Abstract
Objective
One developing strategy for obesity treatment has been to use combinations of differently acting pharmacotherapies to improve weight loss with fewer adverse effects. The purpose of this study was to determine whether the combination of naltrexone, an opioid antagonist acting on the reward system, and exendin-4, a glucagon-like peptide 1 (GLP-1) agonist, acting on satiety signaling, would produce larger reductions in food intake than either alone in rats. Because the anorectic potencies of both compounds have been associated with nausea and malaise, the influence of these drug combinations on the acquisition of a conditioned taste aversion (CTA) was also determined.
Methods
In Experiment 1, the acute anorectic effects of naltrexone (0.32–3.2 mg/kg; IP) and exendin-4 (1–10 µg/kg; IP) were assessed alone or in combination. Combinational doses were further investigated by the repeated daily administration of 1 mg/kg naltrexone + 3.2 µg/kg exendin-4 for 4 days. In Experiment 2, both compounds alone or in combination were used as unconditioned stimuli in a series of CTA tests.
Results
Naltrexone and exendin-4, alone or in combination, suppressed food intake in a dose dependent fashion, and the interaction on food intake between naltrexone and exendin-4 was additive. In the CTA paradigm, naltrexone (1 mg/Kg) alone did not support acquisition, whereas a CTA was evident with doses of Ex-4 (1 or 3.2 µg/Kg). Combinations of naltrexone and exendin-4 also resulted in a more rapid and robust acquisition of a CTA.
Conclusion
Given that the Nal and Ex-4 combination produces additive effects on not only food intake reduction but also food aversion learning, this specific drug combination does not have the benefit of minimizing the adverse effects associated with each individual drug. These data suggest that it is necessary to evaluate both the positive and adverse effects at early stages of combinational drug development.
Keywords: combinational therapy, naltrexone, exendin-4, GLP-1, opioids, conditioned taste aversion, food intake
Introduction
Obesity and its comorbid health conditions, such as type 2 diabetes and cardiovascular diseases, have become worldwide health care burdens and leading causes of death1. Reducing body weight (BW) is commonly regarded as an important aim of obesity treatment because health related complications of obesity can be ameliorated by weight management. The most clinically effective approach in terms of both the magnitude of weight loss and sustained effects is bariatric surgery2, 3. Although the efficacy of bariatric surgery is high, it is expensive and invasive and is targeted at only those with BMI>40 or BMI>35 with obesity-related comorbid conditions4. Effective pharmacological intervention, on the other hand, could potentially provide tremendous benefit for a greater portion of overweight individuals. However, there are few options currently approved by the FDA, Health Canada, or the EMA. In the United States for example, the two medications available for obesity, phentermine and orlistat, are either not approved for long-term use (>12 weeks) or are often not well-tolerated5.
Because food intake and BW are controlled by numerous overlapping physiological mechanisms, recent preclinical advances have been made in utilizing combinational drug therapies to treat obesity5–7. Several combinational therapies for the treatment of obesity are presently in late-stage clinical trials or being further evaluated by the FDA or other regulatory agencies8 . The use of combinational therapy offers the potential for synergistic interactions between compounds to produce a greater degree of weight loss than the sum of the individual effects of each compound. Given that smaller doses of each compound can be used to produce effective weight loss, compounds that interact in an additive or synergistic fashion also have the potential advantage of minimizing associated dose-dependent adverse effects.
In this study, we hypothesized that manipulating both the satiety and reward systems simultaneously would produce additive actions on food intake. We began to test this hypothesis with two clinically approved drugs to ensure individual drug safety. The GLP-1 agonist exendin-4 (Ex-4) was chosen to enhance satiety function. Ex-4 is a naturally occurring peptide 9 that is resistant to dipeptidyl-peptidase IV degradation10. It is an incretin that increases the release of insulin to facilitate clearing blood glucose and so a synthetic version of Ex-4 Byetta (Exenatide) has been approved for treating type 2 diabetes. Clinical reports have indicated that Ex-4 produces progressive weight loss in addition to its action on glucose homeostatsis 11, 12. The opioid receptor antagonist naltrexone (Nal) was chosen to suppress the rewarding value of food. Naltrexone is clinically approved for treating opioid and alcohol dependence. Reports from both human and animal studies have demonstrated that naltrexone can decrease food intake by decreasing food palatability 13–15.
In this study, the anorectic potencies of the two drugs were measured alone or in combination. Additional experiments were performed to determine whether these combinations reduced food intake by aversive actions. Both of these drugs have been associated with aversive side effects11, 16, 17. Conditioned taste aversion (CTA) is a learned behavior that occurs when ingestion of a novel food is followed by visceral illness which can be induced by drug treatment. Once a CTA is formed, the value of the food switches from preference to avoidance18. Thus, the CTA paradigm was employed as a metric of avoidance to determine whether the naltrexone or exendin-4 or their combination devalued food18.
Materials and Methods
Animals
Two sets of male Sprague Dawley (SD) rats (Charles River) weighing 250–275g upon arrival were used in this study. The first set n=8 were used for Experiment 1 to study the effects of Nal and Ex-4 and their combination on food intake. The second set n=30 were used for Experiment 2 to study the aversive effects of the same drug combinations. Rats were single housed with Prolab 5001 rodent chow and water available ad libitum except when stated otherwise. Food intake and BW were measured daily. All animal protocols were approved by the Institutional Animal Care and Use committee of the Johns Hopkins University.
Experiment 1. Food intake
Drugs
Naltrexone hydrochloride (Sigma Aldrich) and Exendin-4 (Bachem) were dissolved in 0.9% NaCl. The drug solutions were injected intraperitoneally (IP) in a volume of 1ml/Kg. The doses used for Nal were 0, 0.32, 1, and 3.2mg/Kg and for Ex-4 were 0, 1, 3.2, and 10µg/Kg. For the combination treatments, the two drugs were dissolved together in saline for a single injection.
Short-term food intake
The effects of Nal or Ex-4 or dose combinations of the two drugs on short-term food intake were tested when the rats were mildly food restricted (4hrs prior to the dark onset). On the test days, either saline or drug solutions were administered 15–10 min before the onset of dark. Food was returned to the rats at the time of dark onset, and intake was measured at 1, 4, and 20hr afterwards. There were at least 2 days between each drug injection test. Once drug treatment began, saline treatment also occurred intermittently. Baseline intake was the mean of 3 initial saline injection tests and 2 additional tests during drug treatment.
Consecutive daily injection
When the rats were about 30 wks old and had an average BW=638 ± 28.5g, a dose combination of Nal(1mg/Kg) and Ex-4(3.2µg/Kg) was administered once daily for 4 consecutive days to examine whether such treatment would produce sustained effects on food intake. The dose combination was chosen based on the results from short-term food intake tests. On average, this dose combination suppressed 75.8% food intake from saline injection at 1hr. Injections and intake measurements were conducted similarly as above.
Experiment 2. Conditioned taste aversion (CTA) learning
During Experiment 1, it was observed that rats showed signs of malaise including lying on their belly and increased salivary secretion around the mouth after injections of the high dose 10µg/Kg Ex-4. Thus, we examined whether the additive effects of food intake suppression were related to the aversive property of Nal and Ex-4, alone or in combination.
Group
Rats were divided into 4 groups that received IP injections of either Saline, Nal, Ex-4, or a mixture of Nal and Ex-4 (Mix) as an unconditioned stimulus (US) for a CTA acquisition. The dose combinations (1mg/Kg Nal+3.2µg/Kg Ex-4, 3.2mg/Kg Nal+1µg/Kg Ex-4, and 1mg/Kg Nal + 1µg/Kg Ex-4) were selected for the three CTA tests based on the short-term intake test results. Thus, there were three different conditioned stimuli (CS) in the order of 0.3M sucrose, 0.15M NaCl, and 0.006M citric acid solutions across the dose combinations.
CTA procedures
The CTA training and testing protocols were adapted from previous studies19, 20. Rats were overnight water deprived. The training included 15 min presentation of fluid and post test 1hr rehydration with water. At baseline training, rats received water during the 15 min session. Once the water intake stabilized, rats received a 15 min presentation of a CS and 10 min after that an IP injection of an US on the conditioning day. There were 3 conditioning trials and then a one 1-bottle (15 min CS presentation alone) test to evaluate the degree of aversion. Each CS presentation trial was separated by 2 days of water presentation. After acquiring a CTA to a CS, rats were returned to water and food ad lib for at least 1 week before the next CTA training with a new CS begun. Rats were re-grouped based on equal average BW for each CTA acquisition.
Statistical analyses
Food intake, BW, and CS intake were analyzed using Statistica 7.1 (Tulsa, OK). Cumulative food intakes were analyzed using mixed-model repeated-measures ANOVA (Experiment 1, doses of drugs as within factors; Experiment 2, US treatment as predictor and conditioning and 1-bottle test trials as within factors). Post hoc comparisons were made with Fisher LSD tests. Food intake data of Experiment 1 were expressed as percentage of saline injection intake. Data from individual drug administration and dose combinations of Nal and Ex-4 were expressed in a quadratic surface plot and, a response surface methodology (RSM) was used to determine whether the range of combinational doses resulted in an infra-additive, additive or supra-additive21–24 effects on food intake. Based on previous RSM, additivity was suggested by a P value ≥0.05 and more than additivity (supra-additivity) P value <0.05 for the interaction term. Data are expressed as means±SE.
Results
Experiment 1 – Food Intake Testing
Individual drug administration
Acute food intake after individual doses of Nal was suppressed dose dependently at all 3 time points measured (Table 1). This is supported by one way repeated measure ANOVA’s revealing significant dose effects [1hr, F(3, 21)=11.5, P<0.001; 4hr, F(3, 21)=11.2, P<0.001; 20hr, F(3, 21)=12.6, P<0.0001]. Similarly, administration of Ex-4 produced dose dependent suppressions of food intake [Table 1, 1hr, F(3, 21)=2.6, P=0.08; 4hr, F(3, 21)=5.6, P<0.006; 20hr, F(3, 21)=6.7, P<0.003]. There was no significant BW reduction in response to any one of these once a day injections (data not shown), likely due to the small level of intake suppression by 20hr. Overall these results are consistent with previous reports that both Nal and Ex-4 reduce acute food intake.
Table 1.
Cumulative intake for naltrexone (Nal) and exendin (Ex-4), alone or in combination
| Drug(s) doses | 1 H | 4 H | 20 H | |
|---|---|---|---|---|
| Nal 0.32 mg/Kg | 80.1 ± 7.0 a | 94.4 ± 7.0 a | 103.7 ± 3.1 a | |
| Nal 1 mg/Kg | 62.9 ± 5.6 b | 68.1 ± 6.2 b | 93.9 ± 3.2 b | |
| Nal 3.2 mg/Kg | 53.6 ± 7.5 b | 60.3 ± 5.6 b | 86.4 ± 1.2 c | |
| Ex-4 1 µg/Kg | 93.6 ± 12.9 | 76.1 ± 5.8 a | 90.6 ± 3.5 a | |
| Ex-4 3.2 µg/Kg | 73.2 ± 12.3 | 78.2 ± 11.2 a | 88.2 ± 4.0 a | |
| Ex-4 10 µg/Kg | 58.3 ± 10.9 | 52.6 ± 7.8 b | 74.2 ± 6.9 b | |
| Nal 0.32 mg/Kg + | Ex-4 1 µg/Kg | 50.5 ± 6.2* | 58.4 ± 5.7$ | 88.2 ± 3.7$ |
| Ex-4 3.2 µg/Kg | 30.8 ± 8.8* | 51.8 ± 7.8 | 72.8 ± 3.7* | |
| Ex-4 10 µg/Kg | 32.1 ± 9.3* | 40.1 ± 10.1$ | 76.9 ± 5.4$ | |
| Nal 1 mg/Kg + | Ex-4 1 µg/Kg | 47.4 ± 8.5# | 60.8 ± 6.6 | 86.9 ± 3.6$ |
| Ex-4 3.2 µg/Kg | 24.2 ± 8.5* | 42.4 ± 9.6* | 78.9 ± 4.9* | |
| Ex-4 10 µg/Kg | 10.5 ± 6.7* | 25.3 ± 7.9* | 66.4 ± 3.7$ | |
| Nal 3.2 mg/Kg + | Ex-4 1 µg/Kg | 25.3 ± 6.8# | 54.5 ± 8.2 | 83.6 ± 3.9 |
| Ex-4 3.2 µg/Kg | 28.6 ± 14.8# | 40.8 ± 12.5# | 77.4 ± 5.6* | |
| Ex-4 10 µg/Kg | 32.9 ± 14.9 | 30.0 ± 12.7 | 65.5 ± 6.4 | |
Comparisons are made down the hour columns. Different letters indicate significant differences between doses at each hour column during individual drug treatment.
vs. individual parent Nal or Ex-4 P<0.05;
vs. individual Ex-4 P<0.05;
vs. individual Nal P<0.05
Dose combinations of Nal and Ex-4
There were 9 dose combinations of Nal and Ex-4. From their respective saline baselines, these combination treatments resulted in dose dependent reductions in food intake at all 3 time points measured [1hr, F(9, 63)=6.9, P<0.0001; 4hr, F(9, 63)=6.2, P<0.0001; 20h, F(9, 63)=8.5, P<0.0001]. Table 1 includes food intake results from each individual or combination dose administration at each time point measured. ANOVA comparing individual and combination (Nal, Ex-4, and Nal+Ex-4) for each dose, Nal+Ex-4 combinations had additive effects on food intake i.e. drug combinations reduced food intake more than did individual drugs alone. Response surface regression analyses also suggested additive effects at all 3 time points measured (Figure 1). At 1hr, P values for the Nal and Ex-4 terms were < 0.001 and for the Nal+Ex-4 interaction term was=0.087. At 4hr, P values were <0.04 for the individual Nal and Ex-4 terms and was =0.61 for the Nal+Ex-4 interaction term. Finally at 20hr, P values were=0.27 and <0.005 respectively for the Nal and Ex-4 terms and was=0.85 for the Nal+Ex-4 interaction term. Here the P values for Nal or Ex-4 indicates the significant dose dependent food intake suppression for the two drugs alone. These results are consistent with the ANOVA results that revealed dose dependent effects on food intake suppression. Based on what has been defined by others using combinational interactions and response surface methodology21, 22, 25, the P values for the drug combination indicate additive or supra-additive effects on food intake. Because the P values for drug combinations at each time point were >0.05, the Nal and Ex-4 combinations produced additive suppressive effects on food intake.
Figure 1.
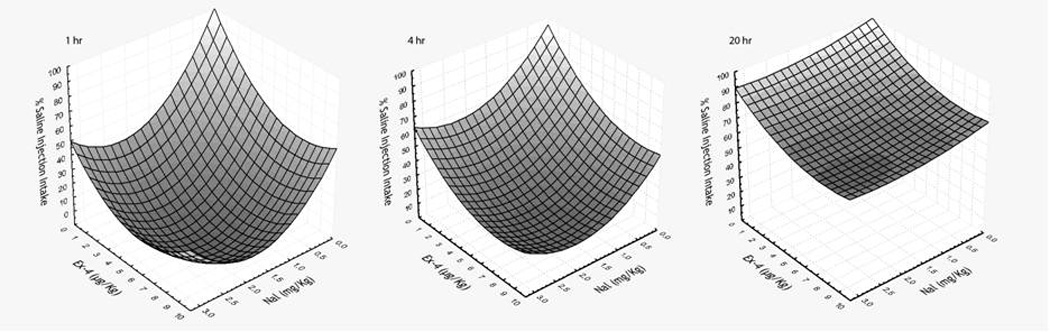
Quadratic surface plots for cumulative food intake at 1, 4, and 20 hr after IP administrations of doses of Nal or Ex-4 alone and the 2 drugs combined. Data are presented as percentage of the corresponding hourly saline injection intake. The bottom tips of all 3 surface plots do not extend to the front junction of the Ex-4 and Nal axis suggesting additive effects on food intake suppression after treatments of Nal + Ex-4 at all time points measured. These additive effects are confirmed by response surface regression (see Experiment 1 Results).
Consecutive daily injection
Results for 4 daily consecutive injections of 1mg/Kg Nal+3.2µg/Kg Ex-4 combination are shown in Figure 2. Consistent with the results from the short-term food intake tests, such dose combinations produced a large suppression of food intake at 1 and 4 hr (50%–90% reduction from their respective saline injection intake, P<0.003). The intake suppression occurred after each daily injection. The reduction in intake from saline injection was smaller at 20hr (15%–25%, P<0.003 from day 1 to 3). However, the suppression effect on food intake at 20hr was not significant on day 4. Overall, the consecutive injections of 1mg/Kg Nal+3.2µg/Kg Ex-4 combination did not result in any reduction of BW (starting average weight=638.5±28.5g vs. ending weight=641.2±30.5g).
Figure 2.
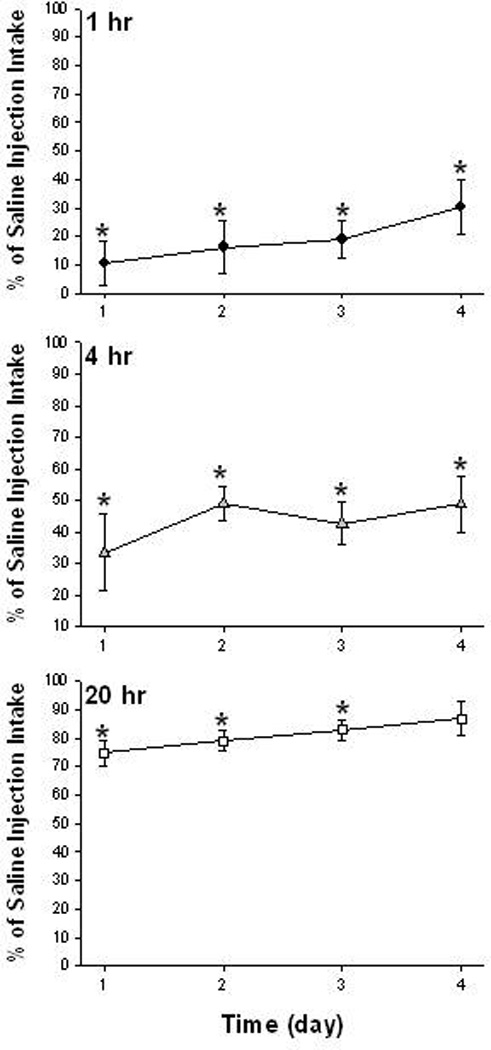
Effects of consecutive daily administration of Nal (1 mg/Kg) + Ex-4 (3.2 µg/Kg) on food intake. Combination of Nal +Ex-4 produced sustained reduction in food intake at 1 hr and 4 hr. The reduction in food intake at 20 hr gradually declined. By the 4th day, food intake was not significantly reduced at 20 hr. (*,vs. saline, P<0.05)
Experiment 2 – CTA Testing
1mg/Kg Nal and 3.2 µg/Kg Ex-4. The results of 1mg/Kg Nal and 3.2µg/Kg Ex-4 alone or combined for conditioned aversion to 0.3M sucrose are shown in Figure 3. Repeated measure ANOVA revealed significant effects of group [F(3, 26)=16.1, P<0.0001], trial [F(3, 78)=7.6, P<0.0002], and group × trial interaction [F(9, 78)=11.0, P<0.0001]. After 3 conditioning sessions, rats that received either Ex-4 or the combination of 1mg/Kg Nal+3.2µg/Kg Ex-4 learned to avoid the sucrose CS in the 1-bottle test trial. The aversive effects of Ex-4 and the Ex-4+Nal combination were also demonstrated by rats lying on their belly after the injection. Nal at 1mg/Kg had no aversive effect. Rats that received this dose maintained large volumes of sucrose intake as did the Saline group. Thus, it appeared that the aversion effect at the dose combination of 1mg/Kg Nal+3.2µg/Kg Ex-4 was mainly from Ex-4.
Figure 3.
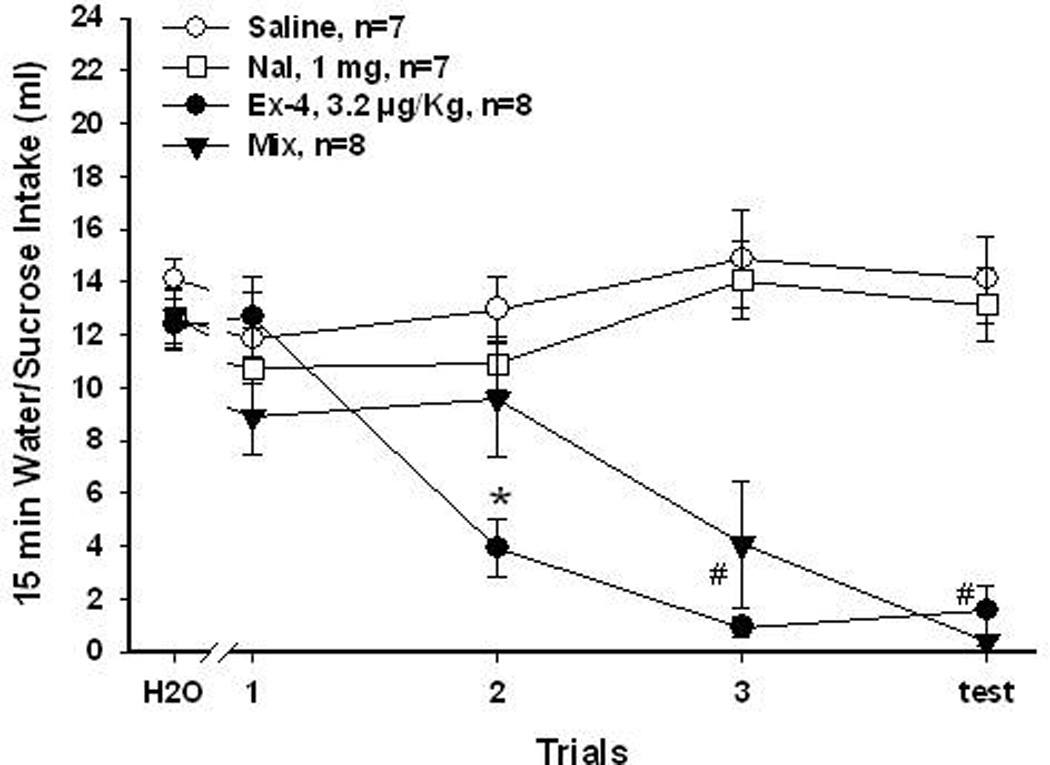
1 mg/Kg Nal and 3.2 µg/Kg Ex-4 for 0.3M sucrose aversion. Mean (±SEM) intake of 15 min presentation of baseline water or sucrose over conditioning and test trials. Rats that received saline and Nal (1 mg/Kg) did not learn to avoid sucrose. Rats that received Ex-4 (3.2 µg/Kg) and Mix (Nal + Ex-4) injections both significantly reduced their intake of sucrose over trials. Rats in the Ex-4 and Mix groups significantly reduced sucrose intake to the same level by the test trial. (*, Ex-4 vs. the other 3 groups, P<0.05; #, Ex-4 or Mix vs. saline or Nal, P<0.05)
3.2mg/Kg Nal and 1µg/Kg Ex-4. In order to test whether using a combination of a higher dose Nal and a lower dose Ex-4 would produce less aversive effects, a dose combination of 3.2mg/Kg Nal+1µg/Kg Ex-4 was used for conditioned aversion to 0.15M NaCl. This combination produced an additive effect on food intake in Experiment 1. Figure 4 summarizes the results of the CTA acquisition. After 3 conditioning trials, rats that received this combination treatment greatly reduced their intake of NaCl. Compared to trial 1 NaCl intake, rats that received Ex-4 also had a significant decrease in NaCl intake during the 1-bottle test (P<0.002). Although the dose combination group drank less NaCl than did the Ex-4 group at 1-bottle test, post hoc tests revealed no significant difference between them (P=0.12). Rats that received Nal during conditioning drank significantly less NaCl during 1-bottle test than those received Saline (P<0.03). However, there was no significant reduction in NaCl intake from initial trial 1 in Nal treated rats (P=0.21). Overall, repeated measured ANOVA confirmed significant effects of group [F(3, 26)=3.2, P<0.04], trial [F(3, 78)=5.3, P<0.003], and group × trial interaction [F(9, 78)=6.2, P<0.0001]. Because rats with this combination treatment reduced NaCl intake significantly sooner than with those that received Ex-4 alone, results in NaCl CTA acquisition suggested that additive effects of Nal and Ex-4 combination may also occur on conditioned food aversion.
Figure 4.
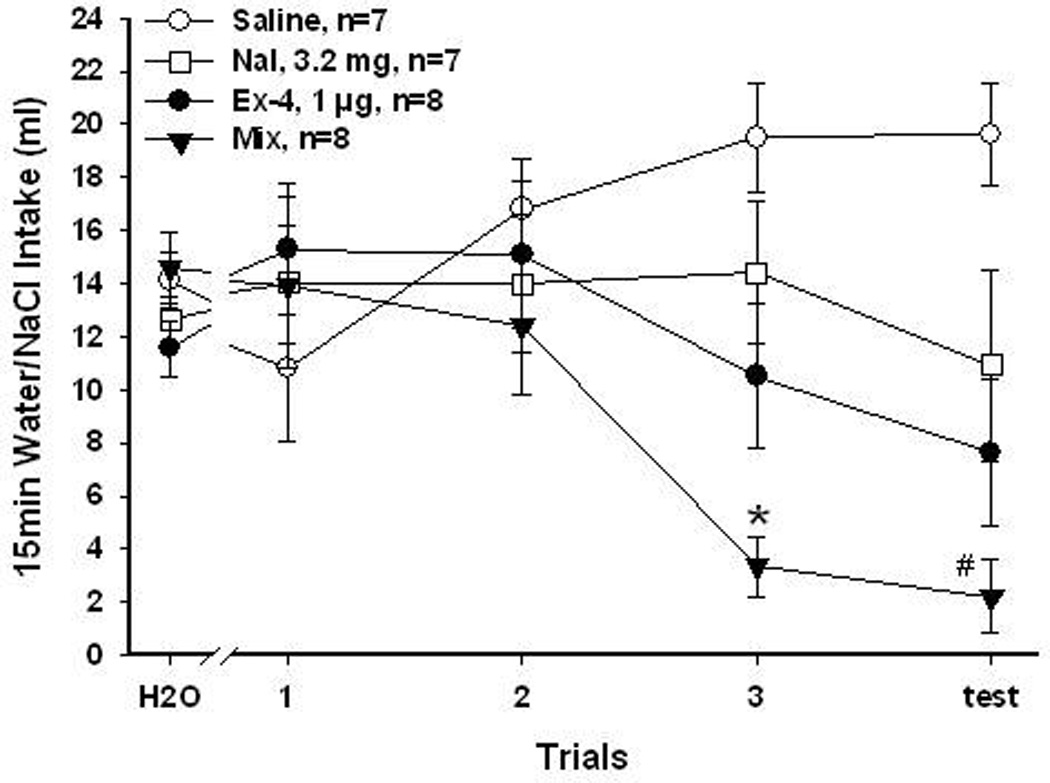
3.2 mg/Kg Nal and 1 µg/Kg Ex-4 for 0.15M NaCl CTA. Mean (±SEM) intake of 15 min presentation of baseline water or NaCl over conditioning and test trials. Rats that received saline and Nal (3.2 mg/Kg) did not learn to avoid NaCl. Rats that received Ex-4 (1 µg/Kg), and Mix (Nal + Ex-4) injections both significantly reduced their intake of NaCl over trials. Rats in the Mix group also significantly reduced NaCl intake more than the Ex-4 rats. (*, Mix vs. the other 3 groups, P<0.05; #, Mix vs. saline or Nal, P<0.05)
1mg/Kg Nal and 1µg/Kg Ex-4. To further assess the aversive effects of combinations of Nal and Ex-4, smaller doses of Nal (1mg/Kg) and Ex-4 (1µg/Kg) that produced either no or mild CTA when given alone were used. In these experiments, the CS was 0.006M citric acid. The results of citric acid CTA after 1mg/Kg Nal and 1µg/Kg Ex-4 are summarized in Figure 5. Repeated measured ANOVA reveled that both Ex-4 and the combination treatment produced conditioned aversion to citric acid [group, F(3, 21)=12.7, P<0.0001; trial, F(3, 63)=5.5, P<0.003; group × trial interaction, F(9, 63)=10.6, P<0.0001]. Rats treated with this combination greatly avoided citric acid after 1 conditioning trial, and thus acquired aversion sooner than rats treated with Ex-4. Consistent with the sucrose CTA results, Nal at 1mg/Kg did not result in avoidance to citric acid after 3 conditioning training. Overall, these results also demonstrated that combinations of Nal and Ex-4 can produce additive effects on not only food intake but also on food aversion learning.
Figure 5.
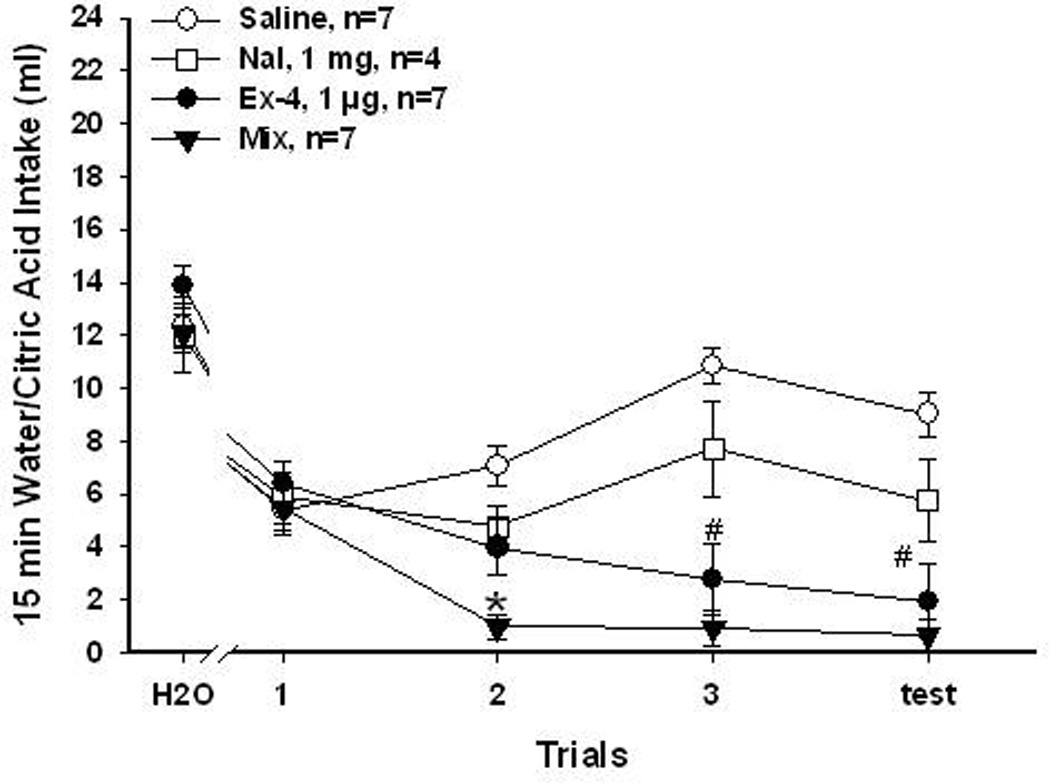
1mg/Kg Nal and 1 µg/Kg Ex-4 for 0.006M citric acid CTA. Mean (±SEM) intake of 15 min presentation of baseline water or citric acid over conditioning and test trials. Rats that received saline and Nal (1 mg/Kg) did not learn to avoid citric acid. Rats that received Ex-4 (1 µg/Kg), and Mix (Nal + Ex-4) injections acquired a CTA and avoided citric acid after 3 pairing trials. Rats in the Mix group also significantly reduced citric acid intake sooner than the Ex-4 rats. (*, Mix vs. the other 3 groups, P<0.05; #, Ex-4 or Mix vs. saline or Nal, P<0.05)
Discussion
The initial hypothesis in this study was that co-administration of compounds acting on different mechanisms involved in controlling food intake would produce larger suppression on food intake than either one of them alone. Furthermore, it was hypothesized that when additive effects of the combination occurred, requiring a smaller dose of individual drugs to decrease food intake, the most common adverse effect of the two drugs, nausea, would be ameliorated. Thus, the acute effects of individual and combined peripheral treatment of Nal and Ex-4 on food intake and CTA were examined. The results revealed that Nal and Ex-4 combination produced greater suppressions of short term food intake than either of them individually. Because the two drugs deviated in their constant relative potency on food intake, there is an inherent difficulty in examining their pharmacological relationship. While RSM can be used where the simple isobolographic method cannot, our assessment of potential synergy is somewhat limited because of the lack of constant relative potency26. However, our conclusion of additivity is conservative and consistent with the results of the ANOVA. Overall, the anorectic effect of the Nal+Ex-4 combination could be associated with an induced avoidance since there was larger and more rapid suppression of the CS intake in CTA with Nal+Ex-4 than when each compound was administered alone.
Combinational therapies have been applied to treat multiple chronic diseases such as hypertension and diabetes, and many drug combinations to treat obesity are under investigation 5. One of them is Contrave which combines Nal and bupropion. In this case, Nal the opioid antagonist, was chosen based on its ability to increase the activity of pro-opiomelanocortin neurons in the arcuate nucleus of the hypothalamus to produce anorectic effects7. In our experiments, we chose Nal based on its effects on the rewarding component of food. Many studies have demonstrated that the opioid system in the striatum contributes to the palatability of foods27,14, 28, 29. Although Nal alone is associated with minimal weight loss30, 31, combining it with bupropion has been reported to result in supra-additive effects on food intake and BW reduction7. In our present research, we combined Nal with the GLP-1 receptor agonist Ex-4. Initially Ex-4 was chosen for its satiety action. However, recent work has also suggested direct effects of GLP-1 on the reward system32, 33. Thus the combination would affect multiple systems underlying feeding control. The combination produced additive effects on short term intake suppression. Moreover, consecutive once a day Nal+Ex-4 treatment for 4 days revealed a persistent effect of short term intake reduction but no effects on BW. It also appeared that some tolerance occurred at this dose combination over 4-day consecutive treatment. Although not statistically significant, a clear trend of reduced intake suppression was found for 20hr accumulative food intake (Figure 2).
While the GLP-1 agonist Ex-4 is an FDA approved anti-diabetic drug, its use has produced significant weight loss in human34. Besides the gut, GLP-1 is expressed in neurons in the nucleus of the solitary tract (NST) and the GLP-1 receptors are expressed there and widely throughout other brain regions35, 36, 37. These NTS GLP-1 containing neurons largely project to the paraventricular nucleus of the hypothalamus (PVN) and, a significant amount project to other forebrain regions including the ventral tegmental area(VTA) and the nucleus accumbens (NAc)32, 33, 38. Central GLP-1 pathway activation results not only in food intake reduction but also in food avoidance. For example, injections of GLP-1 in the amygdala can produce malaise as measured by a CTA test39, 40 while intracerebral ventricular GLP-1 antagonists block CTA induced by LiCl 41. Furthermore, peripheral LiCl injection activates GLP-1 containing neurons in the NTS and those neurons project to the PVN42. These data suggest that central GLP-1 systems are involved in CTA learning. In this study, Ex-4 and Nal+Ex-4 combination were administered peripherally. Previous studies have suggested that the sites of action of peripherally administered GLP-1 agonists can be both peripheral and central43, 44,45. Furthermore, it has been demonstrated that Ex-4 can penetrate the brain rapidly46. Accordingly, our food intake and CTA results with Ex-4 could be induced by both peripheral and central activation of the GLP-1 receptors47. Although it is unclear whether the food intake and avoidance effects here involve the same populations of GLP-1 neurons or neurons that express GLP-1 receptors, recent studies showing food intake affected by GLP-1 neurons and receptors in the VTA and NAc32, 33 suggest that GLP-1 analogs may also be viewed as agents that acts on not only satiety but also reward mechanisms. Given that the opioid receptors are also expressed in similar brain regions, the interactions between Nal and Ex-4 could potentially occur at multiple central or peripheral sites to control ingestion.
The ability of Ex-4 to induce malaise or visceral illness varies across different animal models. In the rat, there have been mixed results with two commonly used methods to examine the aversive properties of anorexigenic drugs, pica and CTA23. Pica refers to ingestion of non-nutritive substances such as clay or Kaolin. Rodents show Kaolin hyperphagia in response to toxic treatments such as LiCl injection48, 49. When using pica behavior to measure the aversive property of the drug in SD rats, none of the tested Ex-4 doses (0.1–10µg/Kg) induced kaolin intake50. In Wistar and SD rats, peripheral Ex-4 can induce a CTA or decrease locomotor activity at a dose much higher than the threshold dose for reducing food intake 51,50. However, Hayes and colleagues recently demonstrated that peripheral Ex-4 induces both CTA (0.25–3.0µg/Kg) and pica (3µg/Kg b.i.d.) 52. Our data are similar to those of Hayes and colleagues. The reasons for the difference among the various experiments are not clear. However, the paradigm we employed with 3 pairings is considered to be especially sensitive.
The aversive effects of Ex-4 have not been as widely reported in experiments with mice and primates. One study demonstrated that the effective peripheral dose to reduce food intake is 50 times lower than the dose to induce CTA in mice53. In nonhuman primates, a dose dependent reduction in meal size in response to Ex-4 has been reported21, 54. At doses (0.1 to 3.0µg/Kg) tested, meal numbers were not changed and the monkeys did not show any obvious signs of malaise54. These data translate better to the responses in humans taking Ex-4 for diabetes treatment. Although nausea is the most commonly reported adverse effect of Exenatide (~38%), the symptom declines or stops over time11, 55. It has also been suggested that the occurrence of nausea can be greatly reduced if the GLP-1 agonist is taken by gradually increasing the dose. Overall, it is suggested that malaises related to Ex-4 can be improved or excluded overtime in humans.
The results of our study, nevertheless, are in line with the rat data revealing that Ex-4 reduces food intake and produces robust conditioned food avoidance. In Experiment 2, group assignment was reorganized for another CTA with a different CS after the initial conditioned avoidance acquisition. With this method, some rats ended up receiving saline or Nal as the US treatment for the 2nd or 3rd CTA acquisition and some rats repeatedly received the US that contained either Ex-4 alone or combined with Nal. At first glance, this design may complicate the interpretation of the results because the intake of the three different CSs at the first trial differed. However, the fact that there were no group differences in the initial intakes of all three CSs supports the following implications. First, across the doses tested, the aversive effect of Ex-4 was greater than that of Nal. When Nal alone was the US, only the higher dose 3.2mg/Kg reduced CS intake (~22%), but this reduction was not statistically significant. At the lower dose 1mg/Kg, Nal did not reduce intake of either NaCl or citric acid CS during the test trial. However, significant CTA was formed at every doses of Ex-4 tested. At the lowest Ex-4 dose tested (1µg/Kg), rats developed a 50% CS intake reduction by the 1-bottle test trial with either NaCl or citric acid as the CS. Second, in contrast to human data, previous experience with Ex-4 did not ameliorate its aversive effects. That is, no matter whether the rats were naïve or experienced with a US that included Ex-4, they developed avoidance to the CS and the rate of CTA acquisition was not decreased or increased with previous Ex-4 experience. It is possible that acclimating rats to Ex-4 treatments for an extended period of time would ameliorate CTA in the future. However, there was no evidence for this in this study. Finally, in the group that received the combination treatments, the rats acquired avoidance sooner and stronger when the low dose Ex-4 (1µg/Kg) was combined with either high or low dose Nal. Thus, these data suggest that the aversive effect of the combined treatment (Nal+Ex-4) was primarily from Ex-4 and, the combination can produce additive effects on conditioned avoidance.
Data presented here support that the Nal and Ex-4 combinations have additive effects on food intake suppression. This is consistent with the idea of using compounds that act on both the satiety and hedonic aspects of food intake to develop combinational therapy for obesity. However, inconsistent with the assumption that compounds that interact in an additive or synergistic fashion may have the advantage of minimizing dose-dependent adverse effects associated with individual compound, the data indicate that these drug combinations actually produce stronger malaise side effects in rat. Such data enhance the importance of examining not only the desired but also the adverse effects of combinational therapy at early stages of drug development.
Acknowledgements
The authors thank Mr. Ryan Purcell, Drs. Gretha Boersma, and Yada Treesukosol for assistance with animal care and conditioned taste aversion experiments. This research is supported by NIH grant DK19302.
Footnotes
Conflict of interests
The authors declare no conflict of interests.
References
- 1.WHO. [accessed September 26, 2011];Obesity and overweight factsheet. 2011 Mar; http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Fontana MA, Wohlgemuth SD. The surgical treatment of metabolic disease and morbid obesity. Gastroenterol Clin North Am. 2010;39:125–133. doi: 10.1016/j.gtc.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009:CD003641. doi: 10.1002/14651858.CD003641.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–190. 215–357, iii–iv. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 5.Greenway FL, Bray GA. Combination drugs for treating obesity. Curr Diab Rep. 2010;10:108–115. doi: 10.1007/s11892-010-0096-4. [DOI] [PubMed] [Google Scholar]
- 6.Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol. 2010;6:255–269. doi: 10.1038/nrendo.2010.19. [DOI] [PubMed] [Google Scholar]
- 7.Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17:30–39. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 8.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010;6:578–588. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pohl M, Wank SA. Molecular cloning of the helodermin and exendin-4 cDNAs in the lizard. Relationship to vasoactive intestinal polypeptide/pituitary adenylate cyclase activating polypeptide and glucagon-like peptide 1 and evidence against the existence of mammalian homologues. J Biol Chem. 1998;273:9778–9784. doi: 10.1074/jbc.273.16.9778. [DOI] [PubMed] [Google Scholar]
- 10.Raufman JP, Jensen RT, Sutliff VE, Pisano JJ, Gardner JD. Actions of Gila monster venom on dispersed acini from guinea pig pancreas. Am J Physiol. 1982;242:G470–G474. doi: 10.1152/ajpgi.1982.242.5.G470. [DOI] [PubMed] [Google Scholar]
- 11.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 13.Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–R96. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- 14.Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, et al. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006;24:1220–1226. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- 15.Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiol Behav. 1996;60:439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 16.Parker LA, Rennie M. Naltrexone-induced aversions: assessment by place conditioning, taste reactivity, and taste avoidance paradigms. Pharmacol Biochem Behav. 1992;41:559–565. doi: 10.1016/0091-3057(92)90373-n. [DOI] [PubMed] [Google Scholar]
- 17.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 18.Norgren R, Grigson P, Hajnal A, Lundy RFJ. Motivational modulation of taste. In: Ono T, et al., editors. Limbic and association cortical systems: basic, clinical and computational aspects. Amsterdam: Elsevier Science; 2003. pp. 319–334. [Google Scholar]
- 19.Mungarndee SS, Lundy RF, Jr, Norgren R. Central gustatory lesions and learned taste aversions: unconditioned stimuli. Physiol Behav. 2006;87:542–551. doi: 10.1016/j.physbeh.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav Neurosci. 1997;111:633–645. [PubMed] [Google Scholar]
- 21.Bello NT, Kemm MH, Ofeldt EM, Moran TH. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2010;299:R945–R952. doi: 10.1152/ajpregu.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gennings C, Carter WH, Jr, Campbell ED, Staniswalis JG, Martin TJ, Martin BR, et al. Isobolographic characterization of drug interactions incorporating biological variability. J Pharmacol Exp Ther. 1990;252:208–217. [PubMed] [Google Scholar]
- 23.Roth JD, Trevaskis JL, Turek VF, Parkes DG. "Weighing in" on synergy: preclinical research on neurohormonal anti-obesity combinations. Brain Res. 2010;1350:86–94. doi: 10.1016/j.brainres.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679–5687. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 25.Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, et al. Combination therapy with amylin and peptide YY[3-36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology. 2007;148:6054–6061. doi: 10.1210/en.2007-0898. [DOI] [PubMed] [Google Scholar]
- 26.Tallarida RJ, Raffa RB. The application of drug dose equivalence in the quantitative analysis of receptor occupation and drug combinations. Pharmacol Ther. 2010;127:165–174. doi: 10.1016/j.pharmthera.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldo BA, Pratt WE, Kelley AE. Control of Fat Intake by Striatal Opioids. 2010 [PubMed] [Google Scholar]
- 28.Taha SA. Preference or fat? Revisiting opioid effects on food intake. Physiol Behav. 2010;100:429–437. doi: 10.1016/j.physbeh.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glass MJ, Billington CJ, Levine AS. Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol. 2000;279:R86–R92. doi: 10.1152/ajpregu.2000.279.1.R86. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson RL, Berke LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL. Effects of long-term therapy with naltrexone on body weight in obesity. Clin Pharmacol Ther. 1985;38:419–422. doi: 10.1038/clpt.1985.197. [DOI] [PubMed] [Google Scholar]
- 31.Malcolm R, O'Neil PM, Sexauer JD, Riddle FE, Currey HS, Counts C. A controlled trial of naltrexone in obese humans. Int J Obes. 1985;9:347–353. [PubMed] [Google Scholar]
- 32.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology. 2011 doi: 10.1210/en.2011-1443. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman JS. Optimizing outcomes for GLP-1 agonists. J Am Osteopath Assoc. 2011;111:eS15–eS20. [PubMed] [Google Scholar]
- 35.Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16:97–107. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology. 1987;121:1076–1082. doi: 10.1210/endo-121-3-1076. [DOI] [PubMed] [Google Scholar]
- 37.Uttenthal LO, Toledano A, Blazquez E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7-36) amide in rat brain. Neuropeptides. 1992;21:143–146. doi: 10.1016/0143-4179(92)90036-v. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- 39.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, et al. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol. 1997;272:R726–R730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- 41.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, et al. The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 43.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and Central GLP-1 Receptor Populations Mediate the Anorectic Effects of Peripherally Administered GLP-1 Receptor Agonists, Liraglutide and Exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 46.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 47.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Jonghe BC, Lawler MP, Horn CC, Tordoff MG. Pica as an adaptive response: Kaolin consumption helps rats recover from chemotherapy-induced illness. Physiol Behav. 2009;97:87–90. doi: 10.1016/j.physbeh.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horn CC, De Jonghe BC, Matyas K, Norgren R. Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1375–R1382. doi: 10.1152/ajpregu.00284.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–1340. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- 51.Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain Activation Following Peripheral Administration Of The Glp-1 Receptor Agonist Exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- 52.Kanoski SE, Hayes MR. Benjamin Franklin Lafayette Seminar. Frejus, France: 2011. Body weight and food intake suppression by glucagon-like-peptide-1 receptor agonists, exendin-4 and liraglutide, may require nausea and malaise. [Google Scholar]
- 53.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 54.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293:R983–R987. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 55.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]


