aoki et al.
Several studies have recently provided evidence that functional changes in the adventitia contribute to vascular remodeling during atherogenesis. Several pro-inflammatory molecules have been proposed to act locally to contribute to activation of the adventitia, ranging from enhanced growth factor activity and increased extracellular matrix synthesis to generation of reactive oxygen species and accumulation of progenitor cells 19. It is not clear to us whether there is a signal from endothelium or smooth muscle cells that affects the adventitia. Additionally, these enzymes maybe localized to inflammatory cells (perhaps macrophages) in adventitia.
We speculate that the presence of mPGES-1 in adventitia of cerebral aneurysm wall may contribute to the headache after subarachnoid hemorrhage and inhibition of this enzyme may ameliorate this headache. This speculation is based on the fact that deletion of mPGES-1 in mice also has been reported to inhibit experimentally evoked pain and inflammation, and to a degree comparable to that observed from treatment with nonsteroidal antiinflammatory drugs (NSAIDs) 20
All tissues from ruptured and non-ruptured aneurysms and STA stained positively for COX-1. Expression of COX-1 was similar in ruptured and non-ruptured aneurysms and in an extracranial artery. This finding is consistent with the fact that COX-1 is present constitutively in several cell lines and that expression does n
mPGES-1 and Vascular Injury
Several studies have reported that deletion or inhibition of mPGES-1 suppresses atherogenesis, angiotensin II-induced aortic aneurysm formation, activation of aortic MMP-2, and attenuates neointimal hyperplasia after vascular injury in mice 7-11. Deletion of mPGES-1 in mice also has been reported to inhibit inflammation 20. These studies suggest a critical role of mPGES-1 in inflammation and in pathogenesis of several vascular pathologies, and suggest a potential benefit of targeting mPGEs-1 in management of these diseases.
Limitation
This study is limited by the small sample size, and generalization of the results may not be appropriate. It also is difficult to determine whether increased expression of COX-2 and mPGES-1 in ruptured cerebral aneurysms (compared to non-ruptured) is due to inflammation that occurs following the rupture of the aneurysm, or whether there was an increase in expression of these molecules which preceeded and led to rupture of the aneurysm. Our finding of localization of these two molecules to the aneurysm wall even in unruptured aneurysms, suggests that COX-2 and mPGES-1 may contribute to formation and rupture of cerebral aneurysms.
Conclusion
COX-2 and mPGEs-1 are expressed in human cerebral aneurysms, and expression increases in ruptured aneurysms. COX-1 is found constitutively in cerebral aneurysms and in an extracranial artery. The findings suggest that COX-2/mPGES-1may play a role in formation and rupture of aneurysms. We also speculate that inhibition of COX-2/mPGEs-1 by aspirin may contribute to protective effects of aspirin in reducing rupture of human cerebral aneurysm.
Acknowledgments
We thank Dr. Miriam B. Zimmerman for assistance with statistical analysis.
Funding Sources: This study was supported by NIH grant # R03NS07922to Dr. Hasan, NIH grant # R01NS055876 to Dr. Hashimoto, and NIH grants # 24621 and HL62984 to Dr. Heistad.
Footnotes
Disclosure: The Authors have no disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45:1137–46. doi: 10.1097/00006123-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture:histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–93. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 3.Jin D, Sheng J, Yang X, Gao B. Matrix metalloprtienases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surg Neurol. 2007;68:S11–S16. doi: 10.1016/j.surneu.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 4.Hasan DM, Mahaney KB, Brown RD, Meissner I, Piepgras DG, Huston J, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke A, Smyth E, FitzGerald GA. Analgesic antipyretic and antiinflammatory agents. In: Goodman, Gilman, editors. The pharmacological basis of therapeutics. New York, NY: McGaw-Hill; 2006. pp. 671–716. [Google Scholar]
- 6.Aoki T, Nishimura M, Matsuoka T, Yamamoto K, Furuyashiki T, Kataoka H, et al. PGE2-EP2 signalling in endothelium is activated by hemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-kB. Br J Pharmacol. 2011;163:1237–1249. doi: 10.1111/j.1476-5381.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Ihida-Stansbury K, Kothappalli D, Tamby MC, Yu Z, Chen L, et al. Microsomal prostaglandin E2 Synthase-1 modulates the response to vascular injury. Circulation. 2010;123:631–639. doi: 10.1161/CIRCULATIONAHA.110.973685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Lee E, Wenliang S, Ricciotti E, Rader DJ, Lawson JA, et al. Microsomal prostaglandin E ynthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 9.Cipollone F, Prontera C, Pini B, Marini M, Fazi M, Cesare DD, et al. Overexpression of functionally coupled cycolooxyegenase-2 and prostaglandin E synthase in symptomatic atherosclerotic palques as a basis of prostaglandin E2-dependant plaque instability. Circulation. 2001;104:921–927. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]
- 10.Cipollone F, Fazia M, Ciabattoni G, Pini B, Cuccurullo C, Ucchino S, et al. Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arteioscler Thromb Vasc Biol. 2004;24:1259–1265. doi: 10.1161/01.ATV.0000133192.39901.be. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Hernandez A, Martin-Venture JL, Sanchez-Galan E, Vidal C, Ortego M, Blanco-Colio LM, et al. Overexpression of COX-2, prostaglandin E synthase-1 and prostaglandin E receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: regulation by nuclear factor-kB. Atherosclerosis. 2006;187:139–149. doi: 10.1016/j.atherosclerosis.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–8. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- 13.Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, et al. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. :2830–40. doi: 10.1161/CIRCULATIONAHA.107.728303. [DOI] [PubMed] [Google Scholar]
- 14.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40:942–51. doi: 10.1161/STROKEAHA.108.532556. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Zhao J, Wang S, Chen Z, Ma L, Zheng Z. Monocyte chemoattractant protein-1 mRNA in human intracranial aneurysm walls. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36:519–21. [PubMed] [Google Scholar]
- 16.Krischek B, Kasuya H, Tajima A, Akagawa H, Sasaki T, Yoneyama T, et al. Network-based gene expression analysis of intracranial aneurysm tissue reveals role of antigen presenting cells. Neuroscience. 2008;154:1398–407. doi: 10.1016/j.neuroscience.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Shi C, Awad IA, Jafari N, Lin S, Du P, Hage ZA, et al. Genomics of human intracranial aneurysm wall. Stroke. 2009;40:1252–61. doi: 10.1161/STROKEAHA.108.532036. [DOI] [PubMed] [Google Scholar]
- 18.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, Rooijen NV, et al. Critical role of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siow RCM, Churchman AT. Adventitial growth factor signaling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]