Abstract
Objective
To quantify spectral power in frequency specific bands and commonly observed types of bursting activities in the EEG during early human development.
Methods
An extensive archive of EEG data from human infants from 35 to 52 weeks postmenstrual age obtained in a prior multi-center study was analyzed using power spectrum analyses and a high frequency burst detection algorithm.
Results
Low frequency power increased with age; however, high frequency power decreased from 35 to 45 weeks. This unexpected decrease was largely attributable to a rapid decline in the number of high frequency bursts.
Conclusions
The decline in high frequency bursting activity overlaps with a developmental shift in GABA's actions on neurons from depolarizing to hyperpolarizing and the dissolution of the gap junction circuitry of the cortical subplate.
Keywords: EEG, bursts, development, infant, subplate, GABA
Introduction
For decades pediatric neurologists have studied electrical signals recorded from the scalp of human infants. Initially, investigators sought to visually characterize patterns of electrical activity that were evident in these signals and to determine developmental changes in these patterns (Andre et al., 2010, Werner et al., 1977). With the advent of improved computer technology, pioneers in the field used bioengineering methodologies to quantify the amount of electrical activity, i.e. power in a frequency-specific manner (Parmelee, 1969). These techniques revealed frequency dependent differences in power with sleep state (Curzi-Dascalova et al., 1988, Parmelee, 1969) and sleep position (Sahni et al., 2005), variance in power with clinical status (Scher, 1994), and differences between term and preterm infants (Scher et al., 1994a, Scher et al., 1994b).
Other investigators have characterized developmental changes in EEG during infancy, A notable study by Sterman and colleagues (Sterman et al., 1977) analyzed EEG data from birth to 24 weeks of age. They described a steady increase in low frequency power with postmenstrual age (PMA), while higher frequency power exhibited a “dip” from 40 to 44 weeks PMA. Although not reported as a “dip”, data from Jenni et al. (Jenni et al., 2004) also showed a transient decrease in EEG power from 2 weeks after term birth to 2 months of age. Scher and colleagues found a transient decrease in high frequency power in both term and preterm infants and over a comparable period around 40 weeks PMA (Scher, 1997) and consistent with a report of a negative correlation between the 12-24 Hz beta of power and PMA from 36 to 43.5 weeks PMA in preterm infants (Scher et al., 1995). Although these data are suggestive of decreasing EEG power at higher frequencies through this early period of development, this pattern is not widely appreciated nor is there an understanding of what might account for this early decrease in high frequency EEG power.
To provide much-needed quantitative data of changes in the EEG during early development we analyzed an extant EEG data base collected during the Collaborative Home Infant Monitoring Effort (CHIME). CHIME was an NICHD-funded multi-site study from the early to mid-90s designed to evaluate markers and risks for Sudden Infant Death Syndrome (SIDS) (Hunt, 1995). As part of this effort, overnight, ∼6 hour, polysomnographic studies were conducted with four channels of EEG in a large subset of infants. Although studied at a mean of 10 weeks after birth, the distribution of postnatal and postmenstrual ages was broad and encompassed the period of development from 35 to 52 weeks PMA. For most of the polysomnograms, sleep states were coded in 30 sec blocks. Although many of the study subjects enrolled in CHIME were at increased risk for SIDS, we selected infants who were in the lower risk categories (i.e. healthy term and preterm infants without apnea and/or bradycardia near discharge) (Crowell et al., 2004, Crowell et al., 2002). Following this selection, there remained an adequate population of comparably studied infants for a robust cross-sectional study of changes in patterns of EEG power during early development.
For years, clinical electrophysiologists have recognized bursting activity in the EEG that is unique to early development. These bursts have been variously named slow or spontaneous activity transients (SATs), delta brush, and sharp transients (Andre, Lamblin, 2010, Biagioni et al., 1999, Biagioni et al., 2007, Hughes and Kohrman, 1989, Lamblin et al., 1999, Milh et al., 2007, Statz et al., 1982, Tolonen et al., 2007, Vanhatalo et al., 2005). These and other bursting patterns have been noted and quantified in EEG recordings in rodents (Seelke and Blumberg, 2008, Yang et al., 2009) and nonhuman primates (Myers et al., 1997, Myers et al., 1993, Stark et al., 1991). Khazipov and Luhmann collectively termed these waveforms as “delta brushes”, apparent at ∼28weeks PMA and mostly gone by term age (Khazipov and Luhmann, 2006). Although generally thought to be of spontaneous origin, it has become evident that at least some bursts are linked to feedback from motor activity (Khazipov et al., 2004, Mohns and Blumberg, 2008). Although bursting activity in neuronal circuits is considered to be vital for normal cortical development (Ben-Ari et al., 2007) there is no consensus on exactly how bursts should be measured and categorized nor what their impact may be on traditional measures of EEG power.
The goal of this study was to quantify changes in power and bursting features of EEG activity unique to early development in order to develop novel methods for tracking specific neurophysiologic mechanisms critical for development of normal brain function. Our results substantiate previous inferences that EEG power above 10Hz does not increase from 34 to 44 weeks PMA as might be expected during this period of rapid brain growth. Further, we find that the contributions to high frequency power attributable to short bursts of activity account for a significant amount of the total high frequency power and that these contributions decline steadily through this period. We discuss the potential linkage between our findings and the developmental shift in mechanisms underlying the ability of GABA to act as an inhibitory neurotransmitter, the dissolution of the circuits of the cortical subplate, and the progressive maturation of sleep states.
Methods
Database and Subject Population
This study is a secondary analysis of the multi-site Collaborative Home Infant Monitoring Evaluation (CHIME) study data set. The CHIME study was conducted from 1994-1998 to assess physiological function and neurodevelopmental outcome in healthy infants and those at increased risk for SIDS. The institutional review boards at each of the clinical sites (Chicago, Cleveland, Honolulu, Los Angeles, and Toledo) approved the study, and parents gave informed consent prior to participation. IRB approval for use of the de-identified data was obtained from the New York State Psychiatric Institute and Dartmouth University. The details of the data collection methods and composition of the Chime data set are available on at the CHIME public access website http://dccwww.bumc.bu.edu/ChimeNisp/.
The analyses of the Chime data set for this current study excluded symptomatic preterm infants with signs of respiratory disturbance, preterm siblings of SIDS, preterm infants with apparent life threatening events (ALTE), full term siblings of SIDS, and full term infants with ALTEs. The remaining study population consisted of 252 infants with 81 full term healthy infants and 171 asymptomatic preterm infants. All of these infants had overnight EEG recordings obtained between 35 and 52 weeks PMA. Characteristics of the study population are in Table 1.
Table 1.
Characteristics of the study population.
| Preterm (N=171) | Term (N=81) | |||
|---|---|---|---|---|
| Variable | median | range | median | range |
| Maternal Age (yr) | 28.2 | 16-42 | 30.5 | 18-45 |
| GA at birth (wk) | 29.6 | 23-34 | 39.4 | 38-42 |
| Birth Weight (g) | 1296 | 466-1750 | 3323 | 2495-3800 |
| PMA at Study (wk) | 39 | 35-52 | 48 | 41-52 |
| Sex (% female) | 54% | 48% | ||
| Multiple Births (%) | 17% | 5% | ||
| Ethnicity = White | 32.2% | 55.6% | ||
| Black | 17.5% | 12.0% | ||
| Hispanic | 28.7% | 2.5% | ||
| other | 20.6% | 24.9% | ||
With regard to neurological status, 16 of the 171 infants born prematurely had grade I IVH and none had greater than grade I. From late cranial ultrasounds 3 infants were diagnosed with periventricular leucomalacia. Five of the 117 premature infants assessed between 35 and 43 weeks of age had seizures diagnosed prior to discharge. None of these infants continued to have seizures after discharge and none were being treated for seizures at the time of the EEG studies. Fifteen of these infants received barbiturates/valium or benzodiazepines but none had been given dilantin. Examination of the data from these infants did not appear markedly different from undiagnosed and untreated infants and thus were not excluded from the final analyses.
Infant Polysomnography (IPSG) and Sleep State
An overnight polysomnographic recording was obtained for each subject at an average postnatal age (PNA) of 9.8 ± 5.8 (SD) weeks. The IPSG included EEG data from central (C3, C4) and occipital (O1, O2) electrode sites recorded with contralateral mastoid references (A1, A2). The CHIME acquisition hardware system (ALICE3) filtered the EEG data from 1 to 40 Hz with a notch filter at 60 Hz, and then digitized the signals with 8 bits per sample at the rate of 100 Hz. For a subset of subject population, CHIME investigators coded sleep state in 30-second epochs from autonomic data in the IPSG (Crowell et al., 1997, Hoppenbrouwers et al., 2005). For infants included in the current study, state data were coded for 174 infants (112 preterm, 62 term).
EEG Processing and Analysis
EEG data were processed in 30-second epochs to facilitate alignment with sleep state data. The root-mean-square (RMS) value of the EEG waveform for each 30-second epoch of data was computed and compared with a threshold to identify epochs containing artifact. Age-specific thresholds were set to define epochs of artifact that were apparent on visual review of the data (35-40wk, 35.8μV; 40-45wk, 39.8μV; and 45-55wk, 40.4μV). Most artifacts were associated with subject movements that were reflected in other IPSG data channels. The percentage of EEG data deemed as artifact varied with state and included 3% of data in quite sleep, 13% in active sleep.
To remove a potential for bias from the contralateral mastoid reference bipolar derivations were formed from the original 4 EEG channels. The bipolar channels were C3 to O1 on the left hemisphere and C4 to O2 on the right hemisphere. For each 30 second epoch of artifact-free data in these 2 channels, power spectra were computed. We used an FFT algorithm in custom MATLAB software that we had previously evaluated (Grieve et al., 2003). EEG power from the left and right hemispheres were computed and summarized as the mean of spectral values in 3Hz bands. For each 30 epoch, for each subject, EEG power was averaged within each of these 3Hz bands. Then, the average of these values was computed for epochs of quiet or active sleep. Outliers in power in each 3Hz band were defined as mean values exceeding 2 times the interquartile range above the 75th percentile or below the 25the percentile. These outliers were deleted prior to data analyses. In the worst case (19-21 Hz, left side), 4% of the data were excluded.
EEG Burst Detection and Characterization
In each 30 second epoch, high frequency bursts in EEG activity were detected and characterized with custom software following an algorithm depicted in Figure 1. For each 30 second epoch of EEG data (lower panel), burst detection was derived from results of spectral analysis of this waveform. Prior to the spectral analysis, the EEG was multiplied by a standard cosine on a pedestal window function that produced side lobes in the spectrum at least 40 dB below the main response (Harris, 1978). Then, the spectrum was calculated for each epoch (zero padded to 3032 points) using FFTs with a sliding 32 time-sample window which overlapped by one data sample and yielded a frequency resolution of ∼ 3Hz. The resultant EEG spectral time series covered 0 to 40 Hz for each 30-sec epoch (middle panel). The highest frequency was limited to 40Hz by characteristics of the acquisition hardware. Next, for detection of high frequency bursts, the sum of power in the spectral time series between 10 and 40 Hz was smoothed with a 2 Hz low pass filter to eliminate EKG artifact (blue curve, upper panel). A 10Hz high pass setting was adopted based on review of the literature describing high frequency oscillations in infant EEG (Milh, Kaminska, 2007, Tolonen, Palva, 2007). Next, for each epoch, a threshold for burst detection (red line, upper panel) was defined as two times the 10th percentile (green line, upper panel) of the smoothed 10-40Hz power time series. This threshold was arrived at by iteratively altering the threshold until the bursts captured by the automated method were in agreement with three experienced EEG analysts who concurred on visually identified high frequency bursts. Results of this algorithm for detecting high frequency bursts were consistent with those from visual inspection by multiple observers.
Figure 1.
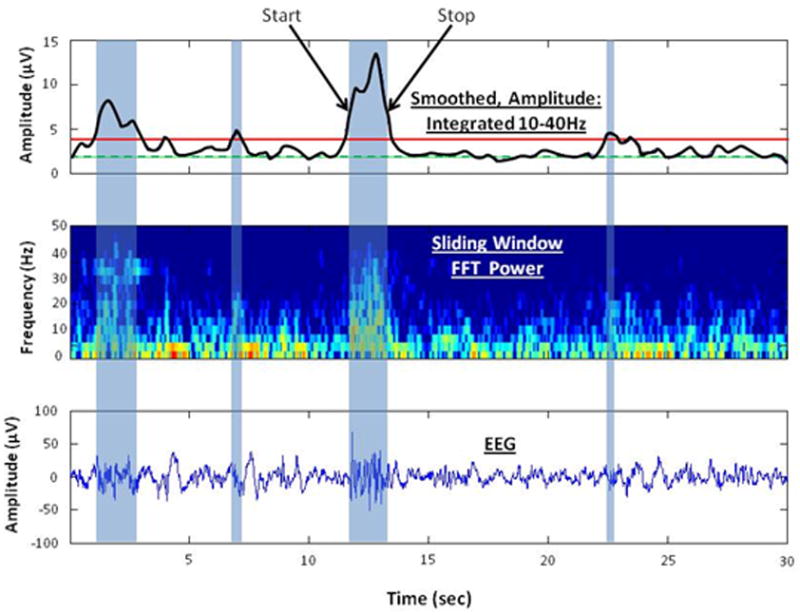
Depiction of the burst detection algorithm to identify bursts of high frequency activity. The lower panel shows 30 sec of EEG and 4 high frequency bursts of varying duration (blue shaded areas). The middle panel shows the sliding window, 32 point FFT spectral time series from 0 to 40 Hz. Increasing amounts of power are depicted with a color scale from blue to red. The top panel shows the sum of power in the spectral time series between 10 and 40 Hz, smoothed with a 2 Hz low pass filter (black curve). The threshold for burst detection (red solid line) was defined as two times the 10th percentile (green dashed line) of the smoothed power. Each high frequency burst was characterized by peak amplitude, start and stop times corresponding to half peak power, and mean spectral frequency within the burst.
Finally, each high frequency burst was characterized by peak amplitude (square root of peak power) and start and stop times corresponding to half peak power. From these measures the mean rate of occurrence (bursts/min), mean peak amplitude, and the mean duration of bursts were computed for all epochs of active and quiet sleep states in each record. We also computed the mean spectral frequency within the bursts. The mean frequency of the spectrum was calculated as the integral of the product of the power spectrum (as a function of frequency) and the frequency variable divided by the total power in the spectrum.
Data Analysis
Preliminary analyses of these data indicated a very rapid fall off in EEG power and minimal effects of age above 24Hz; accordingly, the analyses presented are of power in each of eight 3Hz frequency bands up to 24Hz. All analyses of power used natural log (loge) transformations. We defined outliers as values 1.5 times the interquartile range above the 75th percentile or below the 25th percentile and set these values to missing. This resulted in exclusion of less than 2.5% of the total values. Comparisons between powers recorded in the left versus right hemispheres were made using paired t-tests. Regression analyses were used to test for significance of rates of change in power (slopes) and characteristics of bursts over selected ranges of PMA. Tests for significant differences between slopes with regard to left versus right hemisphere and quiet versus active sleep were done using t-tests.
Results
Our initial approach to ascertain the changes in EEG activity with development was to plot the values from individual infants along with the mean (±SE) for frequency bands in each hemisphere without regard to sleep state. We found three distinct patterns of change with PMA that were dependent upon frequency band. In the 4-6 Hz band, power increased monotonically with PMA (upper panel Figure 2) while at 7-9 Hz there appeared to be little change with age (middle panel Figure 2). In the 13-15 Hz band a biphasic pattern was apparent with power decreasing from 35 to 46 weeks and increasing from 46 to 52 weeks (lower panel Figure 2).
Figure 2.
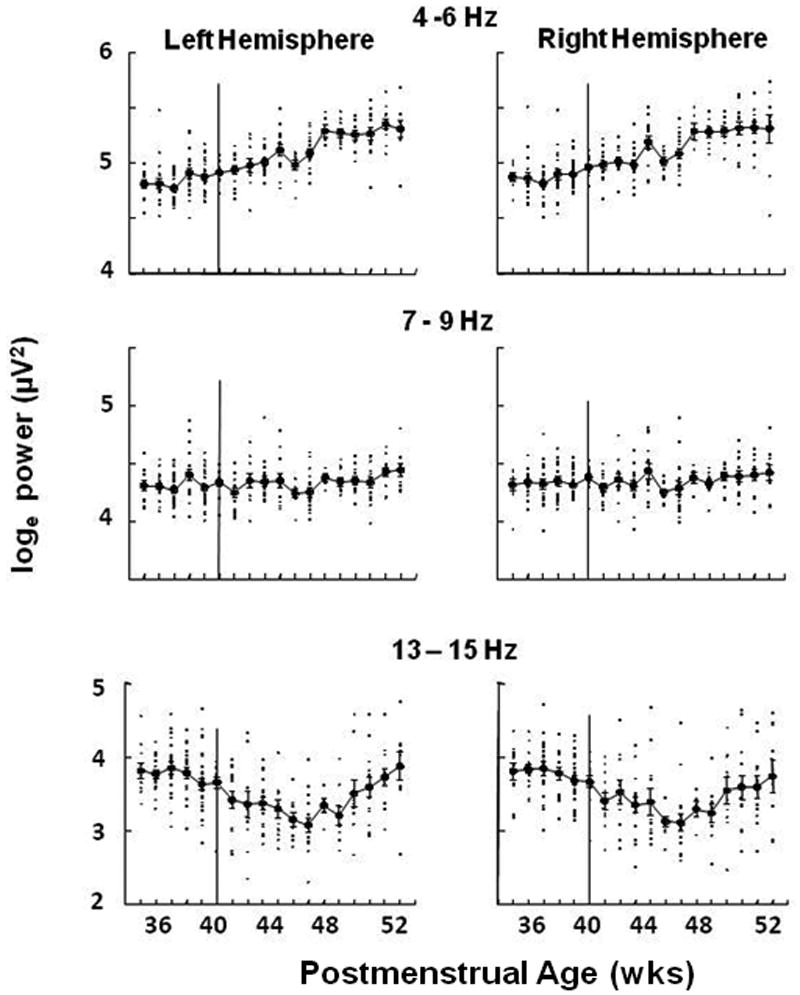
EEG power (loge, μv2) in 3 frequency bands plotted against postmenstrual age in weeks. Individual points (small filled squares) are the mean values of ∼900 30 second epochs during overnight polysomnographic studies for each baby. Each baby was studied at only one age. Also shown are means (larger filled circles) ±SE for babies with recordings at the same age (N ranged from 5 to 29, with 14 of 18 ages having N>10). Data from the left hemisphere bipolar leads (C3, O1) are given in the left panel; data from the right hemisphere bipolar leads (C4, O2) are given in the right panel.
To determine the statistical significance of these observed patterns by frequency band we performed a series of regression analyses of EEG power with PMA for all frequency bands in infants studied earlier (35 to 45 weeks) and later (46 to 52 weeks) ages. Analyses were repeated for data from each hemisphere of the brain. Differences in EEG power between right and left hemispheres and changes in power with PMA within the two age ranges are presented in Table 2.
Table 2.
Differences in EEG power between right and left hemispheres of the brain, and changes in power with postmenstrual age (PMA) within two age ranges (35-45 and 46-52 weeks). With outliers removed (see Methods) Ns varied.
| 35-45 weeks, (N= 166 – 177) | ||||
|---|---|---|---|---|
| Frequency | Left vs Right power | Left: slope with PMA | Right: slope with PMA | Left vs Right slope |
| .3 – 3 Hz | R>L p<.01 | - | ↑ p<.05 | - |
| 4 – 6 Hz | R>L p<.001 | ↑ p<.001 | ↑ p<.001 | - |
| 7 – 9 Hz | R>L p<.05 | - | - | - |
| 10 – 12 Hz | R>L p<.05 | ↓ p<.001 | ↓ p<001 | - |
| 13 – 15HZ | - | ↓ p<.001 | ↓ p<.001 | - |
| 16 – 18HZ | - | ↓ p<.001 | ↓ p<001 | - |
| 19 – 21 Hz | - | ↓ p<.001 | ↓ p<.001 | - |
| 22 – 24 Hz | - | ↓ p<.01 | ↓ p<.001 | - |
|
| ||||
| 46-52 weeks, (N= 74-75) | ||||
| Frequency | Left vs Right power | Left: slope with PMA | Right: slope with PMA | Left vs Right slope |
|
| ||||
| .3 – 3 Hz | - | ↑ p<.05 | ↑ p<.05 | - |
| 4 – 6 Hz | - | ↑ p<.001 | ↑ p<.01 | - |
| 7 – 9 Hz | - | ↑ p<.01 | ↑ p<.05 | - |
| 10 – 12 Hz | - | ↑ p<.001 | ↑ p<.01 | - |
| 13 – 15 HZ | - | ↑ p<.001 | ↑ p<.001 | - |
| 16 – 18 HZ | - | ↑ p<.05 | - | - |
| 19 – 21 Hz | - | - | - | - |
| 22 – 24 Hz | - | - | - | - |
Note: ↑ indicates positive slope, ↓ indicates negative slope, - indicates p>0.05.
Mean power was higher in the right hemisphere for the four bands below 13 Hz, but only during the early PMA age range. The greater power on the right side, in the younger babies, at frequencies below 13Hz was of the order of 5-9% (depending on frequency band). At 4-6Hz, these findings held true with a p<.05 for infants born at term (N=21), for prematurely born infants with GAs at birth between 23 and 29 weeks (N=69), and for prematurely born infants with GAs at birth between 30 and 34 weeks (N=87). These analyses were done without regard to sleep state. When state was taken into account higher power was seen on the right side in both states although in AS the difference did not reach significance and there was a significant state by hemisphere interaction (p<.05).
At 35 to 45 weeks there were robust decreases in power with age for all frequencies above 9 Hz in both hemispheres. In contrast, at 4-6 Hz power increased in both hemispheres with age. At 46 to 52 weeks, in both hemispheres, power in frequency bands below 16Hz increased with age. There were no differences in slope between the right and left hemispheres in any frequency band in either age range.
We then evaluated the developmental changes in EEG spectral power in relation to sleep state. These results are given in Table 3. From 35 to 45 weeks, EEG power. was greater during quiet sleep (QS) than active sleep (AS) in frequency bands between 4 and 12 Hz. In contrast power was lower in QS at 16 to 24 Hz. A similar state-dependent pattern was seen at 46 to 52 weeks during which power at lower frequencies was greater during QS and power at higher frequencies greater during AS.
Table 3.
Differences in EEG Power by sleep state (Active Sleep, AS: Quiet Sleep, QS), and changes in power with postmenstrual age (PMA) within two age ranges (35-45 and 46-52 weeks). With outliers removed (see Methods) Ns varied.
| 35-45 weeks , (N= 109-111) | ||||
|---|---|---|---|---|
| Frequency | QS vs AS power | QS: slope with PMA | AS: slope with PMA | QS vs AS slope |
| .3 – 3 Hz | - | ↑ p<.05 | - | - |
| 4 – 6 Hz | QS >AS p<.001 | ↑ p<.001 | ↑ p<.001. | - |
| 7 – 9 Hz | QS>AS p<.001 | - | - | - |
| 10 – 12 Hz | QS>AS p<.001 | ↓ p<.001 | ↓ p<.001 | - |
| 13 – 15HZ | - | ↓ p<.001 | ↓ p< 001 | - |
| 16 – 18HZ | AS>QS p<.001 | ↓ p<.001 | ↓ p<001 | - |
| 19 – 21 Hz | AS>QS p<.001 | ↓ p<.001 | ↓ p<001 | - |
| 22 – 24 Hz | AS>QS p<.001 | ↓ p<.001 | ↓ p<001 | - |
|
| ||||
| 46-52 weeks, (N=62-63) | ||||
| Frequency | QS vs AS power | QS: slope with PMA | AS: slope with PMA | QS vs AS slope |
|
| ||||
| .3 – 3 Hz | QS>AS p<.001 | ↑ p<.05 | - | - |
| 4 – 6 Hz | QS>AS p<.001- | ↑ p<.01 | - | - |
| 7 – 9 Hz | QS>AS p<.001 | ↑ p<.05 | - | - |
| 10 – 12 Hz | QS>AS p<.001 | ↑ p<.001 | - | QS>AS p<.05 |
| 13 – 15HZ | QS>AS p<.001 | ↑ p<.001 | - | QS>AS p<.001 |
| 16 – 18HZ | - | ↑ p<.01 | - | - |
| 19 – 21 Hz | AS>QS p<.001 | - | - | - |
| 22 – 24 Hz | AS>QS p<.001 | ↑ p<.05 | - | - |
Note: ↑ indicates positive slope, ↓, indicates negative slope, - indicates p>0.05.
At younger ages, in both QS and AS, power increased with age in the 4-6 Hz band, but decreased with age in all frequency bands above 10 Hz. In comparison, infants in the older age group, in QS, increased power with age in all frequency bands except 19-21 Hz. Strikingly, in the older infants during AS there were no significant changes in power with age at any frequency; however, state-dependent differences in these changes with age were found in the 10-12 and 13-15Hz bands. These frequency and state dependent effects on the changes in EEG power with age are depicted in Figure 3. This figure shows that power in both the 13-15 Hz and 19-21 Hz bands declines from 36 to 46 weeks in both sleep states; however in QS, but not AS, and only in the 13-15 Hz band, power increases from 46 to 52 weeks PMA.
Figure 3.
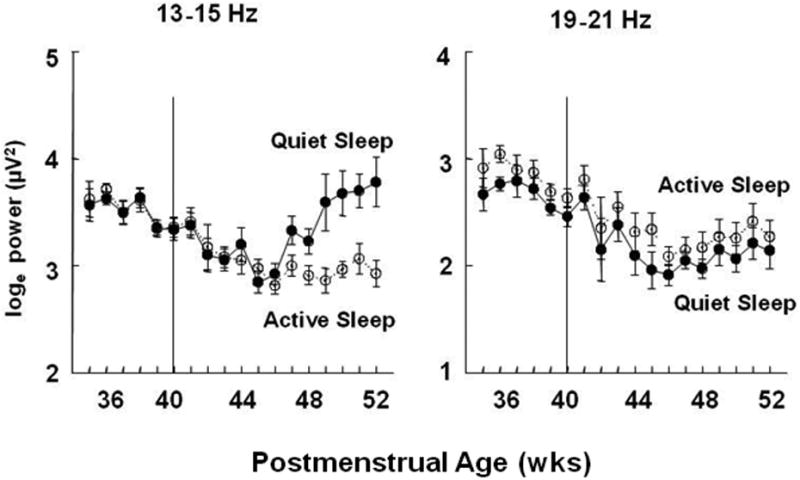
EEG power (loge, μv2) in 2 frequency bands by sleep state. The means ± SE at each postmenstrual age of data obtained in Quiet Sleep (filled circles connected by solid lines) and Active Sleep (open circles connected by dotted lines).
We then conducted a series of analyses to determine if changes in EEG power with age were differentiated by the sex of the infant. For data obtained between 35 and 45 weeks PMA, there were no significant sex related differences in slopes of EEG power at any frequency band. Between 46 and 52 weeks PMA there were no sex related differences except that males had a steeper increase in the 3 Hz band with PMA than females (p<.01)
For an alternative depiction of the frequency and age dependent changes in EEG, we created two groups of infants with distinct ages at EEG study; those from 35 to 37 weeks (n=29) and those from 40 to 43weeks (n=40). It is important to note that all of these infants delivered prematurely <37weeks. For these groups we computed mean (±SE) power in the left hemisphere in quiet and active sleep for each of the 8 frequency bands. Results are plotted as loge power versus the center frequency of 3 Hz bands from 3 to 24 Hz in Figure 4. This figure depicts a dramatic fall off in power with frequency for both the younger and older infants; however, in frequency bands centered at 12, 15, 18 and 21 Hz power is greater in younger than in older infants (all p values for each band in both states <.01). These decreases in high frequency power are highly significant in both sleep states and in both hemispheres (right hemisphere data not shown).
Figure 4.
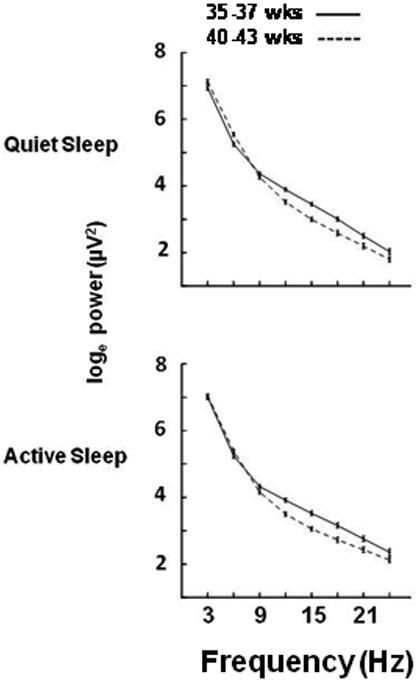
EEG power (loge, μv2) in the left hemisphere in each frequency band in Quiet (upper panel) and Active (lower panel) Sleep. Data are from prematurely born infants studied from 35 through 37 weeks (N=29) postmenstrual age (solid line) and from 40 through 43 weeks (N=40, dashed line).
The results at this stage of analysis suggest there is a component of high frequency EEG power that is present in preterm infant prior to term age that decreases with advancing age. Accordingly, we asked whether this component is continuous or is associated with periodic high frequency events. Visual inspection of the EEG data from younger infants revealed multiple examples of periodic bursts of high frequency activity. Figure 5 depicts four brief EEG tracings from an infant born at 30 weeks gestation studied at 5 weeks after birth. These tracings show bursts of high frequency activity can be found with or without associated low frequency activity.
Figure 5.
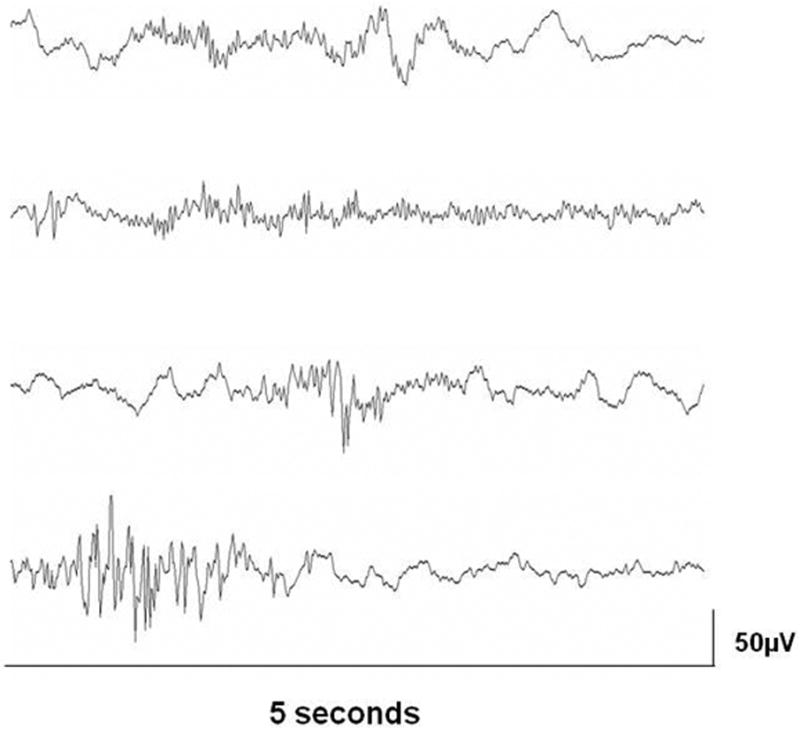
Four examples, each 5 seconds in duration, of high frequency bursts of EEG activity from an infant born at 30 weeks gestation and studied at 5 weeks after birth. The examples show various patterns: high frequency bursts in association with low frequency activity (top tracing), in the absence of low frequency activity (second tracing), coincident with an abrupt transient (third tracing), and coincident with a burst of activity at ∼7Hz (bottom tracing).
Based on these observations we derived a method for detecting bursts of activity in the 10 to 40 Hz range (see Methods) and then assessed the change in characteristics of bursts with age. The top panel of Figure 6 shows the mean ± SE number of bursts detected per minute in the left hemisphere over age in quiet and active sleep. Results from regression analyses with 117 subjects showed, in both sleep states, there is a dramatic decrease in number of bursts detected over this age range (p<.001). The middle panel shows the mean amplitudes of bursts over age and the bottom panel shows the mean duration of bursts. Neither of these parameters had significant linear changes with age. On average, the mean frequency of activity within bursts identified across all epochs of quiet sleep was 17.3±0.7 Hz (mean ± SD) and there was no significant change in frequency with age. For epochs of active sleep, the mean frequency of activity within bursts was 18.5±1.1 Hz. There was a slight upward slope to this measure with age (r=+.22, n=117, slope=0.13Hz/wk, p<.02)
Figure 6.
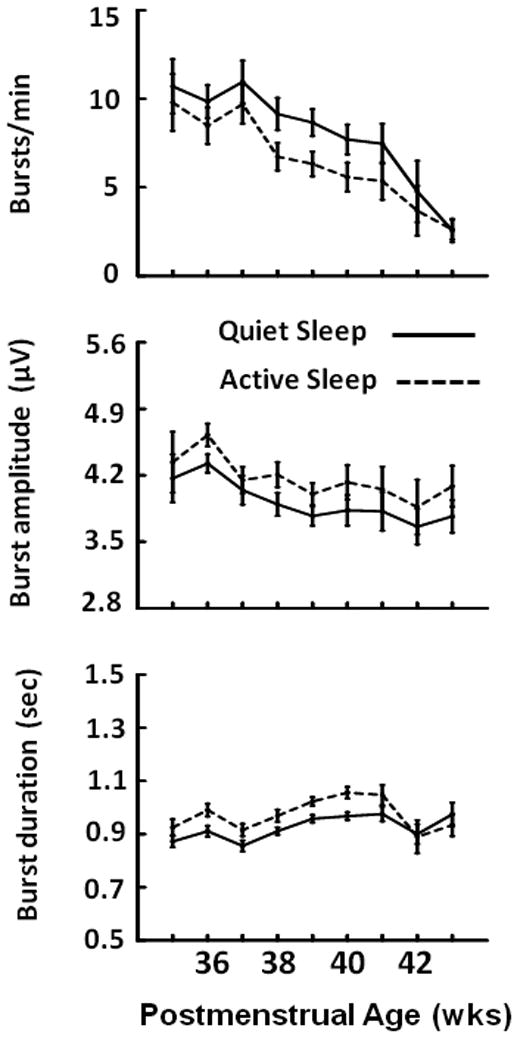
Rate, amplitude and duration of high frequency EEG bursts (mean ± SE) in Quiet (solid lines) and Active (dashed lines) Sleep.
Figure 7 presents results that show the effects of excising burst waveforms from the EEG. At all frequencies from 12 to 21 Hz, removal of bursts results in a reduction of power of at least 20% in both states. Interestingly, even though bursts were identified based on power above 10 Hz, with bursts removed, power at frequencies below 10Hz was significantly reduced by 10 to 20%. This implies that at least some bursts include increases in both high and low frequency power.
Figure 7.
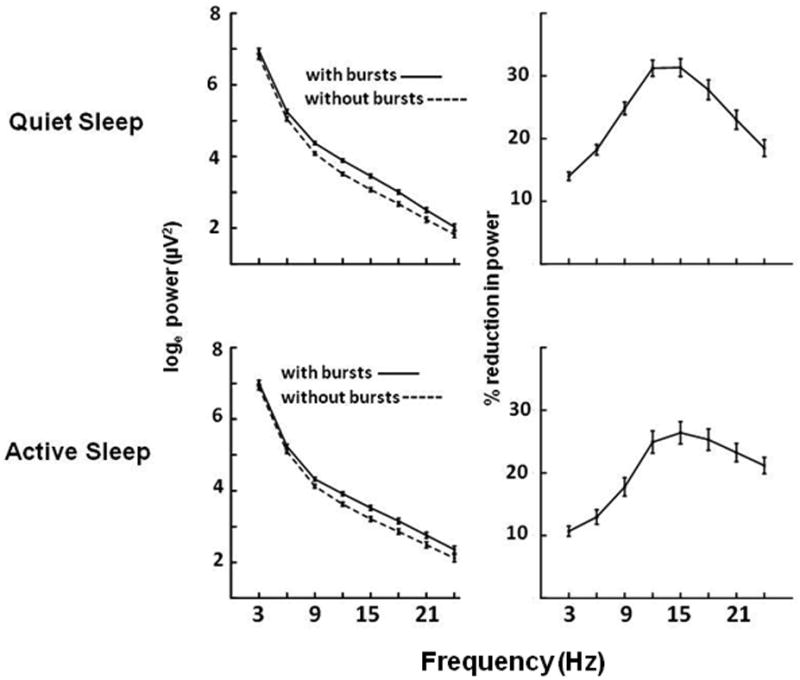
Effects of removing bursting activity on the EEG. In the top left panel the solid line shows the loge of total power (mean ± SE, μV2) in 3Hz bands during Quiet Sleep in 22 infants <37 weeks postmenstrual age. The dashed line shows the power remaining after excluding bursting activity. The panel on the upper right shows the mean (±SE) percent reduction in power caused by burst removal. The lower panels show these results for Active Sleep.
Finally, we estimated how much of the decline in high frequency power is accounted for by the decline in burst activity. We divided the infants into the same PMA groups as use in Figure 4: 35-37 wk (N=29) versus 40-43 wk (N=40). In QS, there was a 37% decline in power in the 17-19 Hz band between these ages and a 37% decline in AS. In the younger group of infants, removing bursts decreased 17-19 Hz power by 28% in QS and 25% in AS. Thus, in QS, burst removal could account for 76% of the decline in high frequency power associated with development (i.e. 28% / 37%). In AS, burst removal could account for 68% of this developmental change (i.e. 25% / 37%).
Discussion
Electrical activity recorded on the scalp emanates from neuronal activity near the outer surface of the cortex and is due to synchronous postsynaptic activity (Nunez and Srinivasan, 2006). During the period over which the majority of the CHIME studies were performed (35-52 weeks PMA) the human brain is growing rapidly in size and forming new circuits involving trillions of new synaptic connections (Khazipov and Luhmann, 2006). Accordingly, increases in EEG activity (power) uniformly across frequencies through this period might be expected. Instead, we found frequency-specific changes in EEG activity that was dependent on sleep state. In general, power below 7 Hz increased throughout the time period assessed while power from 7-9 Hz showed little change with age. In contrast with these linear patterns, power in frequency bands above 9 Hz decreased from ∼36 to ∼45 weeks PMA and then remained relatively stable to 52 weeks (PMA). An exception to this was power between 9 and 15 Hz which increased from ∼45 to 52 weeks (PMA) but only in QS. The state dependent increase in 9 to 15 Hz power starting at 45 weeks, but only in QS, is likely due to the emergence of “sleep spindles” during this period (Jenni, Borbely, 2004). The combination of results reported in other studies is consistent with these diverse patterns of change in EEG power (Jenni, Borbely, 2004, Scher, 1997, Scher, Steppe, 1995, Sterman, Harper, 1977). Our analyses of the CHIME data indicate that the observed decrease of high frequency power prior to term age is largely (74% in AS, 82% in QS) due to a several fold decrease in the frequency of occurrence of bursts of high frequency activity. While not widely recognized this developmental pattern may be understood in the context of the formation of transient microcircuits within the cortical subplate and their modulation by GABA interneurons whose effects during this critical period of development transition from depolarizing to hyperpolarizing.
Following the early developmental concepts of Hebb (Hebb, 1949) many now accept the idea that bursts of activity, whether spontaneous or evoked, are likely critical for circuit formation and subsequent brain development (Allene and Cossart, 2010, Ben-Ari, Gaiarsa, 2007, Katz and Shatz, 1996, Khazipov and Luhmann, 2006). Establishing the precise functions of these bursts and their neurophysiologic underpinning remains an important focus of current research. In the hippocampus of rodents, periodic increases in synchrony can lead to bursts known as giant depolarizing potentials (GDPs). These events can be of several hundred microvolts in amplitude and a few hundred milliseconds (Ben-Ari et al., 1989). Periodic enhancement of synchrony in neuronal firing also occurs in the neocortex and can be measured in the surface EEG (Seelke and Blumberg, 2008) and in cortical layers (Allene et al., 2008, Minlebaev et al., 2007, Rheims et al., 2008, Yang, Hanganu-Opatz, 2009).
GDPs in the hippocampus are seen at the same time during development that GABA has depolarizing effects. GDPs disappear with the transition in expression of chloride transporter-mediated inward (NKCC1) to outward (KCC2) flux and the transition in the effect of GABA to membrane hyperpolarization (Ben-Ari, Gaiarsa, 2007, Rheims, Minlebaev, 2008). Consistent with these findings, bumetanide, a selective inhibitor of NKCC1, blocks GDP in slices during the early postnatal period (Dzhala et al., 2005). Bumetanide also blocks sharp waves in the rat hippocampus at postnatal days 7-9 which are thought to be the in vivo version GDPs (Sipila et al., 2006).
The idea that the transient developmental expression of some types of cortical bursting patterns in EEG might similarly be related to the early depolarizing effects of GABA was first suggested by Vanhatalo and colleagues (Vanhatalo, Palva, 2005). This group described and quantified bursts of activity that they called Spontaneous Activity Transients (SATs) identified primarily by their high amplitude (∼100uV) and low frequency content. It is important to note that these transients also contain power across a broad frequency spectrum including high frequencies (Tolonen, Palva, 2007). The incidence of SATs declines dramatically during the perinatal period, disappearing by a few weeks after birth (Tolonen, Palva, 2007, Vanhatalo and Kaila, 2006, Vanhatalo, Palva, 2005). A similar decline in SAT-like features was reported in the EEG of rats during the early postnatal period (Seelke and Blumberg, 2008). Analyses of postmortem human infant cortical tissue obtained at several ages through this period demonstrated a transition in expression of chloride transporters from the inwardly transporting NKCC1 to the outwardly transporting KCC2 (Dzhala, Talos, 2005). Vanhatalo and colleagues noted that the decline in SATs parallels the transition in the effects of GABA from depolarizing to hyperpolarizing (Vanhatalo, Palva, 2005), thus the conclusion that cortical SATs may be dependent on the depolarizing effects of GABA.
The burst detection method we used quantifies all high frequency bursts, not only those coincident with low frequency bursts of activity (e.g. SATs and delta brush). As can be seen in Figures 1 and 5 we identified bursts of many types. Vanhatalo and colleagues have shown that even though SATs contain high frequency power (Tolonen, Palva, 2007) precise detection of SATs requires that the EEG be recorded with DC amplifiers that allow low frequency transients to be seen (Vanhatalo and Kaila, 2008). However, CHIME data were high pass filtered at 1 Hz which would impede the accurate detection of onset and offset of SATs. Thus, we cannot determine the proportion of high frequency bursts measured by our algorithm that are associated with SATs. Moreover, the minimal spatial resolution (2 bipolar leads) and low sampling rate (100Hz) used in CHIME preclude determination of the distribution of bursts over the cortical surface and whether there are bursts at higher frequencies. In addition, we are unable to quantify bursting activity prior to 35 weeks PMA. Future studies should acquire data at earlier ages, address topographic distribution of these developmental patterns, and assess frequencies above 24 Hz to enrich these analyses. Despite the limitations of the CHIME data set, we show that high frequency bursts decline with age (see Figure 6) over the same time course as others found SATs decline (Tolonen, Palva, 2007, Vanhatalo and Kaila, 2006, Vanhatalo, Palva, 2005). Albeit speculative, this suggests that high frequency bursts might be used as a marker for the transitions in the effects of GABA on membrane polarization.
The speculation that bursts of EEG activity of various forms may be linked to the depolarizing actions of GABA during early stages of brain development is paralleled by an hypothesis that relates the bursting activity to a cortical structure that, like neuronal responses to GABA, undergoes rapid changes during the late prenatal and early postnatal period. This structure is the layer of neurons and interneurons referred to as the subplate. The subplate lies beneath the primitive cortical plate and, at its maximum size, which is achieved in humans between 22 and 34 weeks PMA, is several fold thicker than the cortical plate (Kanold and Luhmann, 2010, Kostovic et al., 1989, Kostovic and Rakic, 1990). The subplate circuitry is formed by interconnections between neurons in the subplate and cortical plate, from ascending thalamic fibers, from GABAergic interneurons, and from ascending monoaminergic fibers (Kanold and Luhmann, 2010, Kostovic and Rakic, 1980, 1990, Luskin and Shatz, 1985, Weber and Andrade, 2010). Numerous studies have shown that the subplate neural circuitry is required for normal cortical development (Allendoerfer and Shatz, 1994, Clowry et al., 2010, Ghosh et al., 1990, Ghosh and Shatz, 1992, Kanold et al., 2003, Soriano et al., 1994, Wood et al., 1992, Xie et al., 2002). Of particular interest to the current findings, many studies have shown that subplate circuits are transient and in humans regress during the last weeks of gestation and early postnatal period although in some regions these circuits may be sustained for up to 6 months of age or longer (Delalle et al., 1997, Kanold and Luhmann, 2010, Kostovic and Judas, 2007).
Although there are no in vivo studies in the subplate, the role of this transient layer in generation of high frequency bursts is supported by electrophysiological studies of early postnatal mouse cortical tissue slices. In one study, brief (2 msec) electrical stimulation of the subplate resulted in high frequency (∼18Hz) oscillations in the cortical plate lasting several hundred msec (Sun and Luhmann, 2007). In another study, also conducted in mice, application of cholinergic agonists to cortical slices produced bursts of activity at a comparable frequency range (∼17Hz) (Dupont et al., 2006). Although these cholinergic-induced bursts were not blocked by a GABA-A antagonist, this study did show that these high frequency bursts were dependent on gap junction based circuitry of the subplate but only up to about 3 days of age. In subsequent studies, it was found that activation by non-synaptic release of GABA facilitates the generation of “up states” during which bursting activity is produced and prolongs cholinergic-induced depolarization of subplate neurons (Hanganu et al., 2009). These findings in mice are of interest in relation to the current study because the frequencies within these cholinergic-induced bursts are essentially the same as the mean spectral frequency of the spontaneous bursts we identified in sleeping infants (17.3Hz in QS, 18.5Hz in AS).
The decrease in bursting activity we quantified in this current study occur over the time interval expected for the transition in GABA effects on membrane polarization and with the dissolution of the cortical subplate. However, these are only temporal associations and do not demonstrate causal, mechanistic links. Other researchers have reported that in both preterm human infants and early postnatal mice high frequency bursting activity in response to visual stimulation. This response disappears with age and is replaced by the typical evoked response seen later in development. These light-induced bursts are not blocked by a GABA-A antagonist (Colonnese et al., 2010) and therefore not dependent on the depolarizing effects of GABA expected during this period of development. These authors also proposed that the disappearance of these bursts was not related to the disappearance of the subplate but no direct evidence of this was shown. It is important to note that the transition in the response to light flashes in human infants occurred from 34 to 36 weeks, prior to the disappearance of bursting activity we quantified. Thus, light-induced bursts and the spontaneous bursting activity we recorded differ in development course and are not likely to be related.
In other studies Marcano-Reik and colleagues showed that in rat pups before 6 days of age high frequency bursting activity in the somatosensory cortex associated with myoclonic twitches is increased following administration of bicuculline, a GABA-A antagonist (Marcano-Reik et al., 2010). Transection of the corpus callosum increased the number of bursts associated with twitches without altering the number of twitches. This increase in bursting activity was also potentiated by bicuculline. These findings are discordant with the hypothesis that depolarizing effects of GABA are permissive if not casually related to bursts since antagonism of GABA-A receptors did not inhibit bursts. These authors proposed that although the effects of GABA on membrane potential at this early age might be depolarizing, the major effect of GABA is nonetheless inhibitory due to shunting. However, it is not clear whether the inhibitory effects of GABA on twitch-related bursts in rats applies to the multiple types of bursting activity we characterized in human infants.
The results provided in Table 2 show an addition finding from this study. Power below 13 Hz is greater in the right hemisphere, but only at the earlier PMA age range (35 to 45 wks). This is interesting in light of findings showing auditory evoked responses have shorter latencies on the right side in fetuses 29 to 38 wks of age (Schleussner et al., 2004), and greater amplitude on the right side in infants born between 30 and 34 wks GA and studied at about 35 PMA (Mento et al., 2010). These data suggest that the right hemisphere may develop more rapidly than the left, and our results provide evidence that resting EEG power may also be a marker of this asymmetry in maturation. We did not find a left/right difference in the rate of bursting through this age range. However, bursts as we measured them are higher frequency phenomena and thus may reflect a different aspect of cortical maturation than low frequency power.
Although developmental timing is not precise, the process of subplate dissolution overlaps with the transition in chloride transporter gene expression and the switch in GABA effects on membrane polarization. It is not clear if these two development phenomena are mechanistically linked. We propose that bursting activity measured in the EEG of premature infants requires intact transient subplate circuits and their connections to the cortical plate. In addition, some types of bursting activity may be related to membrane depolarizing effects of GABA; although, this may not be true for all types of bursts. Disruptions in GABA's actions and subplate function during early development cause structural and functional changes that could underlie dysfunction in sensory processing, emotion regulation, and cognition which are apparent in a wide range of mental disorders including autism and schizophrenia. (Avino and Hutsler, 2010, Ferriero and Miller, 2010, Kostovic et al., 2011, Wang and Kriegstein, 2011). Currently, it is not possible to determine in human infants the progression of these fundamental neurophysiologic processes. Our analyses of the CHIME data set are informative with regard to helping establish norms for EEG development and suggest that systematic quantitative analyses of resting EEG activity can provide a means of tracking specific neurophysiologic mechanisms that are key to normal brain development.
Highlights.
High frequency EEG activity in infants born prematurely unexpectedly declines from 35 to 45 weeks postmenstrual age.
This decline in activity can be accounted for by a decrease in the rate of high frequency EEG bursts.
These changes in EEG activity during early development overlap with a transition in GABA's effects on neurons and the dissolution of the transient circuitry of the cortical subplate.
Significance.
We postulate that quantitative characterization of features of the EEG unique to early development provide indices for tracking changes in specific neurophysiologic mechanisms that are critical for normal development of brain function.
Acknowledgments
The writing of this manuscript was supported by the Sackler Institute of Developmental Psychobiology at Columbia University, the BrainGut Initiative at Columbia University Medical Center, and by National Institute of Health grants R01 HD045653 to RAD, R03 HD060671 to PGG, and 5R37HD32774-13 to WPF.
Footnotes
Financial Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, et al. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28:12851–63. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allene C, Cossart R. Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J Physiol. 2010;588:83–91. doi: 10.1113/jphysiol.2009.178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre M, Lamblin MD, d'Allest AM, Curzi-Dascalova L, Moussalli-Salefranque F, T SNT, et al. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Avino TA, Hutsler JJ. Abnormal cell patterning at the cortical gray-white matter boundary in autism spectrum disorders. Brain Res. 2010;1360:138–46. doi: 10.1016/j.brainres.2010.08.091. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–25. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–84. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Biagioni E, Bartalena L, Boldrini A, Pieri R, Cioni G. Constantly discontinuous EEG patterns in full-term neonates with hypoxic-ischaemic encephalopathy. Clin Neurophysiol. 1999;110:1510–5. doi: 10.1016/s1388-2457(99)00091-7. [DOI] [PubMed] [Google Scholar]
- Biagioni E, Frisone MF, Laroche S, Kapetanakis BA, Ricci D, Adeyi-Obe M, et al. Maturation of cerebral electrical activity and development of cortical folding in young very preterm infants. Clin Neurophysiol. 2007;118:53–9. doi: 10.1016/j.clinph.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Clowry G, Molnar Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;217:276–88. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, et al. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–98. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell DH, Brooks LJ, Colton T, Corwin MJ, Hoppenbrouwers TT, Hunt CE, et al. Infant polysomnography: reliability. Collaborative Home Infant Monitoring Evaluation (CHIME) Steering Committee. Sleep. 1997;20:553–60. [PubMed] [Google Scholar]
- Crowell DH, Brooks LJ, Corwin M, Davidson-Ward S, Hunt CE, Kapuniai LE, et al. Ontogeny of arousal. J Clin Neurophysiol. 2004;21:290–300. doi: 10.1097/01.wnp.0000141754.03598.dc. [DOI] [PubMed] [Google Scholar]
- Crowell DH, Kulp TD, Kapuniai LE, Hunt CE, Brooks LJ, Weese-Mayer DE, et al. Infant polysomnography: reliability and validity of infant arousal assessment. J Clin Neurophysiol. 2002;19:469–83. doi: 10.1097/00004691-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Curzi-Dascalova L, Peirano P, Morel-Kahn F. Development of sleep states in normal premature and full-term newborns. Dev Psychobiol. 1988;21:431–44. doi: 10.1002/dev.420210503. [DOI] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostovic I, Uylings HB. Laminar distribution of neuropeptide Y- immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol. 1997;379:515–22. doi: 10.1002/(sici)1096-9861(19970324)379:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Miller SP. Imaging selective vulnerability in the developing nervous system. J Anat. 2010;217:429–35. doi: 10.1111/j.1469-7580.2010.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–81. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 1992;255:1441–3. doi: 10.1126/science.1542795. [DOI] [PubMed] [Google Scholar]
- Grieve PG, Emerson RG, Fifer WP, Isler JR, Stark RI. Spatial correlation of the infant and adult electroencephalogram. Clin Neurophysiol. 2003;114:1594–608. doi: 10.1016/s1388-2457(03)00122-6. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Okabe A, Lessmann V, Luhmann HJ. Cellular mechanisms of subplate- driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex. 2009;19:89–105. doi: 10.1093/cercor/bhn061. [DOI] [PubMed] [Google Scholar]
- Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proceedings of the IEEE. 1978;66:51–83. [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hoppenbrouwers T, Hodgman JE, Rybine D, Fabrikant G, Corwin M, Crowell D, et al. Sleep architecture in term and preterm infants beyond the neonatal period: the influence of gestational age, steroids, and ventilatory support. Sleep. 2005;28:1428–36. doi: 10.1093/sleep/28.11.1428. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Kohrman MH. Topographic mapping of the EEG in premature infants and neonates. Clin Electroencephalogr. 1989;20:228–34. doi: 10.1177/155005948902000410. [DOI] [PubMed] [Google Scholar]
- Hunt CE. Sudden infant death syndrome and subsequent siblings. CHIME Steering Committee. Collaborative Home Infants Monitoring Evaluation. Pediatrics. 1995;95:430–2. [PubMed] [Google Scholar]
- Jenni OG, Borbely AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–5. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–8. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–61. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–68. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M, Sedmak G. Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: relevance for schizophrenia. Int J Dev Neurosci. 2011;29:193–205. doi: 10.1016/j.ijdevneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Lukinovic N, Judas M, Bogdanovic N, Mrzljak L, Zecevic N, et al. Structural basis of the developmental plasticity in the human cerebral cortex: the role of the transient subplate zone. Metab Brain Dis. 1989;4:17–23. doi: 10.1007/BF00999489. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–42. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–70. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Lamblin MD, Andre M, Challamel MJ, Curzi-Dascalova L, d'Allest AM, De Giovanni E, et al. Electroencephalography of the premature and term newborn. Maturational aspects and glossary] Neurophysiol Clin. 1999;29:123–219. doi: 10.1016/s0987-7053(99)80051-3. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985;5:1062–75. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcano-Reik AJ, Prasad T, Weiner JA, Blumberg MS. An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity. Behav Neurosci. 2010;124:600–11. doi: 10.1037/a0020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mento G, Suppiej A, Altoe G, Bisiacchi PS. Functional hemispheric asymmetries in humans: electrophysiological evidence from preterm infants. Eur J Neurosci. 2010;31:565–74. doi: 10.1111/j.1460-9568.2010.07076.x. [DOI] [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–94. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci. 2008;28:10134–44. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MM, Fifer WP, Grose-Fifer J, Sahni R, Stark RI, Schulze KF. A novel quantitative measure of Trace-alternant EEG activity and its association with sleep states of preterm infants. Dev Psychobiol. 1997;31:167–74. doi: 10.1002/(sici)1098-2302(199711)31:3<167::aid-dev1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Myers MM, Stark RI, Fifer WP, Grieve PG, Haiken J, Leung K, et al. A quantitative method for classification of EEG in the fetal baboon. Am J Physiol. 1993;265:R706–14. doi: 10.1152/ajpregu.1993.265.3.R706. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electrical Fields of the Brain: The Neurophysics of EEG. Second. New York: Oxford University Press; 2006. [Google Scholar]
- Parmelee AH. EEG power spectral analysis of newborn infants' sleep states. Electroencephalogr Clin Neurophysiol. 1969;27:690–1. doi: 10.1016/0013-4694(69)91316-9. [DOI] [PubMed] [Google Scholar]
- Rheims S, Minlebaev M, Ivanov A, Represa A, Khazipov R, Holmes GL, et al. Excitatory GABA in rodent developing neocortex in vitro. J Neurophysiol. 2008;100:609–19. doi: 10.1152/jn.90402.2008. [DOI] [PubMed] [Google Scholar]
- Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Sleeping position and electrocortical activity in low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2005;90:F311–5. doi: 10.1136/adc.2004.055327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher MS. Neonatal encephalopathies as classified by EEG-sleep criteria: severity and timing based on clinical/pathologic correlations. Pediatr Neurol. 1994;11:189–200. doi: 10.1016/0887-8994(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Scher MS. Neurophysiological assessment of brain function and maturation. II. A measure of brain dysmaturity in healthy preterm neonates. Pediatr Neurol. 1997;16:287–95. doi: 10.1016/s0887-8994(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Maturational trends of EEG-sleep measures in the healthy preterm neonate. Pediatr Neurol. 1995;12:314–22. doi: 10.1016/0887-8994(95)00052-h. [DOI] [PubMed] [Google Scholar]
- Scher MS, Sun M, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Comparisons of EEG sleep state-specific spectral values between healthy full-term and preterm infants at comparable postconceptional ages. Sleep. 1994a;17:47–51. doi: 10.1093/sleep/17.1.47. [DOI] [PubMed] [Google Scholar]
- Scher MS, Sun M, Steppe DA, Guthrie RD, Sclabassi RJ. Comparisons of EEG spectral and correlation measures between healthy term and preterm infants. Pediatr Neurol. 1994b;10:104–8. doi: 10.1016/0887-8994(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Schleussner E, Schneider U, Arnscheidt C, Kahler C, Haueisen J, Seewald HJ. Prenatal evidence of left-right asymmetries in auditory evoked responses using fetal magnetoencephalography. Early Hum Dev. 2004;78:133–6. doi: 10.1016/j.earlhumdev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–9. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+ -activated K+ current. Eur J Neurosci. 2006;23:2330–8. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- Soriano E, Del Rio JA, Martinez A, Super H. Organization of the embryonic and early postnatal murine hippocampus. I. Immunocytochemical characterization of neuronal populations in the subplate and marginal zone. J Comp Neurol. 1994;342:571–95. doi: 10.1002/cne.903420406. [DOI] [PubMed] [Google Scholar]
- Stark RI, Haiken J, Nordli D, Myers MM. Characterization of electroencephalographic state in fetal baboons. Am J Physiol. 1991;261:R496–500. doi: 10.1152/ajpregu.1991.261.2.R496. [DOI] [PubMed] [Google Scholar]
- Statz A, Dumermuth G, Mieth D, Duc G. Transient EEG patterns during sleep in healthy newborns. Neuropediatrics. 1982;13:115–22. doi: 10.1055/s-2008-1059609. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Harper RM, Havens B, Hoppenbrouwers T, McGinty DJ, Hodgman JE. Quantitative analysis of infant EEG development during quiet sleep. Electroencephalogr Clin Neurophysiol. 1977;43:371–85. doi: 10.1016/0013-4694(77)90260-7. [DOI] [PubMed] [Google Scholar]
- Sun JJ, Luhmann HJ. Spatio-temporal dynamics of oscillatory network activity in the neonatal mouse cerebral cortex. Eur J Neurosci. 2007;26:1995–2004. doi: 10.1111/j.1460-9568.2007.05819.x. [DOI] [PubMed] [Google Scholar]
- Tolonen M, Palva JM, Andersson S, Vanhatalo S. Development of the spontaneous activity transients and ongoing cortical activity in human preterm babies. Neuroscience. 2007;145:997–1006. doi: 10.1016/j.neuroscience.2006.12.070. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Kaila K. Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med. 2006;11:471–8. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Kaila K. Generation of ‘positive slow waves’ in the preterm EEG: by the brain or by the EEG setup? Clin Neurophysiol. 2008;119:1453–4. doi: 10.1016/j.clinph.2008.02.013. author reply 4-5. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Palva JM, Andersson S, Rivera C, Voipio J, Kaila K. Slow endogenous activity transients and developmental expression of K+-Cl− cotransporter 2 in the immature human cortex. Eur J Neurosci. 2005;22:2799–804. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. Blocking early GABA depolarization with bumetanide results in permanent alterations in cortical circuits and sensorimotor gating deficits. Cereb Cortex. 2011;21:574–87. doi: 10.1093/cercor/bhq124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a Gene and 5-HT(2A) Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front Neurosci. 2010;4:1–2. doi: 10.3389/fnins.2010.00036. Article 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner SS, Stockard JE, Bickford RG. Atlas of Neonatal Electroencephalography. New York: Raven Press; 1977. [Google Scholar]
- Wood JG, Martin S, Price DJ. Evidence that the earliest generated cells of the murine cerebral cortex form a transient population in the subplate and marginal zone. Brain Res Dev Brain Res. 1992;66:137–40. doi: 10.1016/0165-3806(92)90150-u. [DOI] [PubMed] [Google Scholar]
- Xie Y, Skinner E, Landry C, Handley V, Schonmann V, Jacobs E, et al. Influence of the embryonic preplate on the organization of the cerebral cortex: a targeted ablation model. J Neurosci. 2002;22:8981–91. doi: 10.1523/JNEUROSCI.22-20-08981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–25. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


