Abstract
The inhibitor of NF-κB alpha (IκBα) protein is an important regulator of the transcription factor NF-κB. In neurons, IκBα has been shown to play a role in neurite outgrowth and cell survival. Recently, a phosphorylated form of IκBα (pIκBα Ser32/36) was reported to be highly enriched at the axon initial segment (AIS) and was proposed to function upstream of ankyrinG in AIS assembly, including ion channel recruitment. However, we report here that the AIS clustering of ankyrinG and Na+ channels in the brains of IκBα knockout (Nfkbia−/−) mice is comparable to that in wild-type littermates. Furthermore, we found that multiple phospho-specific antibodies against pIκBα Ser32/36 non-specifically label AIS in Nfkbia−/− cortex and AIS in dissociated Nfkbia−/− hippocampal neurons. With the exception of ankyrinG, shRNA-mediated knockdown of known AIS proteins in cultured hippocampal neurons did not eliminate the AIS labeling with pIκBα antibodies. Instead, the pIκBα antibodies cross-react with a phosphorylated epitope of a protein associated with the microtubule-based AIS cytoskeleton that is not integrated into the AIS membrane complex organized by ankyrinG. Our results indicate that pIκBα is neither enriched at the AIS nor required for AIS assembly.
Keywords: Axon initial segment, ankyrinG, NF-κB signaling pathway, neuronal polarity
Introduction
The axon initial segment (AIS) is the site of action potential (AP) initiation in neurons (Khaliq & Raman, 2006; Kole et al., 2008; Hu et al., 2009; Foust et al., 2010; Palmer et al., 2010; Popovic et al., 2011). High-density clusters of voltage gated Na+ channels facilitate action potential (AP) initiation at the distal AIS and are recruited by the cytoskeletal adaptor protein ankyrinG (ankG) (Catterall et al., 1981; Zhou et al., 1998; Jenkins & Bennett, 2001; Garrido et al., 2003; Lemaillet et al., 2003; Kole et al., 2008; Lorincz & Nusser, 2010). Recent reports show that the AIS cytoskeleton plays an important role in AIS assembly and long-term maintenance (Song et al., 2009; Tapia et al., 2010; Leterrier et al. 2011; Maniar et al., 2011; Sanchez-Ponce et al., 2011). For example, the AIS cytoskeleton stabilizes AIS membrane proteins and acts as a molecular sieve to maintain axodendritic polarity (Winckler et al., 1999; Song et al., 2009). In particular, ankG is required for AIS formation and maintenance (Hedstrom et al., 2008; Sobotzik et al., 2009), yet little is known about the proteins that act upstream of ankG in AIS assembly (Rasband, 2010).
Among the proteins enriched at the AIS, pIκBα was the first to be proposed to act upstream of ankG in AIS formation (Sanchez-Ponce et al., 2008). IκBα is an inhibitor of the transcription factor NF-κB. Phosphorylation of IκBα at serines 32 and 36 frees NF-κB to traffic to the nucleus and initiate transcription (Viatour et al., 2005). NF-κB signaling is critically involved in cell growth, and survival and IκBα has been shown to specifically contribute to neurite outgrowth and neuronal plasticity (Chen et al., 2001; Gutierrez et al., 2005). Schultz et al. (2006) reported the enrichment of multiple NF-κB signaling pathway proteins at the AIS. Interestingly, only phosphorylated forms of these proteins (phospho-IκBα (Ser32/36), phospho-IKKα/β (Ser180/Ser181), phospho-NF-κB p65 (Ser536)) were shown to be enriched at the AIS by immunfluorescence. However, these findings were somewhat surprising since IκBα phosphorylated at serines 32 and 36 is reported to be immediately targeted for degradation by the ubiquitin proteasome system (Karin & Ben-Neriah, 2000). Nonetheless, subsequent pharmacological studies using IKKα/β inhibitors in hippocampal neuron cultures revealed a possible role for pIκBα in the recruitment of ankG and voltage-gated ion channels to the AIS (Sanchez-Ponce et al., 2008; Sanchez-Ponce et al., 2010).
In light of pIκBα’s reported role in AIS formation (Sanchez-Ponce et al., 2008), we examined AIS assembly and neuronal development in IκBα null (Nfkbia−/−) mice (Beg et al., 1995). Unexpectedly, Nfkbia−/− brains had normal AIS, with ankG and Na+ channel enrichment at the proximal axon in early development. More surprisingly, immunostaining with three independent phospho-specific antibodies targeting pIκBα (Ser32/36) in Nfkbia−/− tissue and dissociated hippocampal cultures derived from IκBα knockout mice showed that the AIS signal for pIκBα is not specific. Instead, the antibodies detect a phosphorylated epitope of a protein associated with the microtubule-based cytoskeleton at the AIS. The off-target AIS protein does not colocalize with ankG at the membrane, yet it depends on ankG for its AIS accumulation. Our results demonstrate that pIκBα is not required for AIS assembly and the pIκBα antibodies that detect the AIS by immunostaining recognize an unidentified protein associated with the microtubule-based cytoskeleton at the AIS.
Results
AIS assembly is normal in IκBα knockout mice
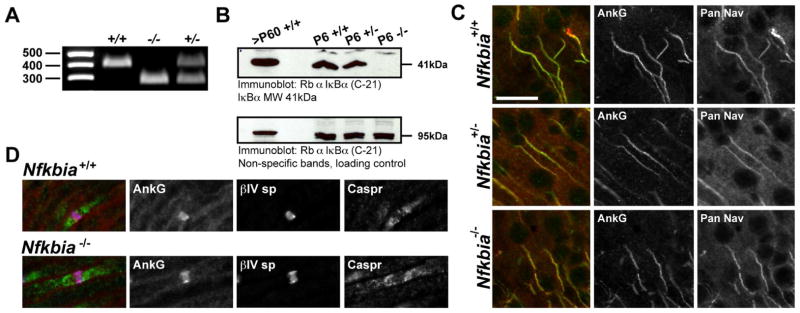
Inhibiting the phosphorylation of IκBα was reported to disrupt AIS assembly and axon outgrowth in cultured hippocampal neurons (Sanchez-Ponce et al., 2008). To study the role of pIκBα in AIS assembly in vivo, we examined the subcellular localization of ankG and voltage-gated Na+ channels in IκBα knockout (Nfkbia−/−) mouse brain (Beg et al., 1995). Nfkbia−/− mice die by P9; therefore, we performed our studies on P6 mice. All mice used in experiments were genotyped (Fig. 1A), and immunoblot analysis of brain membrane homogenates from littermates of each Nfkbia genotype confirmed the absence of IκBα protein expression in the Nfkbia−/− animals (Fig. 1B). However, immunolabeling of P6 brain tissue revealed no difference in AIS formation between control and Nfkbia−/− mice. Specifically, we found no statistically significant difference in ankG and pan-Na+ channel immunofluorescence intensity at P6 cortical neuron AIS between genotypes [AnkG FI (AU): Nfkbia+/+ 92.84±10.3, Nfkbia+/− 87.2±5.1, Nfkbia−/− 89.0±6.7 (n=10), p=0.87; Nav FI (AU): Nfkbia+/+ 101.2±11.7, Nfkbia+/− 123.2±6.6, Nfkbia−/− 124.6±7.8 (n=10), p=0.14] (Fig. 1C). Similarly, AIS length in cortical layer II/III neurons did not differ significantly between genotypes [AIS length (μm): Nfkbia+/+ 26.4±1.7, Nfkbia+/− 25.0±1.5, Nfkbia−/− 24.9±1.6 (n=10), p=0.75]. Since pIκBα immunosignal was also previously reported at a subset of nodes of Ranvier (Politi et al., 2008), we also examined node formation in Nfkbia−/− tissue. However, like the AIS, no differences in node formation or structure were observed among genotypes (Fig. 1D). Together, our data indicate that IκBα is not required for assembly of the AIS or nodes of Ranvier.
Figure 1.
Na+ channel recruitment to the AIS and nodes of Ranvier is normal in Nfkbia−/− cortex, sciatic nerve. (A) Representative genotyping of littermates from Nfkbia+/− crosses used for immunofluorescence studies. (B) Western blot probed with phosphorylation-independent IκBα antibody showing lack of IκBα expression in the Nfkbia−/− knockout brain. Non-specific band from the same blot at 95 kDa is included as a loading control. (C) AnkG and pan-Na+ channel AIS immunofluorescence intensity is similar between WT, Nfkbia+/− heterozygotes and Nfkbia−/− homozygous null cortex. Scale bar represents 20 μm. (D) Assembly of nodes of Ranvier is comparable between WT IκBα mice and IκBα knockouts.
AIS localization of pIκBα depends on ankyrinG expression
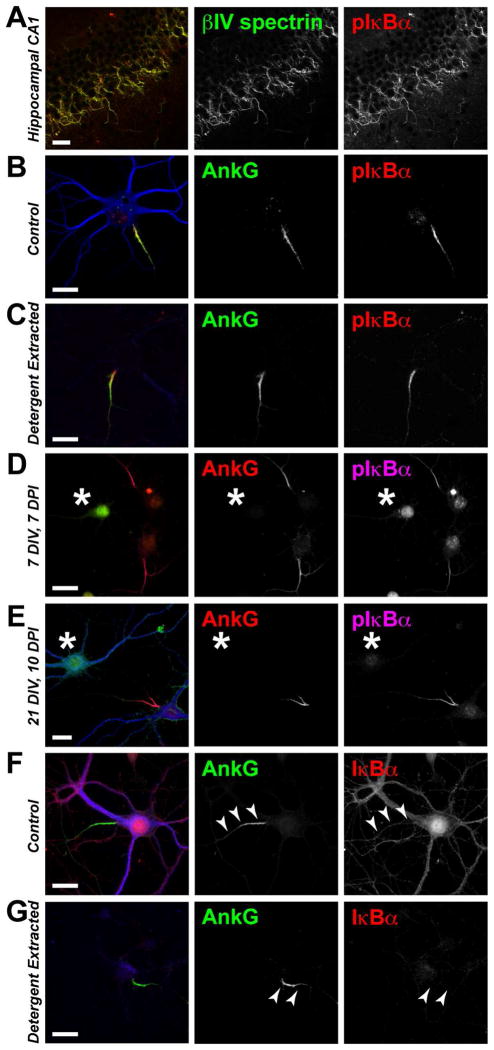
Since ankG and Na+ channels become enriched at the AIS in the absence of IκBα expression, we considered whether the AIS localization of pIκBα depends on ankG. In hippocampal neurons, pIκBα antibody immunoreactivity is highly enriched at the AIS (Schultz et al., 2006; Sanchez-Ponce et al., 2008; Sanchez-Ponce et al., 2010) (Figs. 2A,B). Consistent with previous reports (Schultz et al., 2006; Sanchez-Ponce et al., 2008), we found that pIκBα antibodies strongly label ankG-positive AIS following detergent extraction of dissociated hippocampal cultures (Fig 2C). Since proteins that remain at the AIS following detergent extraction are thought to directly interact with the detergent-resistant AIS cytoskeleton organized by ankG, we decided to test whether pIκBα clustering at the AIS depends on ankG. To this end, we silenced ankG expression during early development by transfecting ankG-targeted shRNAs into cultured hippocampal neurons at the time of plating. We found that pIκBα immunoreactivity was not enriched at the AIS of neurons lacking ankG (Fig. 2D). Therefore, the AIS-localization of pIκBα requires ankG. Furthermore, we found that pIκBα also depends on ankG for its long-term maintenance at the proximal axon. Silencing ankG expression by shRNA-mediated knockdown in mature neurons resulted in the subsequent loss of AIS pIκBα immunosignal (Fig. 2E).
Figure 2.
Phospho(S32/36)-IκBα immunoreactivity is enriched at the AIS and depends on ankG expression. A–C (A) P6 wild-type mouse hippocampal CA1 stained for pIκBα and βIV spectrin. (B,C) Immunostaining of (B) control and (C) detergent-extracted cultured wild-type hippocampal neurons grown 21 DIV. D, E (D) Immunostaining of cultured hippocampal neurons transduced with ankG shRNA-containing adenovirus at the time of plating. (E) Immunostaining of cultured hippocampal neurons in which ankG expression was silenced by adenoviral ankG shRNA delivery at 11 DIV; cells were fixed at 21 DIV, 10 days post-infection (DPI). The transduced neurons are GFP-positive and indicated by an asterisk. F, G Both (F) control and (G) detergent-extracted neurons show a lack of phosphorylation-independent IκBα clustering at the AIS (arrowheads). Scale bars represent 20μm.
Though multiple pIκBα antibodies clearly label the AIS, phosphorylation-independent IκBα antibodies do not show IκBα enrichment at the AIS; rather, these antibodies provide a faint and diffuse labeling of both the somatodendritic and the entire axonal compartment (including the AIS) (Fig. 2F). To confirm that IκBα, regardless of its phosphorylation state, is recruited to and enriched at the AIS, we took advantage of the characteristic detergent-insolubility of the AIS protein complex (Winckler et al., 1999). Proteins associated with the ankG-organized membrane complex of the AIS and the local cytoskeleton are resistant to extraction by 1% TX-100 (Winckler et al., 1999; Boiko et al., 2007; Bréchet et al., 2008; Sanchez-Ponce et al., 2008; Tapia et al., 2010). By contrast, somatodendritic proteins are easily solubilized in 1% TX-100 extraction buffer. Exploiting this property of AIS associated proteins, we hypothesized that if IκBα is enriched at the AIS, then both pIκBα and phosphorylation-independent IκBα immunoreactivity should be detectable at the AIS following detergent extraction. In stark contrast to the clear co-localization of pIκBα immunosignal with ankG following detergent extraction (Fig. 2C), immunostaining with the phosphorylation-independent IκBα antibody was completely abolished by detergent extraction (Fig. 2G). This discrepancy suggested that the AIS antigen detected by antibodies targeting pIκBα (Ser32/36) may not be IκBα, but some other antigen.
pIκBα AIS immunoreactivity is non-specific
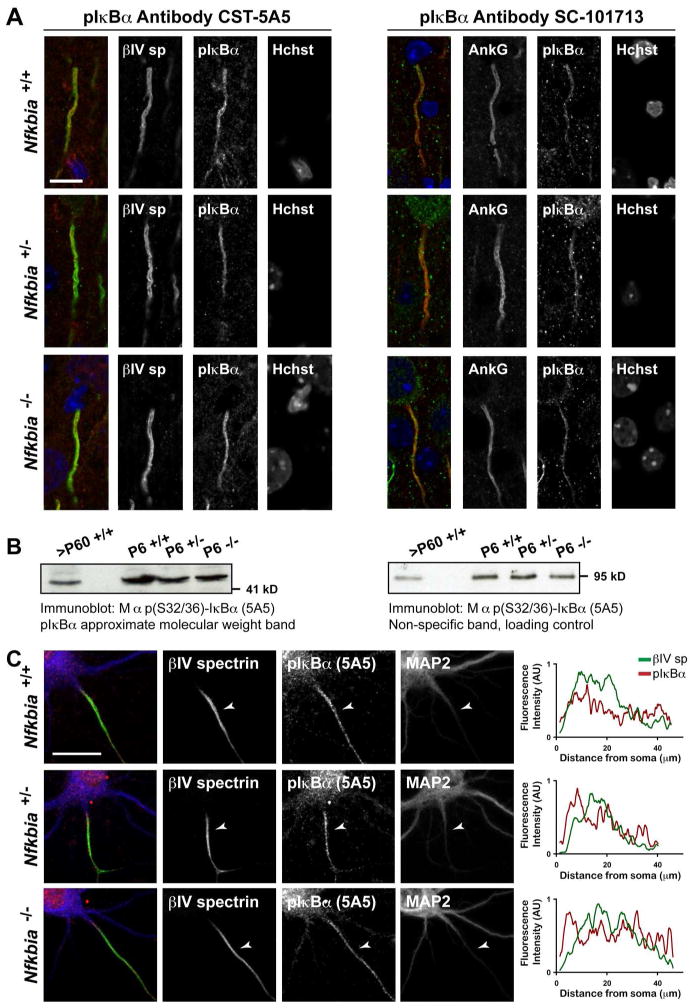
To directly test the specificity of the pIκBα antibodies, we co-immunostained Nfkbia−/− brain tissue with antibodies against ankG and one of three independent, commercially available pIκBα (Ser32/36) antibodies. Surprisingly, these ‘phospho-specific IκBα’ antibodies clearly labeled ankG-positive AIS in tissue lacking IκBα expression (Fig. 3A). When we performed a Western blot analysis of brain homogenates made from Nfkbia−/− brains, we saw a strong band at an IκBα-like molecular weight (~ 41kDa) further confirming the non-specificity of the phospho-IκBα antibodies (Fig. 3B). Finally, AIS immunoreactivity from the pIκBα antibodies was also detected in hippocampal neurons cultured from P0 Nfkbia−/− mice (Fig. 3C). Line scans show comparable pIκBα antibody AIS-immunoreactivity in Nfkbia wild-type, heterozygous, and knockout hippocampal neurons (Fig. 3C). Taken together, our results strongly support the conclusion that pIκBα is not required for AIS assembly and that pIκBα is not enriched at the AIS.
Figure 3.
Non-specific pIκBα immunoreactivity is evident at the AIS of Nfkbia−/− P6 brain tissue and cultured Nfkbia−/− hippocampal neurons. (A) P6 cortex stained for pIκBα and either βIV spectrin or ankG. Two independent antibodies designed to exclusively detect pIκBα (Ser32/36) demonstrate strong AIS immunoreactivity in IκBα KO neurons. Scale bar represents 10μm. (B) Western blot of homogenized brain membrane proteins from adult (>P60) and P6 Nfkbia+/+, +/−, and −/− mice probed with the monoclonal 5A5 pIκBα antibody. A non-specific band of 95 kDa is included as a loading control. (C) Hippocampal neurons cultured at P0 from Nfkbia+/+, +/−, or −/− littermates fixed and immunostained at 10 DIV. pIκBα immunoreactivity co-localizes with βIV spectrin at the AIS in neurons of all genotypes. Fluorescence intensity plots show AIS line-scan fluorescence intensity data for each channel. Scale bar represents 20μm.
AIS antigen is not directly associated with ankyrinG
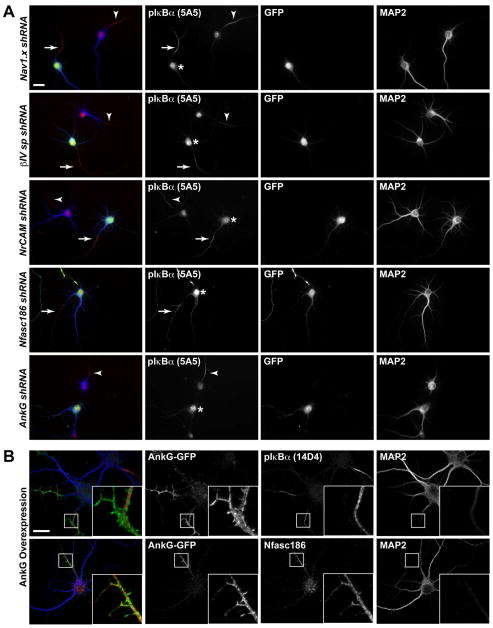
It is possible that the pIκBα antibodies recognize a protein previously reported at the AIS. To test whether the non-specific pIκBα (Ser32/36) antibodies cross-react with known AIS proteins, we transfected cells with shRNA constructs to silence the expression of several AIS proteins. The efficacy of each shRNA construct was validated previously (Hedstrom et al., 2007). We also confirmed protein knockdown by immunostaining for the target protein in parallel with pIκBα immunostaining. pIκBα antibody immunoreactivity was still detected at the AIS of hippocampal neurons lacking Na+ channels, βIV spectrin, NrCAM, or neurofascin-186 (Nfasc186) (Fig. 4A). Thus, the pIκBα antibodies do not cross-react with any of these AIS proteins. Knockdown of ankG, however, eliminated pIκBα immunoreactivity within the proximal axon (Figs. 4A, 2F–G).
Figure 4.
pIκBα antibodies do not cross-react with known AIS proteins. (A) Immunostaining of 8 DIV cultured hippocampal neurons nucleofected with shRNA targeting either Na+ channels, NrCAM, Nfasc186, βIV spectrin, or ankG, as indicated, at the time of plating. Transfected neurons express GFP. (B) Cultured hippocampal neurons transduced with ankG-GFP cDNA at the time of plating and immunostained at 15 DIV for ankG and either pIκBα (14D4) or Nfasc186. Scale bars represent 20μm.
Schultz et al. (2006) demonstrated that pIκBα antibody immunosignal is centrally located within the AIS and is surrounded by AIS membrane protein staining, suggesting an association with the microtubule-based cytoskeleton. To confirm that the pIκBα antibodies do not cross-react with ankG, we overexpressed ankG in cultured hippocampal neurons. Interestingly, we found that the pIκBα antibody immunosignal remained restricted to the interior of the AIS and was not present in the small membrane protrusions that can be seen after ankG overexpression (Fig. 4B). In contrast, Nfasc186 (a direct binding partner of ankG) immunoreactivity co-localized with ankG in the AIS membrane protrusions (Fig. 4B). The absence of pIκBα antibody immunoreactivity in the ankG-positive AIS protrusions demonstrates that the antigen detected by pIκBα antibodies is not ankG. Further, these results suggest that it is not a direct binding partner of ankG but instead is associated with the AIS cytoskeleton.
Non-specific pIκBα antibodies recognize a phosphorylated, MT-associated AIS protein
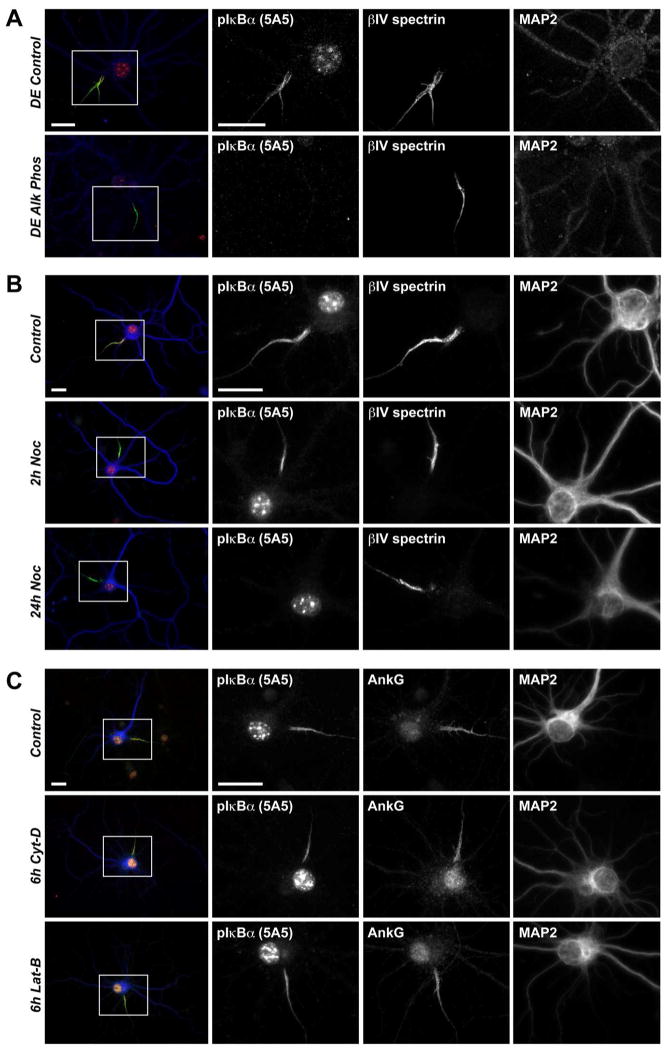
To begin to identify the AIS protein recognized by the non-specific pIκBα (S32/36) antibodies, we first confirmed that the protein of interest is indeed phosphorylated. We found that the pIκBα AIS immunosignal is abolished by alkaline phosphatase treatment of neurons permeabilized prior to fixation (Fig. 5A). Next, we treated the cells with the microtubule depolymerizing agent Nocodazole for up to 24h prior to fixation. Within two hours of Nocodazole treatment, there was a detectable disruption of pIκBα antibody immunofluorescence signal at the AIS (Fig. 5B). At the 24h time-point, pIκBα immunoreactivity was completely absent from the ankG-positive AIS (Fig. 5B). This finding is consistent with previous reports (Schultz et al., 2006). In contrast, actin depolymerization by cytochalasin-D or latrunculin-B treatment had no effect on pIκBα antibody AIS fluorescence intensity (Fig. 5C). Similar results were obtained with a second pIκBα (Ser32/36) antibody (Fig. S1). Therefore, the AIS protein detected by the pIκBα antibodies is phosphorylated and is associated with the microtubule-based cytoskeleton at the AIS.
Figure 5.
pIκBα antibody 5A5 recognizes a phosphorylated protein associated with the microtubule-based AIS cytoskeleton. (A) Immunostaining of 14 DIV detergent-extracted hippocampal neurons treated with either control solution or alkaline-phosphatase for 20 minutes prior to fixation. (B) Immunostaining of 14 DIV cells treated with either DMSO alone for 24h or 25 μM Nocodazole, a microtubule depolymerizing drug, in DMSO over 2 or 24h. (C) Immunostaining of 14 DIV cells treated 6h with either control DMSO solution or 20 μM actin-depolymerizing agents Cytochalasin-D or Latrunculin-B. Scale bars represent 20 μm.
Discussion
Neuronal activity is governed by the AIS. In turn, neuronal activity influences gene regulation. However, neither the molecular mechanisms governing AIS assembly nor the relationship between activity and transcription are well understood (Rasband 2010). Therefore, it was very exciting when components of the NF-κB signaling pathway were reported at the AIS, making it easy to postulate a link between AIS activity and gene regulation (Schultz et al., 2006). In addition, pIκBα was reported to be the first signaling molecule to regulate ankG clustering (Sanchez-Ponce et al., 2008). However, the data presented here argue that pIκBα is dispensable for ankG clustering and AIS assembly. Instead, we demonstrate that the antigen detected by the phospho-specific IκBα antibodies at the AIS is not phosphorylated IκBα but instead is an as yet unidentified, but phosphorylated, AIS protein associated with the local microtubule-based cytoskeleton. Our attempts to isolate and identify the AIS protein detected by the pIκBα antibodies by immunoprecipitation and mass-spectrometry have thus far proven unsuccessful (data not shown). We speculate this is due to the strong detergent-resistant nature of the proteins at the AIS (Boiko et al., 2003) (Figs. 2C, E).
Our results underscore the importance of stringent control experiments when using antibodies that are reportedly ‘phospho-specific.’ Control experiments must include the use of mice that lack the target antigen (Rhodes & Trimmer, 2006; Lorincz & Nusser, 2008). Although our results exclude pIκBα from the AIS, we cannot rule out other members of the NF-κB signaling pathway as components of the AIS. For example, antibodies targeting phosphorylated IKKβ show AIS immunoreactivity and application of the IKKβ inhibitor BMS 345541 disrupts AIS formation in vitro without interfering with axon growth (Schultz et al., 2006; Sanchez-Ponce et al., 2008). Since activated IKKβ is the kinase that phosphorylates IκBα, the latter finding is seemingly contradictory to our report here that pIκBα is dispensable for AIS formation both in vivo and in vitro; however, it is possible that the interpretation of the IKKβ inhibition study results may have been confounded by the off-target effects of BMS 345541 on additional kinases such as ERK8, PKD1, CK1, or CDK2 (Bain et al., 2007). Future experiments must confirm the specificity of the phospho-specific antibodies designed against proteins in the NF-κB signaling pathway. The importance of such experimental rigor is further highlighted by the recent findings of Herkenham et al. (2011) who demonstrate the non-specificity of many commonly used commercial antibodies targeting the NF-κB subunit proteins p65 and p50.
Our findings are consistent with the emerging concept that phosphorylation of AIS ion channels, their auxiliary subunits, and local cytoskeletal proteins may regulate AIS assembly and function (Bréchet et al., 2008; Sanchez-Ponce et al., 2010 Leterrier et al., 2011; Li et al., 2011; Vacher et al., 2011). Consistent with this idea, several kinases have now been reported at the AIS. For example, phosphorylation of Na+ channel α subunits by CK2 at the AIS was reported to promote their interaction with ankG (Bréchet et al., 2008). Cdk-mediated phosphorylation of Kvβ2 at the AIS controls the release of Kvβ2-Kv1 K+ channel complexes from the microtubule-associated end binding protein EB1 for insertion within the AIS membrane (Vacher et al., 2011). Moreover, CamKII was shown to be enriched at Purkinje neuron AIS where it interacts with βIV spectrin (Hund et al., 2010). Thus, the AIS may be a ‘hotspot’ for phosphorylation, which could explain why only phospho-antibodies against NF-κB signaling proteins labeled the AIS (Schultz et al., 2006).
Several recent reports demonstrate a previously unrecognized plasticity in AIS structure that is correlated with changes in neuronal excitability (Grubb & Burrone, 2010; Kuba et al., 2010; Kaphzan et al., 2011). By analogy to the phosphorylation-dependent events that facilitate synaptic remodeling (Evers et al., 2010; Lee et al., 2011), plastic changes at the AIS may also depend on local protein phosphorylation. Future studies to identify the enzymes that regulate dynamic processes at the AIS, including ion channel stability and availability, cytoskeletal dynamics, and axonal trafficking, will improve our understanding of the multi-functional role of the AIS in neuronal development, excitability, and plasticity.
Experimental Methods
Animals
Nfkbia+/− mice were described previously (Beg et al., 1995) and generously provided by Dr. Hui Zheng, Baylor College of Medicine. Nfkbia+/− mice were crossed to obtain Nfkbia+/−, Nfkbia−/−, and Nfkbia+/+ mice. Wild-type C57BL/6 mice were purchased from Jackson Laboratories. Timed-pregnant Sprague Dawley rats were purchased from Harlan Sprague Dawley. Animals were housed and maintained in Baylor College of Medicine’s Center for Comparative Medicine, compliant with the NIH Guide for Care and Use of Laboratory Animals.
Genotyping
Genotyping was performed by PCR of tail snip DNA from either P0 or P6 mice, per experimental requirements. A three-primer multiplex from Nfkbia was used, including a common forward primer (AGTGGCTCATCGCAGGGAGTTTCT), a reverse wild-type (CAGCTCCTTCACCATTTGCTCGTA), and a reverse knockout primer (CGGTATCGATACTGGCTGAA).
Antibodies
The following primary antibodies were used: rabbit polyclonal anti- IκBα (C21) (SC-371, Santa Cruz Biotechnology), mouse monoclonal anti-phospho-IκBα (Ser32/36) (5A5, Cell Signaling Technology), rabbit monoclonal anti-phospho-IκBα (Ser32/36) (14D4, Cell Signaling Technology), rabbit polyclonal anti-phospho-IκBα (Ser32/36) (SC-101713, Santa Cruz Biotechnology), mouse monoclonal anti-ankG (N106/36, UC Davis/NIH NeuroMab Facility), chicken and rabbit polyclonal anti-βIV spectrin (Yang et al., 2004), mouse monoclonal anti-pan-Na+ channel (K58/35, Sigma), rabbit polyclonal anti-Caspr (Schafer et al., 2004), rabbit polyclonal anti-GFP (Invitrogen), chicken polyclonal anti-MAP2 (Encor Biotechnology Inc.), and mouse monoclonal anti-pan-neurofascin (L11A/41.6, Schafer et al., 2004). Alexa-fluorophore-conjugated secondary antibodies were purchased from Invitrogen. AMCA-conjugated goat anti-chicken secondary antibody was purchased from Jackson Immuno Research.
Primary neuron culture
Dissociated hippocampal neurons were cultured essentially as in Kaech & Banker (2006), in the absence of the glial feeder layer. In brief, hippocampi were isolated from the brains of either E17 or P0 mouse embryos or E18 rat emrbyos, trypsin-digested (0.25% trypsin in HBSS), and dissociated by trituration prior to plating on poly-L-lysine and laminin-coated coverslips. Neuronal growth media (97% Neurobasal, 2% B-27 supplement, 1% Glutamax. Invitrogen) was supplemented, in part, with new media every four days.
Transfection of cultured neurons
Immediately following dissection and dissociation, neurons were nucleofected with shRNA or cDNA expression plasmids using the Neon system (Invitrogen). The neurons were suspended in 5ml HBSS and centrifuged 5 minutes at 1300rpm. The supernatant was removed and the cell pellet was subsequently resuspended in resuspenion buffer T at a density of 24,000 cells/μl. A single 1400mV pulse was delivered over 20ms to electroporate the cell membrane and introduce shRNA or cDNA plasmids. Ten microliters of suspended, electroporated cells were plated on poly-L-lysine and laminin-coated coverslips. Media was completely replaced at 4h following transfection. Hippocampal neurons were maintained from one to three weeks as indicated in primary neuron cultures methods section. The efficacy of the Na+ channel, Nfasc186, NrCAM, and βIV spectrin shRNA expression plasmids was reported in Hedstrom et al. (2007). The GFP-tagged ankG cDNA expression plasmids were a gift from Dr. Vann Bennett, Duke University.
Viral transduction of cultured neurons
Adenovirus-mediated RNA interference was used to silence ankG expression in either developing (0 DIV) or mature (11 DIV) cultured hippocampal neurons. At the indicated time points, cells were incubated 4h in media containing virus which was then exchanged completely for virus-free media. Adenoviruses containing ankG shRNA expression plasmids were generated by Hedstrom et al. (2008). Cells were maintained in culture either seven to ten days following infection prior to fixation.
Cytoskeletal depolymerizing drug treatments
Dissociated hippocampal neuron cultures were maintained three weeks at which point the media was supplemented with either 10μM Nocodazole, 20μM cytochalasin-D, 20μM latrunculin-B, or vehicle (DMSO alone). Cells were fixed with ice-cold 4%-PFA at various drug incubation time points including two, six, and 24h and immunostained.
Immunostaining
Mice were deeply anesthetized with isoflurane before transcardial perfusion with ice-cold 4% PFA in 0.1M Na+-phosphate buffer (PB), pH7.2. Brains were post-fixed in 4% PFA 0.1M PB for 1 hour and equilibrated in 20% sucrose 0.1M PB over 48 hours. Afterward, 25 μm coronal slices containing the hippocampal formation were cut on a microtome and washed in 0.1M PB. Slices were blocked in 10% normal goat serum 0.1M PB containing 0.3% TX-100 (PBTgs). Tissue was incubated overnight at 4°C in primary antibodies diluted in PBTgs. Primary antibodies were removed by washing the tissue 3 times for 5 minutes with PBTgs. Secondary antibodies diluted in PBTgs and applied for 1 hour at RT to visualize primary antibodies. Excess secondary antibodies were removed by consecutive 5-minute washes with PBTgs, 0.1M PB, and 0.05M PB. Slices were mounted on gelatin-coated coverslips.
Detergent extraction
Culture media was replaced with an equivalent volume of pre-warmed (37°C) detergent extraction (DE) buffer (2mM MgCl2, 10mM EGTA, 60mM PIPES, 1% TX-100) and returned to the 37°C incubator for 8 minutes. The original DE buffer was aspirated and fresh, pre-warmed DE buffer was added for a second and third extraction. After the third incubation, DE buffer was removed and the cells were immediately fixed with ice-cold PFA.
Alkaline phosphatase treatment of cultured neurons
Culture media was replaced with an equivalent volume of pre-warmed (37°C) DE buffer and the cells were returned to the incubator for 8 minutes. Following permeabilization, the DE buffer was removed and immediately replaced with pre-warmed alkaline phosphatase (AP) diluted to 100U/ml in AP buffer (5mM MgCl2, 100mM NaCl, 100mM Tris-HCl, pH9.5). Cells were returned to the incubator and the dephosphorylation reaction proceeded 20 minutes. The AP solution was aspirated and the cells were immediately fixed with ice-cold PFA.
Imaging
Fluorescence imaging was performed on an AxioImager Z1 microscope (Carl Zeiss MicroImaging) fitted with an AxioCam digital camera (Carl Zeiss MicroImaging). AxioVision acquisition software (Carl Zeiss MicroImaging) was used for collection of images. Comparison of WT, heterozygous Nfkbia+/−, and knockout Nfkbia−/− tissue was performed on slices prepared in parallel and images were acquired at identical exposure times. Experiments were performed at least in triplicate. Fluorescence intensity was measured using ImageJ (NIH). In some images, contrast and brightness were subsequently adjusted in a linear fashion using Photoshop (Adobe). Any adjustments made to images from one genotype were made in all genotypes.
Western blotting analysis
Mouse brains were rapidly dissected and immediately homogenized in ice-cold buffer containing (in mM): 50 Tris-HCl, 64.1 MgCl2, and 320 sucrose, supplemented with protease inhibitor cocktail (Sigma) and the phosphatase inhibitors Na-fluoride (10μM) and Na-orthovandate (10μM). Membrane proteins were isolated by centrifugation. After protein quantification by BCA assay (Pierce), protein samples were denatured in SDS sample buffer containing β-mercaptoethanol and heated to 95°C for 3 minutes. After heating the samples, 10mg of brain membrane protein was loaded onto 7.5% SDS-PAGE gels, resolved, transferred onto nitrocellulose membranes, and probed with the indicated primary antibodies in 5%-milk TBS using standard techniques. HRP-conjugated secondary antibodies were used to visualize primary antibodies and purchased from Invitrogen.
Measurement of AIS parameters
AIS length and protein fluorescence intensity values were determined by line-scan length and gray-value quantification in Image J (NIH). Images were collected at equivalent exposures and were not adjusted subsequent to acquisition.
Statistical analysis
Two-way ANOVA tests with Bonferroni correction were performed on the AIS length and ankG and Na+ channel fluorescence intensity (FI) data sets comparing the three genotypes. Results are reported as mean±s.e.m.
Supplementary Material
Acknowledgments
We thank David J. Shim, PhD, and Hong Lian for providing litters containing Nfkbia wild-type, heterozygous, and knockout mice. Research was supported by NIH grant NS044916 (M.N.R.) and NS073295 (S.A.B.), the Wintermann Foundation (S.A.B.), and Deutsche Forschungsgemeinschaft DFG SCHU 1412/2-1 (S.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Boiko T, Vakulenko M, Ewers H, Yap CC, Norden C, Winckler B. Ankyrin-dependent and –independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J Neurosci. 2007;27:590–603. doi: 10.1523/JNEUROSCI.4302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréchet A, Marie-Pierre F, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Localization of sodium channels in cultured neural cells. J Neurosci. 1981;1:777–783. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol. 2001;159:387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DM, Matta JA, Hoe HS, Zarkowsky D, Lee SH, Isaac JT, Pak DTS. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat Neurosci. 2010;13:1199–1207. doi: 10.1038/nn.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust A, Popovic M, Zecevic D, McCormick DA. Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J Neurosci. 2010;30:6891–6902. doi: 10.1523/JNEUROSCI.0552-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif in sodium channel clustering at the axon initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, Davies A. NF-kappaB signaling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132:1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–40. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Rathore P, Brown P, Listwak SJ. Cautionary notes on the use of NF-κB p65 and p50 antibodies for CNS studies. J Neuroinflammation. 2011;8:141. doi: 10.1186/1742-2094-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–19. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;5:2406–15. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kaphzan H, Buffington SA, Jung JI, Rasband MN, Klann E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of angelman syndrome. J Neurosci. 2011;31:17637–17648. doi: 10.1523/JNEUROSCI.4162-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Lee Y, Rozeboom A, Lee JY, Udagawa N, Hoe HS, Pak DTS. Requirement for Plk2 in orgchestrated Ras and Rap signaling, homeostatic structural plasticity, and memory. Neuron. 2011;69:957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–9. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Vacher H, Fache MP, d’Ortoli SA, Castets F, Autillo-Touati A, Dargent B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc Natl Acad Sci. 2011;108:8826–31. doi: 10.1073/pnas.1018671108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kumar Y, Zempel H, Mandelkow EM, Biernat J, Mandelkow E. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO. 2011;30:4825–4837. doi: 10.1038/emboj.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci. 2008;28:9083–9086. doi: 10.1523/JNEUROSCI.2494-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Molecular identity of denderitic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. UNC-33 (CRMP) and ankyrinG organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat Neurosci. 2011 doi: 10.1038/nn.2970. doi:1038/nn.2970 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Clark BA, Gründemann J, Roth A, Stuart GJ, Häusser M. Initiation of simple and complex spikes in cerebellar Purkinje cells. J Physiol. 2010;588:1709–1717. doi: 10.1113/jphysiol.2010.188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi C, De Turco D, Sie JM, Golinski PA, Tegeder I, Deller T, Schultz C. Accumulation of phosphorylated I kappaB alpha and activated IKK in nodes of Ranvier. Neuropathol Appl Neurobiol. 2008;34:357–365. doi: 10.1111/j.1365-2990.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Popovic MA, Foust AJ, McCormick DA, Zecevic D. The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: a voltage imaging study. J Physiol. 2011;589:4167–4187. doi: 10.1113/jphysiol.2011.209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–20. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Tapia M, Muñoz A, Garrido JJ. New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci. 2008;37:832–844. doi: 10.1016/j.mcn.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Muñoz A, Garrido JJ. Casein kinase 2 and microtubules control axon initial segment formation. Mol Cell Neurosci. 2010;46:222–234. doi: 10.1016/j.mcn.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ponce D, DeFelipe J, Garrido JJ, Muñoz A. In vitro maturation of the cisternal organelle in the hippocampal neuron’s axon initial segment. Mol Cell Neurosci. 2011;28:104–116. doi: 10.1016/j.mcn.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J Neurosci. 2004;24:3176–3185. doi: 10.1523/JNEUROSCI.5427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C, König HG, Del Turco D, Politi C, Eckert GP, Ghebremedhin E, Prehn JH, Kögel D, Deller T. Coincident enrichment of phosphorylated IkappaBalpha, activated IKK, and phosphorylated p65 in the axon initial segement of neurons. Mol Cell Neurosci. 2006;33:68–80. doi: 10.1016/j.mcn.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schultz C. AnkyrinG is required to maintain axo-dendrite polarity in vivo. Proc Natl Acad Sci. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS One. 2010;5:e12908. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kvβ2 auxiliary subunit regulate Kv1 channel axonal targeting. J Cell Biol. 2011;192:813–824. doi: 10.1083/jcb.201007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Winckler B, Forcher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24:7230–40. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.