Abstract
Atrial fibrillation (AF) is a highly prevalent cardiac arrhythmia in clinical practice, affecting approximately 2.3 million people in the USA and 4.5 million people in the European Union. It is unclear whether plasma free fatty acids (FFA) influence the risk of AF among older adults. The aim of this study was to prospectively examine the association between plasma FFA and incident AF in a prospective cohort of 4,175 men and women aged ≥65 years from the Cardiovascular Health Study. Plasma concentrations of FFA were measured in duplicate during the 1992-93 examination. Incident AF was ascertained based on study EKG and hospitalization records during follow up. We used Cox regression to estimate relative risks of AF. The average age at baseline was 74.6 ± 5.1 years. During a mean follow up of 10.0 years, 1,041 new cases of AF occurred. Crude incidence rates of AF were 23.7, 23.3, 23.9, and 29.7 cases/1,000 person-years across consecutive quartiles of plasma FFA. There was a positive association between plasma FFA and the risk of AF. Multivariable adjusted hazard ratios (95% CI) for incident AF were 1.00 (ref), 1.02 (0.85-1.21), 1.05 (0.88-1.26), and 1.29 (1.08-1.55) from lowest to the highest quartile of FFA, respectively. In a secondary analysis restricted to the first five years of follow up, this association persisted. In conclusion, our data show an elevated risk of AF with higher plasma FFA among community dwelling older adults.
Keywords: Free Fatty Acids, Atrial Fibrillation, Risk Factors, Epidemiology
Previous data from the Cardiovascular Health Study (CHS) have demonstrated beneficial effects of light-to-moderate physical activity on AF risk 1, no association between moderate alcohol consumption and AF risk 2, and a positive association between N-terminal pro-B-type natriuretic peptide (NT-BNP) 3 and AF. Other investigators have reported an increased risk of AF with type 2 diabetes (T2D) 4, hypertension (HTN) 5, obesity 6, and inflammation 7. However, the common link between adiposity, T2D, HTN, and sedentary lifestyle and a higher propensity for developing AF is unclear. Elevated levels of plasma free fatty acids (FFA) have been associated with increased insulin resistance and T2D 8,9, HTN 10, physical inactivity11, and inflammation 11, suggesting that FFA may play an important role in the development of AF. However, the association between plasma FFA and incident AF has not been investigated in the general population including older adults, a group extremely vulnerable to AF. Therefore, the current study sought to prospectively assess whether plasma FFA concentration measured late in life was associated with a higher risk of incident AF among community-living older adults.
METHODS
Detailed descriptions of the CHS have been published elsewhere 12,13. Briefly, CHS is a prospective, population-based cohort study of cardiovascular disease in older adults. Between 1989 and 1990, a total of 5,201 ambulatory, non institutionalized men and women ≥65 years of age were recruited from a random sample of Medicare-eligible residents from 4 US communities [Forsyth County, North Carolina (Wake Forest University School of Medicine, Winston-Salem); Sacramento County, California (University of California, Davis); Washington County, Maryland (Johns Hopkins University, Hagerstown); and Allegheny County, Pennsylvania (University of Pittsburgh, Pittsburgh)]. Between 1992 and 1993, a supplemental cohort of 687 predominantly African American men and women was recruited using the same sampling and recruitment methods. The 1992-1993 visit was considered as baseline examination for the current study. Of the 5,265 participants who completed the baseline examination, we excluded people without data on FFA (n= 550), prevalent AF during 1992-93 examination (n=265), and missing data on covariates (n=275). Thus, a final sample of 4,175 participants was used for current analyses. Each participant gave written informed consent and the Institutional Review Board at each of the participating institutions approved the study protocol.
Comprehensive information on health-related variables was collected at baseline and annually thereafter from CHS participants. Clinic examinations including EKG were performed annually from 1989-1990 to 1998-1999 and a clinical examination without EKG was performed between 2005-2006. Standardized questionnaires were administered at a baseline home interview, at annual clinic visits, and during telephone contacts.
Plasma samples collected at the 1992-1993 examination were stored at -70°C until FFA measurements at the Central Laboratory at the University of Vermont. FFA concentration in plasma were measured in duplicates by the Wako enzymatic method and the average of the two measurements was used for current analyses.
Incident AF was defined based on EKG and hospitalization records until year 11 (1998-1999) and then based on hospitalization records without EKG review thereafter. EKGs obtained were reviewed and the diagnosis of AF or atrial flutter was verified at the CHS centralized EKG reading center 14. When AF or atrial flutter was a discharge diagnosis, AF was believed to be present from the day of admission to the hospital. AF or atrial flutter cases that occurred during the same hospitalization for coronary artery bypass graft surgery or valve replacement surgery were excluded from the current analysis. The positive predictive value of hospital discharge diagnosis for AF has been noted to be 98.6% in CHS 14. In another Holter monitoring sub study, only 0.1% of the patients having intermittent or persistent AF were not captured by the above methodology 15.
Data on demographics, anthropometric measures, HTN, T2D, coronary heart disease (CHD), congestive heart failure (CHF), lipid profile, renal function, smoking, and alcohol consumption were recorded at the 1992-93 examination. NT-BNP and C-reactive protein (CRP) were measured using samples from the 1992-93 examination. Age, body mass index (BMI), and systolic blood pressure were analyzed as continuous variables. Physical activity (kcal/day) was determined using modified Minnesota Leisure-Time Activities questionnaire and analyzed as a continuous variable (after logarithmic transformation). Alcohol consumption was classified as none, <7, 7-14, and >14 drinks per week. Smoking status was classified as never, former, and current smokers. HTN was defined as present if average seated systolic blood pressure was >140 mmHg, diastolic blood pressure >90 mmHg, or use of antihypertensive medications by participants who reported a hypertension diagnosis. T2D was present if any of the following conditions was met: fasting glucose ≥126 mg/dl, non-fasting glucose ≥200 mg/dl, or use of insulin/hypoglycemic agents. Plasma levels of total cholesterol, triglycerides, low density lipoprotein (LDL), high density lipoprotein (HDL), and CRP were all analyzed as continuous variables.
Baseline characteristics of the study participants were summarized according to the quartiles of FFA. Continuous variables were presented as means ± standard deviation (SD) or medians [inter-quartile range (IQ)] if the distribution was skewed. Categorical variables were presented as N (%) and incidence rate of AF (per 1,000 person-years) was calculated within each quartile of FFA.
Cox proportional hazard regression was used to estimate the association of FFA with incident AF. FFA were modeled as a continuous variable (per SD of FFA) as well as quartiles. Cubic splines were utilized to assess the linearity of the association between FFA (continuous variable) and incident AF. We computed person-time of follow up from FFA assessment until the first occurrence of a) AF/atrial flutter, b) death, or c) censoring date (i.e., last available follow up). After the crude analysis, we adjusted for demographic variables [age (continuous), race (African-American or other), and sex (model 1)]. Model 2 also controlled for physical activity, alcohol intake, smoking, BMI, CHD, CHF, T2D, HTN, and CRP.
NT-BNP measurements were available on 3,709 (88.8%) subjects. Within this subset, we repeated the final analysis with additional adjustment for log-NT-BNP. In a secondary analysis, we restricted the follow-up time to the first 5 years of follow up. We also tested for effect modification by sex, adiposity, and T2D status. We used Schoenfeld residuals and plots of the residuals over time to examine proportional hazard assumptions and no violations were found. All analyses were conducted using Stata, version 11.2 (StataCorp LP, College Station, Texas). The significance level was set at 0.05.
RESULTS
Table 1 describes the baseline characteristics of the study participants according to the quartiles of plasma FFA. Mean age of the study participants was 74.6 ± 5.1 years. During an average follow up of 10.0 years, 1,041 new cases of AF/atrial flutter were reported. Subjects in the highest FFA quartile were older, more likely to be females, and had higher measures of adiposity, triglycerides, LDL, HDL, NT-BNP, and CRP. Higher FFA levels were also associated with prevalent HTN and T2D.
Table 1.
Baseline characteristics by quartiles of plasma free fatty acids
| Free Fatty Acids Range (mEq/l) | Q1 (≤0.348) (n=1,044) | Q2 (>0.348-0.469) (n=1,047) | Q3 (>0.469-0.610) (n=1,044) | Q4 (>0.610) (n=1,040) |
|---|---|---|---|---|
| Age (years) | 74 ± 4.6 | 75 ± 5.2 | 75 ± 5.3 | 75 ± 5.3 |
| African American | 160 (15%) | 188 (18%) | 184 (18%) | 196 (19%) |
| Male | 628 (60%) | 477 (46%) | 348 (33%) | 251 (24%) |
| Body Mass Index (kg/m2) | 26 ± 4.0 | 27 ± 4.6 | 27 ± 5.0 | 28 ± 5.3 |
| Kcals physical activity, median(IQR) | 1022 (405,2173) | 908 (304,1951) | 769 (263,1768) | 735 (234,1646) |
| Coronary heart disease | 269 (26%) | 202 (19%) | 198 (19%) | 201 (19%) |
| Heart failure | 51 (4.9%) | 46 (4.4%) | 45 (4.2%) | 56 (5.4%) |
| Diabetes Mellitus | 122 (12%) | 134 (13%) | 145 (14%) | 225 (22%) |
| Hypertension | 517 (50%) | 553 (53%) | 615 (59%) | 706 (68%) |
| Low density lipoprotein (mg/dl) | 127 ± 32 | 129 ± 33 | 130 ± 35 | 127 ± 36 |
| High density lipoprotein (mg/dl) | 50 ± 13 | 52 ± 14 | 55 ± 14 | 57 ± 16 |
| Triglycerides (mg/dl) | 115 (84,159) | 123 (90,170) | 127 (91,175) | 133 (96,192) |
| Smoking status | ||||
| Never | 399 (38%) | 460 (44%) | 511 (49%) | 549 (53%) |
| Former | 528 (51%) | 473 (45%) | 428 (41%) | 401 (39%) |
| Current | 117 (11%) | 114 (11%) | 105 (10%) | 90 (9%) |
| Alcohol consumption (drinks/week) | ||||
| None | 531 (51%) | 577 (55%) | 579 (56%) | 615 (59%) |
| <7 | 379 (36%) | 338 (32%) | 352 (34%) | 281 (27%) |
| 7-14 | 73 (7%) | 76 (7%) | 68 (7%) | 72 (7%) |
| >14 | 61 (6%) | 56 (5%) | 45 (4%) | 72 (7%) |
| N-terminal pro-B-type natriuretic peptide, median (IQR) | 125 (63,259) | 130 (63,268) | 135 (72,247) | 146 (74,262) |
| C-reactive protein, median (IQR) | 2.2 (1.0,5.0) | 2.5 (1.3,5.9) | 2.8 (1.4,6.1) | 3.1 (1.3,6.5) |
The crude incidence rates of AF were 23.7, 23.3, 23.9, and 29.7 cases/1,000 person-years from lowest to the highest quartile of FFA (Table 2). Compared with the lowest quartile, there was a positive and statistically significant association between the highest quartile of plasma FFA and incident AF with a hazard ratio (95% confidence interval) [HR (95% CI)] of 1.29 (1.08-1.55) in the fully adjusted analysis (Table 2). Each SD (SD=0.20 mEq/l) of increased FFA was associated with an 11% higher risk of AF (95% CI: 4% to 18%) in the fully adjusted model (Table 2). Evaluation of cubic splines also suggested a linear relationship between plasma FFA levels and incident AF (Figure 1). Upon additional adjustment for NT-BNP (available on 3,704 subjects), the final adjusted HR (95% CI) of AF per SD higher FFA was 1.11 (1.03-1.18).
Table 2.
Incidence rate and relative risk (95% CI) of atrial fibrillation according to quartiles/standard deviation of plasma free fatty acids
| Free Fatty Acids Quartiles | Continuous | ||||
|---|---|---|---|---|---|
|
| |||||
| Free Fatty Acids Range (mEq/L) | Q1 | Q2 | Q3 | Q4 | Per standard deviation |
| ≤0.348 HR (95% CI) | >0.348-0.469 HR (95% CI) | >0.469-0.610 HR (95% CI) | >0.610 HR (95% CI) | (0.20 mEq/L) greater HR (95% CI) | |
| Events/N at Risk | 257/1,044 | 248/1,047 | 245/1,044 | 291/1,040 | |
| Cases/1000 person-years | 23.7 | 23.3 | 23.9 | 29.7 | |
| Unadjusted | 1.00 (Ref) | 0.98 (0.82,1.17) | 1.01 (0.85,1.21) | 1.26 (1.07,1.49) | 1.09 (1.03,1.16) |
| Model 1* | 1.00 (Ref) | 1.02 (0.85,1.21) | 1.08 (0.91,1.30) | 1.43 (1.20,1.70) | 1.15 (1.08,1.22) |
| Model 2† | 1.00 (Ref) | 1.02 (0.85,1.21) | 1.05 (0.88,1.26) | 1.29 (1.08,1.55) | 1.11 (1.04,1.18) |
Adjusted for age, sex, and race
Model 1 variables plus physical activity (log kcals), body mass index, coronary heart disease, congestive heart failure, smoking (current, former, never), alcohol use (0, < 7, 7-14, and > 14 drinks per week), log- C-reactive protein, diabetes mellitus, and hypertension
Figure 1.
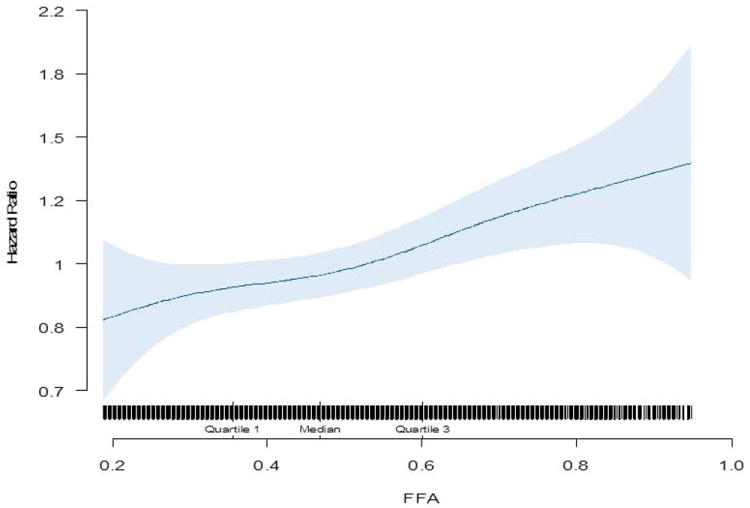
Cubic spline depicting the association of FFA with incident Atrial Fibrillation (adjusted for age, race, sex, physical activity, body mass index, coronary heart disease, congestive heart failure, smoking, alcohol use, log- C-reactive protein, diabetes mellitus, and hypertension)
In a secondary analysis restricted to the first 5 years of follow up, there was a slightly stronger and significant association between the highest quartile of plasma FFA and incident AF (Table 3). There was no evidence of effect modification by sex, adiposity or T2D status (p>0.1).
Table 3.
Relative risk (95% CI) of atrial fibrillation according to quartiles/standard deviation of plasma free fatty acids from 0-5 years of follow up
| Free Fatty Acids Quartiles | Continuous | ||||
|---|---|---|---|---|---|
|
| |||||
| Free Fatty Acids Range (mEq/L) | Q1 | Q2 | Q3 | Q4 | Per standard deviation |
| ≤0.348 HR (95% CI) | >0.348-0.469 HR (95% CI) | >0.469-0.610 HR (95% CI) | >0.610 HR (95% CI) | (0.198 mEq/L) greater HR (95% CI) | |
| Unadjusted | 1.00 (Ref) | 0.87 (0.66,1.16) | 0.97 (0.73,1.28) | 1.35 (1.04,1.75) | 1.14 (1.04,1.25) |
| Model 1* | 1.00 (Ref) | 0.89 (0.67,1.19) | 1.04 (0.78,1.38) | 1.54 (1.17,2.02) | 1.20 (1.09,1.32) |
| Model 2† | 1.00 (Ref) | 0.89 (0.67,1.19) | 1.00 (0.75,1.33) | 1.39 (1.05,1.84) | 1.16 (1.05,1.28) |
Adjusted for age, sex, and race
Model 1 variables plus physical activity (log kcals), body mass index, coronary heart disease, congestive heart failure, smoking (current, former, never), alcohol use (0, < 7, 7-14, and > 14 drinks per week), log- C-reactive protein, diabetes mellitus, and hypertension
DICUSSION
In this cohort of older adults, we found that higher plasma FFA measured late in life were associated with a higher risk of AF. In a secondary analysis restricted to the first 5 years of follow up, the observed elevated risk of AF with higher FFA persisted and was slightly stronger. To the best of our knowledge, this is the first large prospective study to assess the association between plasma FFA and incident AF in community living older adults.
The results of our study are consistent with prior studies that have demonstrated a positive association between higher plasma FFA and the risk of other types of cardiac arrhythmias. Jouven et al 16 in the Paris Prospective Study reported a 70% higher risk of sudden cardiac death (SCD) [multivariable adjusted HR (95% CI) per SD of increased FFA: 1.70 (1.21-2.13)]. Pilz et al 17 demonstrated similar results with a 76% higher risk of SCD when comparing the fourth to the first quartile of FFA among patients referred for cardiac catheterization [HR (95% CI) = 1.76 (1.30-3.00)]. In a study of post -acute myocardial infarction patients, Tansey et al 18 reported that subjects who developed any arrhythmia had higher mean peak FFA levels than those who did not develop arrhythmia. Paolisso et al 19 also observed a positive association between plasma FFA concentration and the incidence of ventricular premature contractions among non- insulin dependent diabetic patients. Furthermore, Soloff et al 20 demonstrated an increased incidence of ventricular arrhythmia following injection of saturated fatty acids in animal models.
As seen in our study, FFA provide information above and beyond standard AF risk factors. Although our analysis showed a positive association between plasma FFA and incident AF, it is possible that within minimal to moderate ranges of high FFA, there may be only modest to no association of FFA with incident AF. If our findings are confirmed by others, it is possible that FFA may be useful in risk stratification for AF among older individuals. While prevention of AF may be difficult, novel therapies are increasingly available to convert and maintain normal sinus rhythm 21,22, and earlier identification of individuals with AF might allow earlier use of anticoagulants to avert cerebrovascular events.
The precise mechanisms by which FFA might lead to cardiac arrhythmias remain unclear, with most of the data obtained from animal studies. A potential mechanism may involve the production of lysophospholipids from a breakdown of membrane lipids and acylcarnitine from circulating FFA that could predispose to cardiac arrhythmias 23. In addition, FFA may inhibit Na+, K+, ATPase pump with subsequent increase in intracellular sodium and calcium 24 that may predispose to arrhythmias.
Our study has some limitations. Study participants were aged ≥65 at baseline, thereby limiting the generalizability of these findings to younger adults. With a single measure of plasma FFA late in life, we were unable to account for longitudinal changes in plasma FFA levels over time. Cases of paroxysmal AF, especially if asymptomatic, could have been missed; however, missed AF cases are likely to bias our results towards the null and would not explain the observed association. We did not have data on specific FFA including trans fatty acids known to adversely affect cardiovascular risk 25,26. Future studies are needed to examine the role of individual FFA on AF risk. Despite above limitations, our study has numerous strengths including its prospective design, a large sample size, lengthy follow up, a review of annual EKGs and hospitalization records to validate AF, and a valid and reproducible way to measure plasma FFA levels.
Acknowledgments
FUNDING:
This work was supported by the National Heart Lung Blood Institute (R01HL094555) to Drs Djousse, Ix, Mukamal, Zieman, and Kizer.
The CHS was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, F C, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukamal KJ, P B, Rautaharju PM, Furberg CD, Kuller LH, Mittleman MA, Gottdiener JS, Siscovick DS. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J. 2007;153:260–266. doi: 10.1016/j.ahj.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Patton KK, E P, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N-Terminal Pro-B-Type Natriuretic Peptide Is a Major Predictor of the Development of Atrial Fibrillation. The Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dublin S, G N, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–858. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnabel RB, S L, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, B EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost L, H L, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Peña JM, M J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33:531–537. doi: 10.1093/eurheartj/ehr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monti LD, L P, Setola E, Rossodivita A, Pala MG, Galluccio E, Lacanna G, Castiglioni A, Cannoletta M, Meloni C, Zavaroni I, Bosi E, Alfieri O, Piatti PM. Effects of chronic elevation of atrial natriuretic peptide and free fatty acid levels in the induction of type 2 diabetes mellitus and insulin resistance in patients with mitral valve disease. Nutr Metab Cardiovasc Dis. 2012;22:58–65. doi: 10.1016/j.numecd.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, T H, Yu H, Zhang X, Suo L, Lu Z, Pu S, Yu Y. Impairment of insulin action in non-obese, normal-glucose tolerant, first-degree relatives of Chinese type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91:67–71. doi: 10.1016/j.diabres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Fagot-Campagna A, B B, Simon D, Warnet JM, Claude JR, Ducimetière P, Eschwège E. High free fatty acid concentration: an independent risk factor for hypertension in the Paris Prospective Study. Int J Epidemiol. 1998;27:808–813. doi: 10.1093/ije/27.5.808. [DOI] [PubMed] [Google Scholar]
- 11.Belotto MF, M J, Rodrigues HG, Vinolo MA, Curi R, Pithon-Curi TC, Hatanaka E. Moderate exercise improves leucocyte function and decreases inflammation in diabetes. Clin Exp Immunol. 2010;162:237–243. doi: 10.1111/j.1365-2249.2010.04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tell GS, F L, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidmiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, B N, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG for the Cardiovascular Health Study (CHS) Collaborative Research Group. The Cardiovascular Health Study: design and rationale. Ann Epidmiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Psaty BM, M T, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, P B, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jouven X, C M, Desnos M, Ducimetiere P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;104:756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 17.Pilz S, S H, Tiran B, Wellnitz B, Seelhorst U, Boehm BO, Marz W. Elevated plasma free fatty acids predict sudden cardiac death: a 6.85-year follow-up of 3315 patients after coronary angiography. Eur Heart J. 2007;28:2763–2769. doi: 10.1093/eurheartj/ehm343. [DOI] [PubMed] [Google Scholar]
- 18.Tansey MJ, O L. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet. 1983;2:419–422. doi: 10.1016/s0140-6736(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 19.Paolisso G, G P, Daniela Manzella MD, Rizzo MR, Tagliamonte MR, Gambardella A, Verza M, Gentile S, Varricchio M, D’Onofrio F. Association of Fasting Plasma Free Fatty Acid Concentration and Frequency of Ventricular Premature Complexes in Nonischemic Non–Insulin-Dependent Diabetic Patients. Am J Cardiol. 1997;80:932–937. doi: 10.1016/s0002-9149(97)00548-1. [DOI] [PubMed] [Google Scholar]
- 20.LA S. Arrhythmias following infusions of fatty acids. Am Heart J. 1970;80:671–674. doi: 10.1016/0002-8703(70)90012-8. [DOI] [PubMed] [Google Scholar]
- 21.Oral H, C A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Frederick M, Fortino J, Benloucif-Moore S, Jongnarangsin K, Pelosi F, Jr, Bogun F, Morady F. Radiofrequency Catheter Ablation of Chronic Atrial Fibrillation Guided by Complex Electrograms. Circulation. 2007;115:2606–2612. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 22.Noheria A, K A, Wylie JV, Jr, Josephson ME. Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation. Arch Intern Med. 2008;168:581–586. doi: 10.1001/archinte.168.6.581. [DOI] [PubMed] [Google Scholar]
- 23.Kurien VA, O M. A metabolic cause of arrhythmias during acute myocardial hypoxia. Lancet. 1970;1:813–815. doi: 10.1016/s0140-6736(70)92412-8. [DOI] [PubMed] [Google Scholar]
- 24.Kelly RA, OH D, Canessa ML, Mitch WE, Smith TW. Characterization of digitalis-like factors in human plasma. Interactions with NaK-ATPase and cross-reactivity with cardiac glycoside-specific antibodies. J Biol Chem. 1985;260:11396–11405. [PubMed] [Google Scholar]
- 25.Mozaffarian D, K M, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 26.Lemaitre RN, K I, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]


