Abstract
Background
Subclinical atherosclerotic plaque is an important marker of increased vascular risk. Identifying factors underlying the variability in burden of atherosclerotic carotid plaque unexplained by traditional vascular risk factors may help target novel preventive strategies.
Methods
As a part of the carotid substudy of the Northern Manhattan Study (NOMAS), 1,790 stroke-free individuals (mean age 69±9; 60% women; 61% Hispanic, 19% black, 18% white) were assessed for total plaque area (TPA) burden using 2D carotid ultrasound imaging. Multiple linear regression models were constructed. Model 1 used pre-specified traditional risk factors: age, sex, LDL-cholesterol, diabetes mellitus, pack-years of smoking, blood pressure (BP), and treatment for BP; and Model 2, an addition of socioeconomic and less traditional risk factors. The contributions of the components of the Framingham heart risk score (FRS) and the NOMAS global vascular risk score (GVRS) to the TPA were explored.
Results
Prevalence of carotid plaque was 58%. Mean TPA was 13±19mm2. Model 1 explained 19.5% of the variance in TPA burden (R2=0.195). Model 2 explained 21.9% of TPA burden. Similarly, FRS explained 18.8% and NOMAS GVRS 21.5% of the TPA variance.
Conclusions
The variation in preclinical carotid plaque burden is largely unexplained by traditional and less traditional vascular risk factors, suggesting that other unaccounted environmental and genetic factors play an important role in the determination of atherosclerotic plaque. Identification of these factors may lead to new approaches to prevent stroke and cardiovascular disease.
Keywords: preclinical atherosclerosis, carotid plaque, plaque area, carotid ultrasound, epidemiology, risk factors
Introduction
Carotid plaque is an important risk factor for stroke, myocardial infarction and vascular death.1,2,3 Asymptomatic and preclinical carotid plaque is biologically and genetically distinct from other phenotypic presentations of atherosclerosis.4,5 Preclinical carotid plaque is a better predictor of vascular events than carotid intima-media thickness (CIMT), another subclinical marker of atherosclerosis.2,6,7,8,9,10 Assessment of carotid plaque and measurement of plaque area by 2D carotid ultrasound is a safe, inexpensive, easy, reliable and reproducible method of detection of atherosclerosis. Measures of subclinical atherosclerosis are useful tools for assessing vascular risk beyond traditional vascular risk factors, risk factor management, genetic and environmental research, and evaluation of new therapies.6,7
The traditional vascular risk factors associated with carotid plaque area are age, sex, pack-years of smoking, systolic blood pressure (SBP), diastolic blood pressure (DBP), diabetes mellitus (DM), lipid-lowering and blood pressure (BP) lowering medication, and high-density lipoprotein (HDL) cholesterol. These risk factors explained about 50% of the variance in total plaque area in prior studies.1,11 Data on the variability of carotid plaque area based on vascular risk factors in population-based cohorts is limited. Therefore, the aim of this study was to identify traditional and less traditional vascular risk factors associated with carotid plaque area, to estimate their contribution to the variance in carotid plaque area, and to validate the contribution of previously determined traditional vascular risk factors on carotid plaque burden1,11 in an urban, multi-ethnic community cohort.
Subjects and Methods
Subjects
The Northern Manhattan Study (NOMAS) is an ongoing population-based study designed to determine the incidence of stroke and the role of novel risk factors in a multiethnic urban community.12 The race–ethnic distribution in NOMAS is 61% Hispanics, 19% blacks, and 17% whites.
Selection of the prospective cohort
The details of the NOMAS design have been described previously.13 In brief, subjects were identified by random digit dialing and enrolled in a prospective study between 1993 and 2001 if they had never been diagnosed with stroke, older than age 39 years, and resided in northern Manhattan for more than 3 months. A total of 3,298 individuals have been enrolled in the NOMAS. NOMAS was approved by the Institutional Review Boards of Columbia University Medical Center and University of Miami. All participants gave written informed consent for participation in the study. The carotid plaque imaging ancillary study to NOMAS started in 1999. All NOMAS subjects who have been enrolled in NOMAS since 1999 received carotid ultrasound at the time of baseline enrollment to NOMAS. There were no separate visits to Doppler laboratory at different time points and there were not specific selection criteria for the carotid ancillary study. Overall, 1,790 stroke-free subjects have been enrolled into the NOMAS carotid imaging substudy.5
Baseline evaluation
Baseline data were collected through interviews using standardized collection instruments, review of the medical records, and physical and neurological examinations.13 Race-ethnicity was based on self-identification through a series of questions modeled after the US Census and the standard definitions outlined by Directive 15. Hypertension was defined as a SBP ≥140 mm Hg or a DBP ≥90 mm Hg or a patient’s self-report of a history of hypertension or use of antihypertensive medications. Cigarette smoking was categorized as non-smoker, former, or current smoker (within the last year). Pack-years of smoking were calculated. Mild to moderate alcohol use was defined as current drinking of >1 drink per month and ≤2 drinks per day. Diabetes was defined as fasting blood glucose ≥126 mg/dL or the patient’s self-report of such a history or use of insulin or hypoglycemic medications. Completion of high-school was a proxy for socioeconomic status.
Assessment of carotid plaque area
Carotid ultrasound was performed according to standard scanning and reading protocols by a trained and certified sonologist as detailed previously.5,14 The left and right carotid bifurcations, the internal and the common carotid arteries were examined for wall thickness and the presence of plaque. Plaque was defined as an area of focal wall thickening 50% greater than surrounding wall thickness. All plaques were scanned in long axes from multiple angles and a digital sequence of 5–10 seconds was recorded in the cineloops. The plaque boundaries were traced off-line from the digitized images using an automatic edge detection system M’Ath (Paris, France). 2D carotid plaque area (mm2) was measured for each plaque according to the validated protocol using M’Ath (Figure 1). The sum of plaque areas in all carotid arteries from both sides of the neck was expressed as a total carotid plaque area. High reproducibility of M’Ath measurements has been reported previously.15,16,17 The image normalization and multiangle compound imaging method was performed in order to reduce angle dependence and random variation (speckle).18,19 In addition, high validity of measurements was achieved by implementation of the M’Ath quality index (MQI)15. Only images with MQI of 80% or greater were accepted for the analyses.
Figure 1.
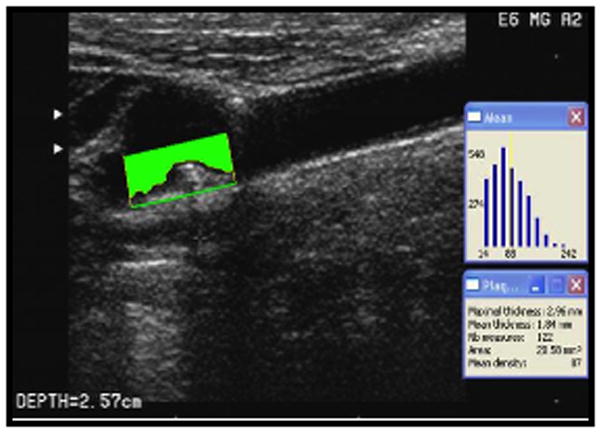
Carotid Plaque Area Measurements (carotid plaque area in mm2 in the far wall of the carotid bifurcation measured by automated edge detection image software).
Statistical Analysis
The primary assessment of total plaque area (TPA) burden was calculated as the sum of plaque area and values of zero were assigned to those with no plaque area. The variable was then transformed using a cube root function (x1/3) to meet the normality assumption. Given that age is a well-known major risk factor for TPA, we explored the age-adjusted association of each demographic and vascular risk factor with TPA based on generalized linear regression models for categorical factors and partial correlation for continuous factors. To validate the previously published plaque area model using traditional vascular risk factors1, we first regressed TPA on 9 traditional risk factors (age, sex, cigarette smoking pack-years, DM status, SBP, DBP, HDL, use of BP-lowering medications, and use of lipid-lowering medications). To investigate whether more variation of TPA can be explained by adding other potential risk factors, we then performed a multiple regression with a forward stepwise modeling by setting a selection criterion of p-value <0.1 for each term in the model. The modified model includes risk factors age, sex, pack-years smoking, SBP, DBP, DM, LDL:HDL ratio, homocysteine, high school completion, LDL, lipid-lowering medication, moderate alcohol drinking, and white blood cell (WBC) count. In addition, 2 separate models were constructed using the components of Framingham Risk Score (FRS)20 and the components of NOMAS Global Vascular Risk Score (GVRS)21 as independent variables. We conducted all analyses using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Carotid ultrasound was performed among 1,790 stroke-free subjects at baseline. Demographics of this group did not differ from the characteristics of the parent cohort.10 The mean age in the carotid sample was 69±9 years; 60% were women; 61% Caribbean Hispanics, 19% black, 17% white. Clinical characteristics and the age-adjusted associations with TPA burden are shown in Table 1. The following factors were significantly associated with TPA burden in univariate models: age, sex, race-ethnicity, high school completion, diabetes, BP-lowering and lipid-lowering medication, waist-to-hip ratio, pack-years of smoking, SBP, LDL, HDL, triglycerides, LDL:HDL ratio, WBC count, and homocysteine.
Table 1.
Demographics and Clinical Characteristics of the Study Population and Relationships with the Total Plaque Area
| Characteristics | Sample N (%) |
TPA* Mean±SD |
P-value |
|---|---|---|---|
| All | 1790 (100) | 1.45±1.38 | |
| Sex | <0.0001 | ||
| Female | 1074 (60) | 1.37±1.34 | |
| Male | 716 (40) | 1.58±1.44 | |
| Race-Ethnicity | 0.004 | ||
| Black | 341 (19) | 1.63±1.44 | |
| Hispanic | 1094 (61) | 1.28±1.33 | |
| White | 313 (17) | 1.90±1.37 | |
| High school completion | 0.005 | ||
| No | 943 (53) | 1.35±1.37 | |
| Yes | 847 (47) | 1.56±1.39 | |
| Moderate alcohol drinking | 0.05 | ||
| No | 1081 (60) | 1.44±1.39 | |
| Yes | 709 (40) | 1.47±1.37 | |
| Physical Activity | 0.08 | ||
| No | 767 (43) | 1.46±1.38 | |
| Yes | 1003 (57) | 1.46±1.38 | |
| Diabetes | <0.0001 | ||
| No | 1443 (81) | 1.38±1.37 | |
| Yes | 347 (19) | 1.74±1.41 | |
| Blood pressure medication | 0.001 | ||
| No | 1062 (59) | 1.33±1.34 | |
| Yes | 728 (41) | 1.62±1.42 | |
| Lipid medication | 0.01 | ||
| No | 1493 (83) | 1.41±1.37 | |
| Yes | 297 (17) | 1.66±1.40 | |
| History of sibling stroke or heart disease | 0.87 | ||
| No | 1415 (0.79) | 1.43±1.38 | |
| Yes | 375 (0.21) | 1.54±1.37 | |
| Mean±SD | Correlation | ||
| Age, years | 69.40±9.26 | 0.37 | <0.0001 |
| Body mass index (BMI), kg/m2 | 28.16±5.03 | 0.01 | 0.71 |
| Waist-to-hip ratio (WHR) | 0.90±0.09 | 0.09 | <0.0001 |
| Smoking, pack-years | 12.16±23.06 | 0.16 | <0.0001 |
| Systolic blood pressure (SBP), mmHg | 140.97±20.21 | 0.11 | <0.0001 |
| Diastolic blood pressure (DBP), mmHg | 83.01±10.93 | 0.01 | 0.78 |
| LDL-C, mg/dL | 128.01±35.09 | 0.06 | 0.009 |
| HDL-C, mg/dL | 46.69±14.43 | −0.06 | 0.01 |
| TG, mg/dL | 134.68±79.19 | 0.05 | 0.04 |
| LDL/HDL ratio | 2.98±1.20 | 0.08 | 0.0005 |
| White blood cell count (WBC), 1000/mm3 | 6.20±2.01 | 0.11 | <0.0001 |
| Estimated glomerular filtration rate (eGFR), ml/min | 75.09±19.89 | −0.02 | 0.35 |
| Homocysteine, μmol/L | 9.42±4.62 | 0.08 | 0.002 |
| C-reactive protein (CRP), mg/L | 4.68±7.21 | 0.02 | 0.46 |
TPA burden was cube root transformed; P-values were adjusted for age for all variables
Using the “traditional” risk factors model1, forward stepwise multiple regression model (Table 2) showed the R2 (coefficient of determination) of 0.195 (19.5%). Age (13.8%), smoking (2.2%), SBP (1.3%), and diabetes (0.9%) contributed the most to the variance in TPA burden. Of the variables in the “traditional” model, both HDL and lipid medication were not significantly associated with TPA burden (p=0.08, 0.80, respectively).
Table 2.
Variation of Total Atherosclerotic Carotid Area Explained by Traditional Vascular Risk Factor Model and Modified Risk Factor Model
| Parameter estimate | SE | β | Partial R2 | P-value | |
|---|---|---|---|---|---|
| Traditional model | |||||
| Age | 0.048 | 0.003 | 0.332 | 0.138 | <.0001 |
| Smoking, pack-years | 0.008 | 0.001 | 0.1128 | 0.022 | <.0001 |
| SBP | 0.011 | 0.002 | 0.161 | 0.013 | <.0001 |
| Diabetes | 0.269 | 0.077 | 0.077 | 0.009 | <.0001 |
| DBP | −0.012 | 0.004 | −0.095 | 0.006 | 0.0006 |
| Male sex | 0.238 | 0.066 | 0.084 | 0.004 | 0.002 |
| Lipid medication | 0.155 | 0.084 | 0.042 | 0.002 | 0.08 |
| BP medication | 0.115 | 0.066 | 0.041 | 0.001 | 0.02 |
| HDL-C | 0.000 | 0.002 | −0.006 | 0.000 | 0.80 |
| R2=0.195 | |||||
| Modified model | |||||
| Age | 0.048 | 0.004 | 0.317 | 0.135 | <.0001 |
| Smoking, pack-years | 0.008 | 0.001 | 0.133 | 0.028 | <.0001 |
| SBP | 0.011 | 0.002 | 0.159 | 0.011 | <.0001 |
| Diabetes | 0.318 | 0.085 | 0.091 | 0.008 | 0.0003 |
| LDL-C/HDL-C ratio | 0.038 | 0.042 | 0.033 | 0.007 | 0.0005 |
| Homocysteine | 0.024 | 0.008 | 0.077 | 0.007 | 0.0005 |
| DBP | −0.013 | 0.004 | −0.104 | 0.006 | 0.001 |
| Completed high school | 0.161 | 0.068 | 0.059 | 0.005 | 0.003 |
| White blood cell count | 0.047 | 0.016 | 0.071 | 0.004 | 0.007 |
| LDL-C | 0.003 | 0.001 | 0.075 | 0.003 | 0.03 |
| Male sex | 0.168 | 0.075 | 0.060 | 0.002 | 0.09 |
| Lipid medication | 0.193 | 0.092 | 0.050 | 0.002 | 0.06 |
| Moderate alcohol drinking | 0.107 | 0.068 | 0.038 | 0.002 | 0.05 |
| R2=0.219 | |||||
β: indicates standardized parameter estimate; R2: coefficient of determination; P-values are based on the multiple linear regression models with a forward stepwise selection.
The modified model which included age, sex, pack-years of smoking, SBP, SBP, diabetes, LDL:HDL ratio, homocysteine levels, high school completion, LDL, lipid-lowering medication and WBC count (Table 2) showed the R2 of 0.219 (21.9%), with most contribution by age (13.5%), smoking (2.8%), SBP (1.1%), diabetes (0.8%), LDL:HDL ratio (0.7%), and homocysteine (0.7%). All variables included in this model were significantly associated with TPA burden (p <0.05) except male sex, lipid medication and moderate alcohol drinking (p = 0.09, 0.06, 0.06, respectively).
Clustering of risk factors within an individual did not improve prediction models. The FRS (age, sex, smoking, BP, DM and total cholesterol) explained 18.8% and the NOMAS GVRS (which includes all the FRS variables plus race-ethnicity, waist circumference, physical activity, alcohol consumption, peripheral arterial disease symptoms, and fasting glucose instead of DM) explained 21.5% of the variance in TPA burden.
Discussion
In an urban multi-ethnic cohort from northern Manhattan, traditional vascular risk factors explain only 19.5% of the variance in the TPA burden. The explanatory risk factors include age, sex, pack-years of smoking, SBP, DBP, BP-lowering and lipid-lowering medications, and diabetes. An inclusion of less traditional risk factors such as LDL:HDL ratio, homocysteine, high school completion, WBC count, moderate alcohol drinking and LDL cholesterol to the traditional model contributed an additional 2.4%, explaining 21.9% of the variance in TPA burden. Therefore variation in subclinical carotid plaque burden is largely unexplained by many known vascular risk factors. Our results suggest that unaccounted factors, both environmental and genetic, play an important role in the determination of subclinical atherosclerosis. Identification of these factors is of great importance for successful prevention of stroke and cardiovascular disease (CVD).
Age, sex, pack-years of smoking, SBP, DBP, DM, HDL, BP-lowering and lipid-lowering medication were the most significant determinants of carotid plaque area in a previous study1, explaining 52% of the total plaque area variance. Our results however, found considerably lower contribution of these risk factors (19.5%) to TPA burden. This difference is most likely attributed to different characteristics of the study populations and study designs. Our cohort is a population-based sample as opposed to the subjects enrolled to a hospital-based clinic in the Premature Atherosclerosis Clinic (PAC) registry1, which represents a highly selected patient population at high risk for CVD. Furthermore, subjects in PAC were predominately white men with significant proportion of symptomatic carotid disease and had a strong family history of premature atherosclerosis. Our population however was comprised of predominately Hispanic women who were free of stroke or other CVD. The TPA burden also differed between our study and PAC (after cube root transformation, 1.45 mm2 in our study vs. 8.73 mm2 in PAC). Nevertheless, traditional vascular risk factors could not explain approximately half of the variance in plaque burden even in the PAC registry.
Age is the primary contributing factor to the atherosclerotic plaque burden in both the traditional and modified model in our as well as in prior studies.1,11 A marked increase in carotid plaque area was found between the ages of 45–70 years, leveling off after age 7011 most likely due to survival bias. Smoking is another important risk factor for plaque burden.2,22,23 Systolic and diastolic BP have been significantly associated with total plaque area and plaque progression.8,24,25 Diabetes mellitus provides an environment that enhances the effect of other environmental and genetic risk factors, and therefore is an important risk factor for increased atherosclerotic plaque burden.26 Increased total cholesterol is associated with plaque burden and plaque progression, and intensive lipid-lowering reduces plaque volume, and improve carotid plaque stability and morphology.2,27,28 Moreover, the LDL:HDL ratio is associated with increased plaque burden, while LDL may have the strongest relation with carotid plaque29; although contributing only 0.2% of TPA variation in our study, LDL remains an important risk factor to treat clinically. Men have more plaque compared to women and are at higher risk of stroke and CVD.30 In addition to having less plaque, women have more stable and less inflammatory carotid plaques compared to men.31 Low socioeconomic status has been associated with subclinical atherosclerosis.32 Individuals with lower education have larger carotid plaques even after adjusting for vascular risk factors.32,33 Despite the fact that traditional risk factors explain only a small proportion of the variance in plaque burden, most of them are modifiable and their adequate control is still of utmost importance for prevention of atherosclerosis and its devastating clinical consequences.
Several factors previously identified that influence carotid atherosclerosis were also noted in our study. Increased levels of homocysteine are associated with greater risk of plaque burden4,34 and vitamin B6 and B12 therapy may be effective in lowering homocysteine and plaque progression.35 Another novel vascular risk factor is increased WBC count. Relative elevations of WBC levels have been associated with presence of subclinical carotid plaque in our population36 and with plaque progression in other populations37; however, from a practical point of view even though elevated WBC count is relevant to development of TPA, its potential for modification is limited. Although several studies have found positive associations with serum C-reactive protein38 and estimated glomerular filtration rate39, we have not observed an association between these factors and carotid plaque burden. The exact role of these novel risk factors in atherosclerosis is yet to be fully elucidated.
If only 19.5% of the carotid plaque burden can be explained by the contribution of traditional and less traditional vascular risk factors, what explain the remaining variance in plaque burden? Clustering of risk factors within an individual may provide a better explanation of the variation in atherosclerotic plaque burden. However, our results remained relatively unchanged when using FRS20 or NOMAS GVRS21. The effects of some unaccounted and less known factors such as inflammation and infection, innate immunity, psychosocial or behavioral factors may contribute to plaque burden, but it is less likely that they would account for a large proportion of the plaque burden variance. Recently, we have identified a contribution of variation in genes involved in inflammation, endothelial function and lipid metabolism to carotid plaque burden.40,41 The investigations of novel genetic factors and their effects in a combination with environmental factors hold the promise of future research, treatment and prevention of atherosclerosis, stroke, and CVD.
Limitations of our study need to be acknowledged. The study population consists of an elderly and predominantly Hispanic population limiting generalizability of our results. The cross-sectional nature of the current findings does not allow inference of causality. Our study mainly included well-known traditional atherosclerotic risk factors along with some behavioral and less traditional factors while other factors of possible importance for atherosclerosis such as diet or endothelial function were not considered.
Summary
Carotid plaque burden is a significant predictor of CVD and 2D ultrasound measurement of plaque area is an inexpensive tool to identify individuals with increased atherosclerotic burden at risk for CVD, evaluate the effects of current and novel therapies, and investigate new contributing factors. Modifiable behaviors such as smoking and physical activity can influence BP, glycemic control, and cholesterol levels, which are important risk factors for plaque burden. Their modification should be strongly recommended. Our study also suggests that many unaccounted factors play an important role in the determination of atherosclerosis, underscoring the importance of further research.
Acknowledgments
Sources of Funding
This research was supported by the grants from the National Institutes of Health/National Institute of Neurological Diseases and Stroke (R01 NS 065114; R37 NS 29993; and K24 NS 062737).
Footnotes
Disclosure
Frank Kuo analyzed, interpreted the data, and drafted and revised the manuscript. Hannah Gardener and Chuanhui Dong performed statistical analyses, analyzed and interpreted the data. Digna Cabral acquired the data. David Della-Morte analyzed and interpreted the data. Susan Blanton interpreted the data and made revision of the manuscript. Ralph L. Sacco, Mitch SV Elkind, and Tatjana Rundek conceived and designed the research, supervised data collection, statistical analyses, handled funding, and made critical revision of the manuscript for important intellectual content.
References
- 1.Lanktree MB, Hegele RA, Schork NJ, Spence JD. Extremes of unexplained variation as a phenotype: An efficient approach for genome-wide association studies of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:215–221. doi: 10.1161/CIRCGENETICS.109.934505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: A tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–2922. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 3.Hulthe J, Wikstrand J, Emanuelsson H, Wiklund O, de Feyter PJ, Wendelhag I. Atherosclerotic changes in the carotid artery bulb as measured by b-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;28:1189–1194. doi: 10.1161/01.str.28.6.1189. [DOI] [PubMed] [Google Scholar]
- 4.Spence JD. Technology insight: Ultrasound measurement of carotid plaque--patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol. 2006;2:611–619. doi: 10.1038/ncpneuro0324. [DOI] [PubMed] [Google Scholar]
- 5.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: The northern Manhattan study. Neurology. 2008;70:1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: The British regional heart study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 7.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Lochen ML, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The tromso study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 9.Mathiesen EB, Johnsen SH, Wilsgaard T, Bonaa KH, Lochen ML, Njolstad I. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: A 10-year follow-up of 6584 men and women: The tromso study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 10.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 11.Spence JD, Barnett PA, Bulman DE, Hegele RA. An approach to ascertain probands with a non-traditional risk factor for carotid atherosclerosis. Atherosclerosis. 1999;144:429–434. doi: 10.1016/s0021-9150(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and hispanic residents of an urban community: The northern Manhattan stroke study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 13.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: The northern Manhattan study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Beecham A, Slifer S, Wang L, Blanton SH, Wright CB, et al. Genome wide linkage and peakwide association analyses of carotid plaque in Caribbean Hispanics. Stroke. 2010;41:2750–2756. doi: 10.1161/STROKEAHA.110.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touboul PJ, Prati P, Scarabin PY, Adrai V, Thibout E, Ducimetiere P. Use of monitoring software to improve the measurement of carotid wall thickness by B-mode imaging. J Hypertens Suppl. 1992;10:S37–41. [PubMed] [Google Scholar]
- 16.Touboul PJ, Vicaut E, Labreuche J, Belliard JP, Cohen S, Kownator S, et al. Design, baseline characteristics and carotid intima-media thickness reproducibility in the PARC study. Cerebrovasc Dis. 2005;19:57–63. doi: 10.1159/000081913. [DOI] [PubMed] [Google Scholar]
- 17.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the advisory board of the 3rd and 4th watching the risk symposium, 13th and 15th European stroke conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 18.Denzel C, Balzer K, Muller KM, Fellner F, Fellner C, Lang W. Relative value of normalized sonographic in vitro analysis of arteriosclerotic plaques of internal carotid artery. Stroke. 2003;34:1901–1906. doi: 10.1161/01.STR.0000081982.85010.A8. [DOI] [PubMed] [Google Scholar]
- 19.Lal BK, Hobson RW, 2nd, Pappas PJ, Kubicka R, Hameed M, Chakhtoura EY, et al. Pixel distribution analysis of b-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg. 2002;35:1210–1217. doi: 10.1067/mva.2002.122888. [DOI] [PubMed] [Google Scholar]
- 20.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, et al. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (northern Manhattan cohort study) J Am CollCardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman DJ, Roman MJ, Devereux RB, Fabsitz RR, MacCluer JW, Dyke B, et al. Prevalence of smoking and its relationship with carotid atherosclerosis in Alaskan Eskimos of the Norton Sound region: The Gocadan study. Nicotine Tob Res. 2008;10:483–491. doi: 10.1080/14622200801901955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang LR, Wong ND, Shi P, Zhao LC, Wu LX, Xie GQ, et al. Cross-sectional and longitudinal association of cigarette smoking with carotid atherosclerosis in Chinese adults. Prev Med. 2009;49:62–7. doi: 10.1016/j.ypmed.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Spence JD, Hegele RA. Noninvasive phenotypes of atherosclerosis: Similar windows but different views. Stroke. 2004;35:649–653. doi: 10.1161/01.STR.0000116103.19029.DB. [DOI] [PubMed] [Google Scholar]
- 25.Delcker A, Diener HC, Wilhelm H. Influence of vascular risk factors for atherosclerotic carotid artery plaque progression. Stroke. 1995;26:2016–2022. doi: 10.1161/01.str.26.11.2016. [DOI] [PubMed] [Google Scholar]
- 26.Bowden DW, Lehtinen AB, Ziegler JT, Rudock ME, Xu J, Wagenknecht LE, et al. Genetic epidemiology of subclinical cardiovascular disease in the Diabetes Heart Study. Ann Hum Genet. 2008;72:598–610. doi: 10.1111/j.1469-1809.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhatriwalla AK, Nicholls SJ, Nissen SE. The ASTEROID trial: coronary plaque regression with high-dose statin therapy. Future Cardiol. 2006;2:651–4. doi: 10.2217/14796678.2.6.651. [DOI] [PubMed] [Google Scholar]
- 28.Makris GC, Lavida A, Nicolaides AN, Geroulakos G. The effect of statins on carotid plaque morphology: ALDL-associated action or one more pleiotropic effect of statins? Atherosclerosis. 2010;213:8–20. doi: 10.1016/j.atherosclerosis.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Gardener H, Della Morte D, Elkind MS, Sacco RL, Rundek T. Lipids and carotid plaque in the northern Manhattan study (NOMAS) BMC Cardiovasc Disord. 2009;9:55. doi: 10.1186/1471-2261-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35:477–481. doi: 10.1161/01.STR.0000110981.96204.64. [DOI] [PubMed] [Google Scholar]
- 31.Hellings WE, Pasterkamp G, Verhoeven BA, De Kleijn DP, De Vries JP, Seldenrijk KA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg. 2007;45:289–296. doi: 10.1016/j.jvs.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Nash SD, Cruickshanks KJ, Klein R, Klein BE, Nieto FJ, Ryff CD, et al. Socioeconomic status and subclinical atherosclerosis in older adults. Prev Med. 2011;52:208–212. doi: 10.1016/j.ypmed.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutsey PL, Diez Roux AV, Jacobs DR, Jr, Burke GL, Harman J, Shea S, et al. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Public Health. 2008;98:1963–1970. doi: 10.2105/AJPH.2007.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spence JD, Malinow MR, Barnett PA, Marian AJ, Freeman D, Hegele RA. Plasma homocyst(e)ine concentration, but not mthfr genotype, is associated with variation in carotid plaque area. Stroke. 1999;30:969–973. doi: 10.1161/01.str.30.5.969. [DOI] [PubMed] [Google Scholar]
- 35.Barnett PA, Spence JD, Manuck SB, Jennings JR. Psychological stress and the progression of carotid artery disease. J Hypertens. 1997;15:49–55. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL Northern Manhattan Stroke S. Elevated white blood cell count and carotid plaque thickness: The northern Manhattan stroke study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 37.Salonen R, Salonen JT. Progression of carotid atherosclerosis and its determinants: A population-based ultrasonography study. Atherosclerosis. 1990;81:33–40. doi: 10.1016/0021-9150(90)90056-o. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn R, Giral P, Bruckert E, Andre JM, Gonbert S, Bernard M, et al. Elevated c-reactive protein constitutes an independent predictor of advanced carotid plaques in dyslipidemic subjects. Arterioscler Thromb Vasc Biol. 2001;21:1962–1968. doi: 10.1161/hq1201.099433. [DOI] [PubMed] [Google Scholar]
- 39.Choi SW, Kim HY, Lee YH, Ryu SY, Kweon SS, Rhee JA, et al. EGRF is associated with subclinical atherosclerosis independent of albuminuria: The Dong-Gu study. Atherosclerosis. 2010;212:661–667. doi: 10.1016/j.atherosclerosis.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Yanuck D, Beecham A, Gardener H, Slifer S, Blanton SH, et al. A candidate gene study revealed sex-specific association between the olr1 gene and carotid plaque. Stroke. 2011;42:588–592. doi: 10.1161/STROKEAHA.110.596841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardener H, Beecham A, Cabral D, Yanuck D, Slifer S, Wang L, et al. Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanics from northern Manhattan. Stroke. 2011;42:889–896. doi: 10.1161/STROKEAHA.110.591065. [DOI] [PMC free article] [PubMed] [Google Scholar]


