Abstract
Reconstruction of complex craniofacial deformities is a clinical challenge in situations of injury, congenital defects or disease. The use of cell-based therapies represents one of the most advanced methods for enhancing the regenerative response for craniofacial wound healing. Both Somatic and Stem Cells have been adopted in the treatment of complex osseous defects and advances have been made in finding the most adequate scaffold for the delivery of cell therapies in human regenerative medicine. As an example of such approaches for clinical application for craniofacial regeneration, Ixmyelocel-T or bone repair cells are a source of bone marrow derived stem and progenitor cells. They are produced through the use of single pass perfusion bioreactors for CD90+ mesenchymal stem cells and CD14+ monocyte/macrophage progenitor cells. The application of ixmyelocel-T has shown potential in the regeneration of muscular, vascular, nervous and osseous tissue. The purpose of this manuscript is to highlight cell therapies used to repair bony and soft tissue defects in the oral and craniofacial complex. The field at this point remains at an early stage, however this review will provide insights into the progress being made using cell therapies for eventual development into clinical practice.
Keywords: Stem Cells, Cell Therapy, Tissue Engineering, Bone Regeneration, Bone Marrow
2. Introduction
The craniofacial region is essentially composed of a framework of bone and cartilage giving support to muscles, ligaments, glands, and various layers of skin and subcutaneous structures. All these elements are innervated by the body’s most sophisticated neurovascular network, which allows for function and sensorial capacities [1]. Injuries caused by trauma, tumor or cyst resection, infectious diseases, and also congenital and developmental conditions (i.e., cleft palate defects) may result into serious functional, aesthetical and psychological sequelae [2, 3]. In such situations, absence of hard and soft tissues can be disfiguring and often compromise basic functions such as mastication, speech, swallowing, and also lead to limited thermal and physical protection of important anatomical structures (i.e. brain, nerves, arteries, veins) [4–7]. The progression of certain oral conditions may also result in craniofacial defects of difficult resolution. Periodontitis is a chronic inflammatory disease of bacterial etiology, characterized by the loss of support around teeth, including alveolar bone resorption and soft tissue alterations [8–10]. Dental implant tooth replacements, one of the most popular therapies for total or partial edentulism, may be affected by a similar condition known as peri-implantitis [11]. Achieving predictable regeneration in the treatment of craniofacial defects is remarkably challenging in most clinical scenarios (Figure 1), given the loss of structural support and different embryologic origins of the affected tissues, among other factors.
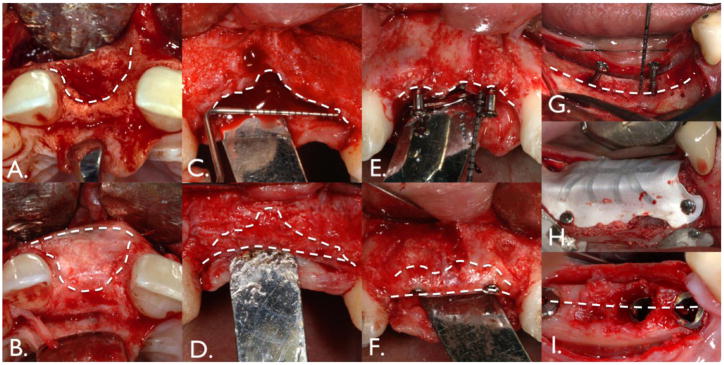
Figure 1. Tissue engineering applications in the craniofacial complex.
Several clinical scenarios can benefit from the application of tissue engineering approaches, such as cell therapy. In order to accommodate dental implants deficient maxillary and mandibular alveolar ridges can be expanded horizontally (A, B), vertically (C, D), or both vertically and horizontally (E, F, G, H).
Autogenous tissues have been widely used and are still considered as the gold standard to which all other biomaterials are compared [12]. Nevertheless, even the most advanced reconstructive techniques using autologous materials are often insufficient to restore extensive or complex maxillofacial defects [1]. Autografts contain all of the basic elements necessary to induce effective tissue regeneration, provided cells, extracellular matrix and cytokines [13, 14]. However, the use of autogenous tissue involves the need of harvesting it from a donor site, with the consequent drawbacks in terms of costs, procedure time, patient discomfort and possible complications. Additionally, oftentimes the volume of harvested tissues is not sufficient to fill or cover a defect, given the limited availability of autogenous tissues [15, 16]. To overcome these limitations, a variety of exogenous substitute materials, including allografts, xenografts and alloplasts, have been introduced in clinical practice over the last three decades [17, 18]. These materials primarily act as scaffolds, supporting the migration of cells from the periphery of the grafted area. Substitutes are indicated in the treatment of cases where the application of autografts alone may not be possible [19]. Unfortunately, when comparing these biomaterials to autografts other limitations emerge. The presence of cellular populations, orchestrate the release of growth factors, maintenance of a stable scaffold, and stimulate angiogenesis and are key for successful tissue regeneration as they play a fundamental role on the healing process [20]. Controlling the dynamics of these elements allows for a more predictable treatment of challenging craniofacial defects.
Novel tissue engineering therapies aimed at enabling clinicians to achieve predictable regeneration have been recently developed. These include, but are not limited to, the delivery of growth factors incorporated in carriers, the stimulation of the selective production of growth factors using gene therapy, and the delivery of expanded cellular constructs [21–67]. Approaches utilizing this latter strategy are known under the general name of cell therapy.
The incorporation of agents with biological properties into scaffolding materials has been proposed to modulate the behavior of precursor cells, that would ultimately contribute to the formation of new tissue [68, 69]. These agents can be grouped into two main categories: growth factors and morphogens. Growth factors primarily have mitogenic and chemotactic properties, while morphogens act through the alteration of cellular phenotype [70, 71]. Bone morphogenic proteins (BMPs) are an example of morphogens; they have the ability of inducing the differentiation of stem cells into bone forming cells in a process known as osteoinduction [72]. Other growth factors used in craniofacial regeneration include platelet-derived growth factor (rh PDGF-BB), transforming growth factor-beta 1 (TGF-β1), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), endothelial cell growth factor (ECGF), and fibroblast growth factor-2 (FGF-2). Although the application of these mediators has shown promising results in preclinical and clinical studies, suboptimal tissue response might occur as a consequence of the short half-life these molecules exhibit in vivo due to proteolytic degradation, rapid diffusion, or inadequate solubility of the carrier within the treated lesion [73].
In order to address growth factor delivery issues, the induction of a sustained release of growth factors via gene therapy was proposed [74–76]. Gene therapy basically consists of the insertion of genes into cells of the host, either directly or indirectly. This strategy was originally aimed at supplementing a defective mutant allele with a functional one in a therapeutic approach for some congenital conditions, but it can also be used to induce a more favorable host response [77, 78]. Targeting cells for gene therapy requires the use of vectors or direct delivery methods to transfect them [20]. In craniofacial regeneration, tissue engineering using gene therapeutic approaches may offer potential for optimizing the release of growth-promoting molecules, such as BMPs, in osseous defects [73, 79]. Although this approach is per se unique and relies on host cells for the new tissue production, several concerns regarding its safety have arisen [80, 81].
Another branch of tissue engineering has adopted the use of transplanted cells in order to promote and direct wound healing (Figure 2). Cell therapy approaches provide an additional source of cells in the area of interest, with the intent to be used as grafted cells (which will integrate into the patients body) or, when not intended for integration, as a source of growth factors [82, 83]. Cell therapy has a great potential in the clinical arena for the regeneration of both hard and soft tissues and could represent a new important instrument to enhance wound healing in different scenarios.
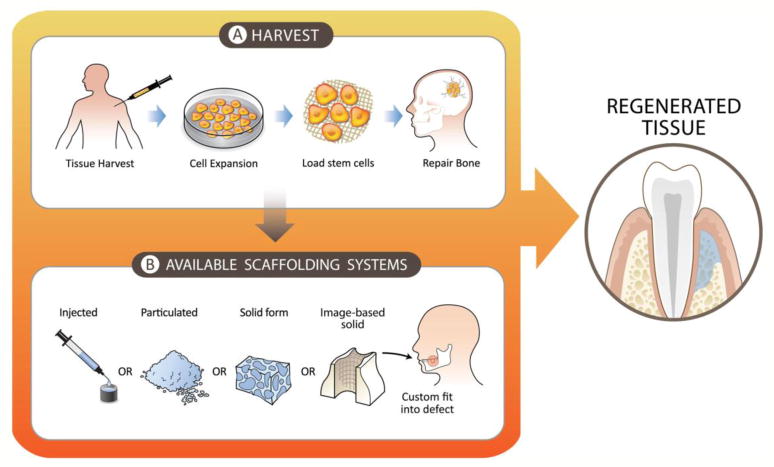
Figure 2. Cell therapy technologies for regenerative medicine.
A) Cell therapy provides an additional source of cells in the area of interest. After harvesting a tissue sample, the cells are expanded, manipulated, and loaded onto a carrier. Similarly, Ixmyelocel-T is harvested from the own patient, expanded through a completely automated and closed SPP system and loaded into a scaffolding material (i.e. gelatin foam, β-TCP). When grafted in a bone defect, Ixmyelocel-T promotes enhanced bone regeneration and maturation.
B) Injected, particulated, prefabricated solid, or image-based solid scaffolds are available in tissue engineering. Thanks to the integration of these newly available technologies new perspectives for enhanced outcomes in the regeneration of craniofacial structures can be explored.
The purpose of this review is to examine the existing literature on the treatment of craniofacial defects adopting cell therapy approaches, to assess the validity of the different strategies, and to propose a path that can be shared by the research community toward which to direct future research efforts.
3. Cell Therapy Applications for Craniofacial Regeneration
Both somatic and stem cells can be used in cell based therapy (Table 1). Somatic cells can be harvested, cultured and implanted with the aim of engineering new tissues. Limitations in their use are related to the lack of self-renewal capability and limited potency; characteristics that are exclusive of stem cells [84]. Somatic cell delivery and stem cell therapy cells have been evaluated in different areas of regenerative medicine; their adoption in craniofacial regeneration will be discussed in the following paragraphs. Moreover, a recently developed cellular approach making use of both cell and gene therapies for the production of induced pluripotent stem (iPS) cells will be highlighted.
Table 1.
Cell therapy applications for periodontal/craniofacial tissue engineering.
| Regenerative cell construct | Study model | References | |
|---|---|---|---|
| Autologous Stem Cells | Bone block allografts impregnated with autogenous bone marrow | Patients with severely atrophic maxillary and mandibular ridges | Soltan et al. 2007 [29] |
| Autologous MSCs isolated from a bone marrow aspirate and expanded in vitro | Periodontal regeneration in class III furcations in a dog model | Kawaguchi et al. 2004 [30] | |
| Engineered porous scaffold seeded with BMSCs | Postextraction socket in rabbits | Marei et al., 2005 [31] | |
| PRP + MNCs from bone marrow aspirate | Alveolar ridge augmentation in humans | Filho Cerruti et al., 2007 [32] | |
| PRP + in vitro-expanded bone marrow derived MSCs | Trephined defects in dog mandibles | Yamada et al., 2004 a,b,c [33–35] | |
| PRP + in vitro-expanded bone marrow derived MSCs | Periodontal defects in humans | Yamada et al., 2006 [36] | |
| Adipose-derived stem cells | Periodontal defects in Wistar rats | Tobita et al., 2007 [37] | |
| BMSCs incorporated with a PLCL scaffold | Osteochondral defect on the medial femoral condyles at a high load-bearing site on a rabbit’s knee joint | Xie et al., 2010 [38] | |
| NELL-1 modified autogenous BMSCs in PLGA scaffold | Surgically-created osteochondral defects in goats’ mandibular condyles | Zhu et al., 2011 [39] | |
| Autologous periodontal ligament cells from extracted teeth in a hyaluronic acid carrier | Dehiscence defects in beagle dogs | Akizuki et al., 2005 [40] | |
| PDL stem cells from extracted teeth | Surgically-created periodontal defects in miniature pigs | Liu et al., 2008 [41] | |
| Bmp2-supplemented dental pulp stem cells | On amputated pulp to stimulate reparative dentin formation | Iohara et al. 2006 [42] | |
| BMSCs cryopreserved for 1 month and freshly isolated BMSCs (control) | Periodontal fenestration on beagle dogs | Li et al. 2009 [43] | |
| Allogenic Somatic Cells | Fibroblast-like cells from expanded regenerated periodontal ligament cells | Artificial class II furcal defect in a dog model | Dogan et al., 2002, 2003 [44, 45] |
| Periodontal ligament cells | Periodontal defects created in Sprague-Dawley male rats | Lekic et al., 2001 [46] | |
| Cultured cementoblasts, periodontal ligament fibroblasts, and dental follicle cells | Ectopic tissue regeneration in mice using 3-D poly lactic-co-glycolic acid (PLGA) scaffolds | Jin et al., 2003 [47] | |
| Cultured primary follicle cells and immortalized cementoblasts | Buccal periodontal defects in mandibular molarf of athymic rats | Zhao et al., 2004 [48] | |
| Syngeneic skin fibroblasts transduced by the BMP-7 gene | Periodontal ligament regeneration at sites with periodontal bone defects in rats | Jin et al., 2003[49] | |
| Living human fibroblast-derived dermal substitute (Allogenic foreskin fibroblasts and keratinocytes) | Patients with insufficient attached gingiva | McGuire et al., 2005 [50] | |
| Living human fibroblast-derived dermal substitute (Allogenic foreskin fibroblasts and keratinocytes) | Multi center study treating patients with insufficient attached gingiva but no need for root coverage | McGuire et al., 2007 [51] | |
| Autologous Somatic Cells | Periodontal ligament cell sheets with reinforced hyaluronic acid carrier | Surgically create dehiscence defects | Akizuki et al., 2005 [40] |
| Cultured and expanded autologous fibroblasts | Injections for papilla priming procedure to augment open interproximal spaces | McGuire et al., 2007 [52] | |
| Ex vivo produced oral mucosa equivalent (EVPOME, Autogenous keratinocytes seeded on Alloderm®) | Patients with either a premalignant or cancerous mucosal oral lesion | Izumi et al., 2003[53] | |
| Ex vivo produced oral mucosa equivalent (EVPOME, Autogenous keratinocytes seeded on Alloderm® | Patients affected by squamous cell carcinoma of the tongue, leukoplakia of the tongue, gingiva, and buccal mucosa or hypoplasia in the alveolar ridge | Hotta et al., 2007 [54] | |
| Autogenous chondrocytes expanded in presence of FGF-2 and TGFβ1 | Cartilage defects in the knee | Brittberg et al., 1994, Jakob et al., 2001, Dozin et al., 2002, 2005[55–58] | |
| Engineered cartilage generated in vitro from chondrocytes cultured on a biodegradable scaffold | Osteochondral defect in a rabbit knee joint | Schafer et al., 2002 [59] | |
| PDL-derived cells cultured and placed on the surface of Ti pins | Implantation on nude mice, beagle dogs and human patients | Gault et al., 2010 [60] |
Somatic Cells
In the craniofacial region, fibroblast-like cells derived from the periodontal ligament have been used to promote periodontal regeneration [44, 45]. As demonstrated through in vivo investigations using a labeling technique, oral-derived periodontal cells are able to stimulate alveolar bone formation [46]. Cloned tooth-lining cementoblasts, periodontal ligament fibroblasts, and dental follicle cells seeded onto three-dimensional polylactic-co-glycolic acid scaffolds, exhibit mineral formation in vitro [47]. Immortalized cementoblasts delivered to large periodontal defects via biodegradable PLGA polymer sponges contributed to complete bone bridging and PDL formation, while dental follicle cells inhibited bone formation [48]. Another study showed that skin fibroblasts transduced by the BMP-7 gene promoted the regeneration of periodontal defects including new bone, functional PDL and tooth root cementum [49].
In the management of soft tissue defects cultivated fibroblasts have also been used for the treatment of interdental papillary insufficiency [52]. A human oral mucosa equivalent, made of autogenous keratinocytes on a cadaveric dermal carrier (Alloderm®) was able to favor wound healing when compared to the dermal carrier alone [53]. An ex vivo synthesized oral mucosa equivalent (EVPOME) produced without using animal-derived serum or feeder layer cells [54, 85] has demonstrated its ability to promote early initiation of epithelialization, short healing period and minimal scar contraction. This can be partially explained by the ability of this living construct to secrete growth factors as VEGF, promoting initial vascularization, which is critical to subsequent graft survival [86, 87]. EVPOME has been successfully used to treat patients affected by squamous cell carcinoma of the tongue, leukoplakia of the tongue, gingiva, and buccal mucosa or hypoplasia of the alveolar ridge [54]. In other soft tissue applications, allogenic foreskin fibroblasts have been utilized to promote keratinized tissue formation at mucogingival defects [50]. A tissue-engineered living cellular construct comprised of viable neonatal keratinocytes and fibroblasts rendered similar clinical outcomes when compared to conventional gingival autografts [51]. This construct has a strong potential to promote tissue neogenesis through the stimulation of angiogenic signals [88]. Another interesting product consists of the application of neonatal keratinocytes and fibroblasts for increasing keratinized gingiva around teeth [89]. This cell construct can stimulate the expression of angiogenic-related biomarkers as compared with autogenous free gingival grafts during early wound-healing stages [88] and, therefore, constitutes a promising material for gingival grafting without the need of a donor site.
The benefits of using somatic cells for the regeneration of soft and hard tissues in the craniofacial district have been illustrated by several preclinical and clinical studies [82]. Although, the lack of self-renewal capability and their commitment toward a single cellular phenotype limit their use in the treatment of more challenging craniofacial defects, in which a more orchestrated cellular response may be critical to gain success. Given their higher characteristics, stem cells might have a greater potential in this arena.
Stem cells
Stem cells retain the ability to perpetuate through mitotic cell division (self-renewal) and can differentiate into a variety of specialized cell types (potency). The features of the tissue that will result from the regenerative process will be dictated by the cell-to-cell interaction at the defect site. For example, stem cell populations have the ability to differentiate into osteogenic cells as well as into ‘supportive osteogenic cells’, which may be of capital importance in the treatment of severely compromised bone defects. Supportive osteogenic cells are defined as cells that do not directly create bone, but that facilitate bone deposition by creating structures needed to allow this process (i.e., vascular network).
The bone marrow stroma contains hematopoietic stem cells and mesenchymal stem cells called Bone marrow stromal cells (BMSC) [90]. Hematopoietic stem cells give rise to blood cells of all lineages, while Bone marrow stromal cells (BMSC) are characterized by elevated renewal potency and by the ability of differentiating into osteoblasts, chondroblasts, adipocytes, myocytes and fibroblasts when transplanted in vivo [91]. From a single progenitor cell, limitative or inductive stimuli in the differentiation pathway may lead to cells characterized by lower renewal capacity and by an increased potential of differentiation [90]. This path seems to be reversible so that an adult adipocyte may de-differentiate back to levels with differentiation capabilities and then progress through the osteogenic pathway [92]. Studies have reported bone formation in ectopic places where MSCs were implanted [93] suggesting a possible role of MSCs in the production of different osteoinductive molecules [94].
MSCs can be obtained from a variety of sources [43, 95, 96]. Autologous MSCs isolated from a bone marrow aspirate from the iliac crest have been used to promote periodontal regeneration preclinically. The treatment allowed for the regeneration of cementum, periodontal ligament, and alveolar bone [30, 97]. Bone marrow mesenchymal stem cells seeded in an engineered porous poly-L-lactic acid - polyglycolic acid composite scaffold have been adopted to graft extraction sockets in an animal model resulting in better preservation of alveolar bone walls than in control groups [31]. Bone block allografts impregnated with bone marrow aspirated from the anterior iliac crest offered a predictable and a cost effective therapy for the treatment of severely atrophic maxillary and mandibular ridges when compared to harvesting autogenous bone [29].
Clinical studies have demonstrated excellent results when combining bone marrow aspirations and platelet-rich plasma (PRP), an autologous product that contains supraphysiologic levels of platelets, in alveolar ridge augmentation procedures [32]. A similar combination therapy was able to improve osseointegration of dental implants [33, 34], to enhance bone regeneration during implant site development techniques [35] and for the treatment of periodontal angular defects [36].
MSCs may also be harvested from adipose tissue with the advantage of a high ease of access with low morbidity, because of the large amounts of human lipoaspirates readily available [98, 99]. Adult adipocytes are able to de-differentiate back to levels with higher generative capabilities. Studies on rats suggested that adipose-derived stem cells mixed with PRP can promote periodontal tissue regeneration [37]. Following the positive results of tissue engineering strategies for the reconstruction of long bones and large osseous defects in orthopedic surgery [100–102], significant efforts were made in regenerating cartilage. Since adult chondrocytes are characterized by a limited proliferative potential, focal chondral lesions (that do not contain a vascular network) do not heal and osteochondral lesions (which receive partial vascularization from the osseous tissue) typically heal by formation of non-functional fibrous tissue [103]. An attempt to produce human articular chondrocytes in vitro has been successfully performed and the cultured cells have been tested in a knee-healing model [55–58]. Despite the encouraging results, chondrocyte culture conditions and graft fixation methods still present limitations. Given their high proliferation and differentiation potential, the adoption of stem or progenitor cells to treat cartilage defects could represent a promising approach and would not require resection of healthy cartilage tissues. Tissue engineered cartilage formed in bioreactors [104] and osteochondral composite tissues have been generated in vitro [105, 106] and successfully utilized in vivo [59, 107]. Repair of osteochondral defects at high load-bearing sites in adult rabbits was achieved by using PLCL-based sponge scaffolds and BMSCs [38]. Recently, the use of a composite material consisting of NELL-1 (NEL-like molecule-1)-modified autogenous bone marrow mesenchymal stem cells (BMMSCs) and poly lactic-co-glycolic acid (PLGA) scaffolds has been tested in the treatment of surgically-created osteochondral defects in goats’ mandibular condyles [39]. This approach could be potentially applied for the resolution of temporomandibular joint injuries, which is often challenging with conventional treatments.
Bone marrow, adipose tissue, liver and muscle are known sources of postnatal stem cells, but even intraoral sites, such as the dental pulp and the periodontal ligament, can be used as a source of MSCs [108–110]. Cells derived from various dental tissues were shown to maintain the ability to differentiate into osteoblasts, adipocytes, and other cell types [111]. Perry and collaborators suggested the possibility of banking MSCs through cryopreservation of extracted third molars, because cells recovered from the pulp maintained the characteristics of MSCs [112]. Similar characteristics were observed on cells isolated from deciduous dental pulp including the ability of generating dentin and dental pulp [113–115]. Also cells from human periodontal ligament were found to have MSCs features and were able to generate PDL-like structure in vivo [116]. Post-natal stem cells were also recovered from cryopreserved periodontal ligament of previously extracted teeth [117]. Periodontal regeneration was more robust when using autologous periodontal ligament cells obtained from extracted premolars and prepared in sheets using temperature-responsive cell culture dish technique and hyaluronic acid carrier [40]. PDL stem cells have also shown the potential to regenerate periodontal attachment apparatus in vivo in a porcine model including new bone, cementum and PDL [41]. PDL stem cells express several mesenchymal stem cell markers, such as STRO-1 and CD44, and maintain the ability to differentiate into osteogenic, adipogenic, and chondrogenic pathways [82, 118].
After extracting tooth germ progenitor cells from discarded third molars, Ikeda and co-workers suggested the possible use for regeneration of fatal disorders as for cell-based therapy to treat liver diseases [119]. Iohara et al. demonstrated reparative dentin formation in a dog model using BMP2-treated pellet culture of pulp progenitor/stem cells [42].
In vivo generation of a tissue-engineered natural tooth, including all of its supporting structures and capable of completely replace functionally and aesthetically its missing counterpart, is a current utopia. Thanks to advances in cell therapy materialization of that concept seems to be getting closer and closer. Sonoyama et al. were able to generate a “bio-root” structure encircled with PDL tissue by combining PDL stem cells with stem cells from the root apical papilla of human teeth [120]. Recently, cell transplantation of PDL progenitor cells, collected from extracted teeth, expanded in bioreactors and delivered in the surface of titanium implants, has shown the proof-of-principle to generate hybrid ligament-dental implant constructs [60]. For the first time in a case series of human participants it was possible to induce formation of a biological ligament at the interface between these “ligaplants” allowing them to withstand functional loading for extended periods of time [60, 121, 122].
As such, there is significant potential for the use of either stem cells or PDL progenitor cells to form both soft and hard periodontal tissues in vivo.
The latest advancement in stem cell therapy is related to the use of induced pluripotent stem (iPS) cells. These cells populations have the similar characteristics of embryonic stem cells in terms of cell morphology, proliferation, surface antigens, gene expression, telomerase activity, and epigenetic status of pluripotent cell specific genes [123–125]. The generation of iPS cells usually requires the combined adoption of cell and gene therapies. The use of retroviruses, lentiviruses, adenoviruses, plasmid transfection, transposons, and recombinant proteins are among the different strategies to produce iPS cells [126]. The potential of iPS cells is remarkable, as they might allow for the use of stem cells without the hassle and possible complications of the surgical maneuvers needed for harvesting cells from the patient bone marrow.
A Japanese group reprogramed mouse somatic cells and adult human dermal fibroblasts to generate iPS cells [123, 124]. Both at the Genome Center of Wisconsin [125] at the Children’s Hospital Boston and at the Dana Farber Cancer Institute [127] researchers were able to derive iPS cells from human somatic cells reprogram somatic cell nuclei to an undifferentiated state. Collaborations between Kyoto and Gifu Universities for the establishment of an iPS cell bank of various human leukocyte antigen (HLA) types generated 2 cell lines, which are estimated to cover approximately 20% of the Japanese population with a perfect match [128].
To date, several concerns have arisen related to the use of iPS cells in humans, including the limited efficiency of reprogramming primary human cells (making it difficult to generate patient-specific iPS cells from initially small cell populations), the possible integration of viral transgenes into the somatic genome, which may potentially induce tumorogenesis [129], and iPS cell teratoma formation [130–132]. Even a small number of undifferentiated cells can result in the formation of a teratoma. Therefore, regardless of the advances demonstrated thus far, the potential for tumor formation has not yet been eliminated [126] and the use of autologous cell sources remains the safest approach to Stem Cell Therapy.
Scaffolds for Cell Therapy Delivery to Oral and Craniofacial Defects
Scaffolds play a pivotal role in providing a three-dimensional template for tissue neogenesis [133]. Scaffolds can not only be used as carriers for cell delivery but they serve as synthetic extracellular-matrix environments to define a 3D geometry for tissue regeneration and provide an adequate microenvironment in term of chemical composition, physical structure and biologically functional moieties [134, 135]. Thus far, the most widely adopted scaffolds for craniofacial bone regeneration are xenogenic and allogenic bone substitutes, hydroxyapatite, calcium phosphates, and gelatin or collagenous sponges [30, 46, 62–64]. Limitations in their use are related to the lack of degradability of certain materials or too fast degradability of others, poor processability into porous structures, brittleness, inability to generate structures to be tailored to the specific needs of the patient or inability to maintain the desired volume under mechanical stimuli. In order to overcome these limitations synthetic scaffolds specifically designed to mimic the wound healing extracellular matrix are being evaluated.
This biomimetic concept applied to materials synthesis intends to generate biodegradable scaffolds with a highly porous structure and adequate mechanical properties for bone engineering [133]. Ideally, a scaffold material should be degradable at a rate similar to that of the new tissue formation, large interconnected pores are required to allow for cell incorporation, migration, and proliferation [136]. Bone formation occurs over a structured collagen matrix with fiber bundle diameter varying from 50 to 500 nm [137, 138], therefore nanofibrous scaffolds appear to provide better cellular attachment [139], increased differentiation of osteoblastic cells [140], and enhanced mineral deposition compared to solid-walled scaffolds [141].
Electrospinning, self-assembly, and phase separation are three different methods employed in the fabrication of nano-fibrous polymeric scaffolds for tissue engineering. Electrospinning is a simple method, which utilizes an electric field to draw a polymer solution from an orifice to a collector, producing polymer fibers [142, 143]. It can be used is used to produce thin two-dimensional sheets, while three-dimensional nanofibrous scaffolds have been fabricated by layering these 2D sheets [144] or by combining electrospinning with 3D printing [145]. Molecular self-assembly uses non-covalent bonds such as hydrogen bonds, van der Waals interactions, electrostatic interactions, and hydrophobic interactions for fabricating supramolecular architectures [146]. Limitations in the use of self-assembly methods are related to difficulties in forming macropores and limited mechanical properties [147]. Finally, thermally induced phase separation (TIPS) technique can be used to fabricate nano-fibers through polymer dissolution, phase separation and gelation, solvent extraction, freezing, and freeze-drying under vacuum [148]. This technique can also be combined with processing techniques such as particulate leaching or 3D printing to design complex 3D structures with well-defined pore morphologies [140, 149, 150].
Another interesting aspect of polymer scaffolds is that CAD/CAM technologies can be applied to create patient-specific, anatomically shaped scaffolds. As craniofacial defects and anatomical stuctures may greatly vary among different individuals a scaffold unique to each patient can be helpful in regenerating defects with complex geometry [133].
Polymers have great design flexibility and their composition and structure can be designed to match the specific needs of the tissue to be engineered. Moreover, benefits can be reached by adding nano-crystalline hydroxyapatite to the scaffolds as it has a strong potential for attracting osteoblasts (osteoconductivity), it improves its mechanical properties [151], and may reduce adverse effects associated with the degradation of some synthetic polymers [147]. Hydroxyapatite crystals can be incorporatied during processing of polymer scaffolds or they could be biomimetically grown onto a prefabricated polymer scaffold. Since all interactions with biological components occur at the pore surface, the non-exposed ceramic is in effect wasted [147] and could affect biodegradability and mechanical properties of the scaffold. It is therefore recommended to allow apatite to form as a coating of the polymer scaffold in order to enhance its surface characteristics. An interesting technology has been described in which prefabricated polymer scaffold are soaked in simulated body fluid in order to allow apatite crystals to grow onto its pore surfaces [152, 153].
Growth factors can easily be incorporated in polymeric scaffolds [154–156], which would allow for a more sustained release of the molecules and better properly orchestrated tissue formation. As such, 3D porous, nanofibrous scaffolds have supported various stem cells and differentiated cells to regenerate many hard and soft tissues. It should be pointed out that significant technical challenges remain for the synergistic integration of structural cues with biological cues for cell-based therapies to achieve functional dental and craniofacial tissue regeneration [157]. However, it is likely that the continuous expansion of biomimetic approaches in the scaffolding materials design will substantially advance the field of tissue engineering and regenerative medicine. Recently, a biomimetic fiber-guiding scaffold using solid free-form fabrication methods that custom fit complex anatomical defects to guide functionally-oriented ligamentous fibers in periodontal regeneration has been successfully tested in vivo [158] and work is being done to incorporate biomimetic scaffolds in cellular delivery for craniofacial bone regeneration in many other clinical scenarios (Table 2).
Table 2.
Scaffolding Matrices for Delivery of Cells for Craniofacial Tissue Engineering.
| Biomaterial | Scaffold | Cell Therapy | |
|---|---|---|---|
| Naturally Delivered | Allografts | Bone block allografts | Extraoral MSCs (Bone marrow MSC) [29] |
| Xenografts | Collagen sponge | Oral/craniofacial MSCs (Pulp cells) [61] | |
| Gel/Gelatin | Oral/craniofacial MSCs (PDL cells) [46] | ||
| Extraoral MSCs (Bone marrow MSC) [30] | |||
| Oral/craniofacial MSCs (PDL cells) [62] | |||
| Extraoral Expanded Stem Cells (BRCs) [63, 64] | |||
| Synthetic/Alloplasts | Polymers | PLLA (polylactic acid) | Oral MSCs (PDL fibroblasts) [65] |
| PLGA (poly[lactide-co-glycolide]) (co-polymer of PLLA & PGA) | Oral/craniofacial MSCs (cementoblasts) [47] | ||
| Extraoral MSCs (Bone marrow MSC) [31] | |||
| Ca-P based ceramics | Tricalcium phosphate (β-TCP), calcium phosphate cement | Extraoral Expanded Stem Cells (BRCs) | |
| Hydroxyapaptite-based scaffolds | Hydroxyapaptite dense HA, porous HA, resorbable HA, Non-porous non-resorbable granular HA | Oral/craniofacial MSCs (PDL cells) [60] | |
| Hyaluronic acid ester | Oral/craniofacial MSCs (PDL cells) [40] | ||
| Porous HA | Expanded bone marrow MSCs [66] | ||
| Hydroxyapaptite/ Tricalcium phosphate | Bone Marrow MSCs [67] |
4. Bone Repair Cells (Ixmyelocel-T)
Bone repair cells (generic term Ixmyelocel-T) are a patient-specific multicellular therapy manufactured from a small sample of autologous bone marrow marrow in a proprietary, closed-system bioreactor. Ixmyelocel-T has been evaluated both clinically and preclinically, in multiple applications in cardiovascular, neurological and orthopedic surgery [159–166]. For example, a recent case report showed successful results in regenerative facial reconstruction of terminal stage osteoradionecrosis and other serious craniofacial conditions [167]. This expanded mixture of cells contains MSCs with bone-forming potential in preclinical animal models [168], however this mixture has not yet been fully-optimized for bone regeneration applications, and in fact is currently in clinical trials for critical limb ischemia and dilated cardiomyopathy. In these models ectopic bone formation has not been reported widely using many different bone marrow derived cells and MSCs from various sources. Thus the impact of the heterogeneous cell populations specifically on bone formation remains to be fully understood.
When using a simple autologous bone marrow aspiration for a bone grafting procedure some of the cells populating bone marrow have strong osteogenic capacity, while others have essentially no intrinsic bone-forming potential (eg. monocytes and macrophages) though they may regulate indirectly or in a paracrine manner [169], though this is primarily hypothetical at this stage. Culturing protocols, therefore, should be aimed at expanding those cells with osteogenic capacity while reducing inhibiting cells. The characterization of ixmyelocel-T cell populations has been previously described [168] and we previously reported on the phenotypic characterization of ixmyelocel-T samples from patients treated in a recently conducted clinical study [64]. Significant to note was the finding that these cell populations were highly enriched for CD90 and CD105 positive cells. CD105 was originally identified as a marker of mesenchymal stem cells [170], and subsequently associated with vascular endothelia in angiogenic tissues [171]. CD90 is expressed by bone marrow subpopulations of colony-forming mesenchymal stem cells (CFU-F, colony-forming unit–fibroblasts) [172]. It was also demonstrated that Ixmyelocel-T populations produced significant concentrations of angiogenic cytokines and showed the ability to differentiate into endothelial cells [64]. The therapeutic implications of these findings are that Ixmyelocel-T may not only serve to provide a source of stem and progenitor cells to a wound healing site, but may also be actively involved in the establishment of a supportive, vasculature which can support and sustain tissue regeneration.
Our group recently completed a Phase I/II, proof-of-concept, feasibility study where we randomly assigned 24 subjects to either a control group (Guided Bone Regeneration [GBR] with gelatin carrier alone) or to a test group (cell therapy with Ixmyelocel-T adsorbed into the gelatin sponge with GBR). For more information, see www.clinicaltrials.gov: NCT00755911. After either 6 or 12 weeks of healing, bone core biopsies were harvested and dental implants were installed. The test groups allowed more bone formation with lower degree of ridge resorption. Bone density was measured by tactile means during clinical assessment, micro-computed tomography (micro-CT) and, additionally, histological analyses were carried out. Bone regenerated with this cell therapy was found to be of higher density than bone regenerated using GBR. Histological evaluation of the biopsy specimens revealed formation of highly vascular, mature bone as early as 6 weeks after implantation.. Our study demonstrated that cell therapy for regeneration of alveolar bone defects is safe and accelerates the early stages of osteogenesis. It also establishes preliminary evidence for consideration of larger scale clinical studies for the use of Ixmyelocel-T therapy in the treatment of more complex craniofacial defects [63, 64]. In addition to this pilot study, our group is conducting an ongoing Phase I/II placebo controlled, randomized human clinical trial investigating the potential of Ixmyelocel-T to stimulate bone formation in severely resorbed alveolar ridges in the maxillary arch. In this investigation (www.clinicaltrials.gov: NCT00980278), patients requiring maxillary sinus floor augmentation and dental implant placement are randomized to receive beta tricalcium phosphate (β-TCP) bone filler as a control, standard-of-care therapy, or β-TCP loaded with Ixmyelocel-T. The patients will be followed over a one-year period and total bone volume and oral implant success will be assessed.
Important considerations to utilization of ixmyelocel-T as a cell therapy are the cost, the need to harvest a bone marrow sample from the iliac crest, and the time required to expand the cells (12–14 days) prior to their use. The regenerative potential of ixmyelocel-T may also be affected by the biomaterial used as a carrier to deliver the cells to the regenerative site. In general, when osseous defects are localized and well-contained, as in the case of a tooth extraction socket, a non-mineralized carrier (ie. gelatin sponge) may be more appropriate to use in that it has good handling properties and easily conforms to the defect site. However, many larger more complex defects that could benefit from ixmyelocel-T treatment are not self-contained. It is these clinical situations, often requiring vertical and horizontal osseous augmentation, that are among the most difficult to treat in that the structural integrity of the graft is paramount in providing the maintenance of the space required for regeneration of the tissue [173]. Generally, mineralized blocks or mineralized particulate bone grafts in combination with resorbable membranes or titanium-reinforced ePTFE membranes are used for the treatment of these types of defects. However, the recent emergence of 3D polymer scaffolding technologies, could represent an optimal solution for the delivery of ixmyelocel-T, once technical limitations are overcome [133, 147, 157].
5. Expert’s Outlook
Advances in tissue engineering open the possibility of utilizing new therapeutic protocols for the treatment of large osseous defects in the craniofacial area including the cranium, jaws and localized periodontal deformities. Bioengineering strategies using stem cells may allow predictable therapeutic approaches with the potential of reducing the limitations of current state-of-the-art clinical protocols. To date, the use of cell therapies for oral craniofacial regeneration is quite limited and reserved to orphan product status for most indications. Some of the first cell therapies receiving FDA approval are limited to the use of neonatal fibroblasts/keratinocytes that received FDA panel review (NCT00587834), but awaiting FDA full approval for oral application. For bone regeneration, the use of cell therapies have many of the practical challenges of harvest, procurement and expansion via bioreactors. The steps involved make the regenerative therapies more expensive and time-consuming as compared to the use of growth factors that have received approval in the craniofacial complex such as rhPDGF-BB or BMP-2 [174]. However, given some of the limitations of protein-based therapies in providing predictable bone regeneration (in terms of consistency of result and extent of bone volume), cell therapies indeed offer a viable alternative to protein-based growth factors and allograft tissue transplants. Clinical trials ongoing using Ixmeyelocel-T in alveolar ridge (NCT00755911) and sinus floor augmentation (NCT00980278) offer potential for the use of stem cells for these application, however these studies are at the Phase 1/2 stage and will require further validation in larger, multi-center investigations.
At this point in time our expert outlook is that cell therapies will have a place in regenerative medicine, and in particular will be most highly used in the treatment of advanced defects in facial reconstruction. These cellular therapies lend themselves to delivery using image-based, CAD approaches to repair major craniofacial defects of complex morphologies where cells will have the unique potential to form into multiple tissues to address the complex form and function required in the oral and craniofacial complex.
Acknowledgments
This work has been supported by NIH/NCRR UL1RR024986, NIH/NIDCR DE 13397 and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
G Pagni, Email: giorgio.pagni@gmail.com.
D Kaigler, Email: dkaigler@umich.edu.
G Rasperini, Email: giulio@studiorasperini.it.
G Avila-Ortiz, Email: gustavo-avila@uiowa.edu.
R Bartel, Email: rbartel@aastrom.com.
WV Giannobile, Email: wgiannob@umich.edu.
References
- 1.Susarla SM, Swanson E, Gordon CR. Craniomaxillofacial Reconstruction Using Allotransplantation and Tissue Engineering: Challenges, Opportunities, and Potential Synergy. Annals of plastic surgery. 2011;67:655–661. doi: 10.1097/SAP.0b013e31822c00e6. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MM., Jr Perspectives on craniofacial asymmetry. III. Common and/or well-known causes of asymmetry. International journal of oral and maxillofacial surgery. 1995;24:127–133. doi: 10.1016/s0901-5027(06)80085-8. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JA, Hobar PC. Common craniofacial anomalies: conditions of craniofacial atrophy/hypoplasia and neoplasia. Plastic and reconstructive surgery. 2003;111:1497–1508. doi: 10.1097/01.PRS.0000049646.25757.BE. quiz 1509–1410. [DOI] [PubMed] [Google Scholar]
- 4.Davis RE, Telischi FF. Traumatic facial nerve injuries: review of diagnosis and treatment. The Journal of cranio-maxillofacial trauma. 1995;1:30–41. [PubMed] [Google Scholar]
- 5.Kadota C, Sumita YI, Wang Y, Otomaru T, Mukohyama H, Fueki K, Igarashi Y, Taniguchi H. Comparison of food mixing ability among mandibulectomy patients. Journal of oral rehabilitation. 2008;35:408–414. doi: 10.1111/j.1365-2842.2007.01814.x. [DOI] [PubMed] [Google Scholar]
- 6.Curtis DA, Plesh O, Miller AJ, Curtis TA, Sharma A, Schweitzer R, Hilsinger RL, Schour L, Singer M. A comparison of masticatory function in patients with or without reconstruction of the mandible. Head & neck. 1997;19:287–296. doi: 10.1002/(sici)1097-0347(199707)19:4<287::aid-hed7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Urken ML, Buchbinder D, Weinberg H, Vickery C, Sheiner A, Parker R, Schaefer J, Som P, Shapiro A, Lawson W, et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: a comparative study of reconstructed and nonreconstructed patients. The Laryngoscope. 1991;101:935–950. doi: 10.1288/00005537-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Genco RJ. Host responses in periodontal diseases: current concepts. Journal of periodontology. 1992;63:338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 9.Kinane DF, Mark Bartold P. Clinical relevance of the host responses of periodontitis. Periodontology 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 10.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontology 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Misch CE. Rationale for implants. In: Misch CE, editor. Contemporary implant dentistry. Mosby; St. Louis: 2008. [Google Scholar]
- 12.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pape HC, Evans A, Kobbe P. Autologous bone graft: properties and techniques. J Orthop Trauma. 2010;24(Suppl 1):S36–40. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- 14.Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. The Journal of the American Academy of Orthopaedic Surgeons. 2005;13:77–86. [PubMed] [Google Scholar]
- 15.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Zouhary KJ. Bone graft harvesting from distant sites: concepts and techniques. Oral Maxillofac Surg Clin North Am. 2010;22:301–316. v. doi: 10.1016/j.coms.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clinical orthopaedics and related research. 2000:10–27. [PubMed] [Google Scholar]
- 18.De Long WG, Jr, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. The Journal of bone and joint surgery American volume. 2007;89:649–658. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 19.Finkemeier CG. Bone-grafting and bone-graft substitutes. The Journal of bone and joint surgery American volume. 2002;84-A:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Taba M, Jr, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthodontics & craniofacial research. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. Journal of periodontology. 2003;74:1282–1292. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 22.Gille J, Dorn B, Kekow J, Bruns J, Behrens P. Bone substitutes as carriers for transforming growth factor-beta(1) (TGF-beta(1)) Int Orthop. 2002;26:203–206. doi: 10.1007/s00264-002-0353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossa C, Jr, Marcantonio E, Jr, Cirelli JA, Marcantonio RA, Spolidorio LC, Fogo JC. Regeneration of Class III furcation defects with basic fibroblast growth factor (b-FGF) associated with GTR. A descriptive and histometric study in dogs. Journal of periodontology. 2000;71:775–784. doi: 10.1902/jop.2000.71.5.775. [DOI] [PubMed] [Google Scholar]
- 24.Chang PC, Cirelli JA, Jin Q, Seol YJ, Sugai JV, D’Silva NJ, Danciu TE, Chandler LA, Sosnowski BA, Giannobile WV. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 2009;20:486–496. doi: 10.1089/hum.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Annals of plastic surgery. 1999;42:488–495. [PubMed] [Google Scholar]
- 26.Laurencin CT, Attawia MA, Lu LQ, Borden MD, Lu HH, Gorum WJ, Lieberman JR. Poly(lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: a regional gene therapy approach to bone regeneration. Biomaterials. 2001;22:1271–1277. doi: 10.1016/s0142-9612(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 27.Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D’Andrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. Journal of periodontal research. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 28.Tatakis DN, Wikesjo UM, Razi SS, Sigurdsson TJ, Lee MB, Nguyen T, Ongpipattanakul B, Hardwick R. Periodontal repair in dogs: effect of transforming growth factor-beta 1 on alveolar bone and cementum regeneration. Journal of clinical periodontology. 2000;27:698–704. doi: 10.1034/j.1600-051x.2000.027009698.x. [DOI] [PubMed] [Google Scholar]
- 29.Soltan M, Smiler D, Prasad HS, Rohrer MD. Bone Block Allograft Impregnated With Bone Marrow Aspirate. Implant dentistry. 2007;16:329–339. doi: 10.1097/ID.0b013e31815c8ef4. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. Journal of periodontology. 2004;75:1281–1287. doi: 10.1902/jop.2004.75.9.1281. [DOI] [PubMed] [Google Scholar]
- 31.Marei MK, Nouh SR, Saad MM, Ismail NS. Preservation and regeneration of alveolar bone by tissue-engineered implants. Tissue engineering. 2005;11:751–767. doi: 10.1089/ten.2005.11.751. [DOI] [PubMed] [Google Scholar]
- 32.Filho Cerruti H, Kerkis I, Kerkis A, Tatsui NH, da Costa Neves A, Bueno DF, da Silva MC. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: clinical case reports. Artificial organs. 2007;31:268–273. doi: 10.1111/j.1525-1594.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: from basic research to clinical case study. Cell transplantation. 2004;13:343–355. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]
- 34.Yamada Y, Ueda M, Naiki T, Nagasaka T. Tissue-engineered injectable bone regeneration for osseointegrated dental implants. Clinical oral implants research. 2004;15:589–597. doi: 10.1111/j.1600-0501.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue engineering. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 36.Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: A clinical case report. The International journal of periodontics & restorative dentistry. 2006;26:363–369. [PubMed] [Google Scholar]
- 37.Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14:945–953. doi: 10.1089/ten.tea.2007.0048. [DOI] [PubMed] [Google Scholar]
- 38.Xie J, Han Z, Naito M, Maeyama A, Kim SH, Kim YH, Matsuda T. Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(L-lactide-co-epsilon-caprolactone): In vivo performance in adult rabbits. J Biomed Mater Res B Appl Biomater. 2010;94:80–88. doi: 10.1002/jbm.b.31627. [DOI] [PubMed] [Google Scholar]
- 39.Zhu S, Zhang B, Man C, Ma Y, Hu J. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthritis Cartilage. 2011;19:743–750. doi: 10.1016/j.joca.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. Journal of periodontal research. 2005;40:245–251. doi: 10.1111/j.1600-0765.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem cells (Dayton, Ohio) 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem cells (Dayton, Ohio) 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Yan F, Lei L, Li Y, Xiao Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells, tissues, organs. 2009;190:94–101. doi: 10.1159/000166547. [DOI] [PubMed] [Google Scholar]
- 44.Dogan A, Ozdemir A, Kubar A, Oygur T. Assessment of periodontal healing by seeding of fibroblast-like cells derived from regenerated periodontal ligament in artificial furcation defects in a dog: a pilot study. Tissue engineering. 2002;8:273–282. doi: 10.1089/107632702753725030. [DOI] [PubMed] [Google Scholar]
- 45.Dogan A, Ozdemir A, Kubar A, Oygur T. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: a preliminary study. Tissue engineering. 2003;9:1189–1196. doi: 10.1089/10763270360728099. [DOI] [PubMed] [Google Scholar]
- 46.Lekic PC, Rajshankar D, Chen H, Tenenbaum H, McCulloch CA. Transplantation of labeled periodontal ligament cells promotes regeneration of alveolar bone. The Anatomical record. 2001;262:193–202. doi: 10.1002/1097-0185(20010201)262:2<193::AID-AR1028>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Jin QM, Zhao M, Webb SA, Berry JE, Somerman MJ, Giannobile WV. Cementum engineering with three-dimensional polymer scaffolds. J Biomed Mater Res A. 2003;67:54–60. doi: 10.1002/jbm.a.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. Journal of periodontology. 2004;75:154–161. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. Journal of periodontology. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: a randomized controlled pilot study. Journal of periodontology. 2005;76:867–880. doi: 10.1902/jop.2005.76.6.867. [DOI] [PubMed] [Google Scholar]
- 51.McGuire MK, Scheyer ET, Nevins M, Neiva R, Cochran DL, Mellonig JT, Giannobile WV, Bates D. Living Cellular Construct for Increasing the Width of Keratinized Gingiva. Results from a Randomized, Within-Patient, Controlled Trial. Journal of periodontology. 2011;82:1414–1423. doi: 10.1902/jop.2011.100671. [DOI] [PubMed] [Google Scholar]
- 52.McGuire MK, Scheyer ET. A randomized, double-blind, placebo-controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. Journal of periodontology. 2007;78:4–17. doi: 10.1902/jop.2007.060105. [DOI] [PubMed] [Google Scholar]
- 53.Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. International journal of oral and maxillofacial surgery. 2003;32:188–197. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 54.Hotta T, Yokoo S, Terashi H, Komori T. Clinical and histopathological analysis of healing process of intraoral reconstruction with ex vivo produced oral mucosa equivalent. The Kobe journal of medical sciences. 2007;53:1–14. [PubMed] [Google Scholar]
- 55.Dozin B, Malpeli M, Camardella L, Cancedda R, Pietrangelo A. Response of young, aged and osteoarthritic human articular chondrocytes to inflammatory cytokines: molecular and cellular aspects. Matrix Biol. 2002;21:449–459. doi: 10.1016/s0945-053x(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 56.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. The New England journal of medicine. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 58.Dozin B, Malpeli M, Cancedda R, Bruzzi P, Calcagno S, Molfetta L, Priano F, Kon E, Marcacci M. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15:220–226. doi: 10.1097/01.jsm.0000171882.66432.80. [DOI] [PubMed] [Google Scholar]
- 59.Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed LE. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46:2524–2534. doi: 10.1002/art.10493. [DOI] [PubMed] [Google Scholar]
- 60.Gault P, Black A, Romette JL, Fuente F, Schroeder K, Thillou F, Brune T, Berdal A, Wurtz T. Tissue-engineered ligament: implant constructs for tooth replacement. Journal of clinical periodontology. 2010;37:750–758. doi: 10.1111/j.1600-051X.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 61.d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. European cells & materials. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 62.Nakahara T, Nakamura T, Kobayashi E, Kuremoto K, Matsuno T, Tabata Y, Eto K, Shimizu Y. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue engineering. 2004;10:537–544. doi: 10.1089/107632704323061898. [DOI] [PubMed] [Google Scholar]
- 63.Kaigler D, Pagni G, Galloro A, Park C-H, Bartel RL, Giannobile W. Acceleration Of Human Oral Osseous Regeneration Using Bone Repair Cells. 2010 AADR Annual Meeting; Washington, DC. 2010. [Google Scholar]
- 64.Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and Osteogenic Potential of Bone Repair Cells for Craniofacial Regeneration. Tissue Eng Part A. 2010;16:2809–2820. doi: 10.1089/ten.tea.2010.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mei F, Zhong J, Yang X, Ouyang X, Zhang S, Hu X, Ma Q, Lu J, Ryu S, Deng X. Improved biological characteristics of poly(L-lactic acid) electrospun membrane by incorporation of multiwalled carbon nanotubes/hydroxyapatite nanoparticles. Biomacromolecules. 2007;8:3729–3735. doi: 10.1021/bm7006295. [DOI] [PubMed] [Google Scholar]
- 66.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29:3053–3061. doi: 10.1016/j.biomaterials.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 67.De Kok IJ, Drapeau SJ, Young R, Cooper LF. Evaluation of mesenchymal stem cells following implantation in alveolar sockets: a canine safety study. The International journal of oral & maxillofacial implants. 2005;20:511–518. [PubMed] [Google Scholar]
- 68.Ripamonti U, Reddi AH. Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med. 1997;8:154–163. doi: 10.1177/10454411970080020401. [DOI] [PubMed] [Google Scholar]
- 69.Kaigler D, Avila G, Wisner-Lynch L, Nevins ML, Nevins M, Rasperini G, Lynch SE, Giannobile WV. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert opinion on biological therapy. 2011;11:375–385. doi: 10.1517/14712598.2011.554814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cochran DL, Wozney JM. Biological mediators for periodontal regeneration. Periodontology 2000. 1999;19:40–58. doi: 10.1111/j.1600-0757.1999.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 71.Smith DM, Cooper GM, Mooney MP, Marra KG, Losee JE. Bone morphogenetic protein 2 therapy for craniofacial surgery. The Journal of craniofacial surgery. 2008;19:1244–1259. doi: 10.1097/SCS.0b013e3181843312. [DOI] [PubMed] [Google Scholar]
- 72.Lynch SE. Tissue engineering : applications in oral and maxillofacial surgery and periodontics. Quintessence Pub; Hanover Park, IL: 2008. p. xvi.p. 296. [Google Scholar]
- 73.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 74.Winn SR, Hu Y, Sfeir C, Hollinger JO. Gene therapy approaches for modulating bone regeneration. Advanced drug delivery reviews. 2000;42:121–138. doi: 10.1016/s0169-409x(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 75.Baum BJ, Kok M, Tran SD, Yamano S. The impact of gene therapy on dentistry: a revisiting after six years. Journal of the American Dental Association (1939) 2002;133:35–44. doi: 10.14219/jada.archive.2002.0019. [DOI] [PubMed] [Google Scholar]
- 76.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3:129–139. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 77.Ke J, Zheng LW, Cheung LK. Orthopaedic gene therapy using recombinant adeno-associated virus vectors. Archives of oral biology. 2011;56:619–628. doi: 10.1016/j.archoralbio.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 78.Scheller EL, Krebsbach PH. Gene therapy: design and prospects for craniofacial regeneration. Journal of dental research. 2009;88:585–596. doi: 10.1177/0022034509337480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nature biotechnology. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 80.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 81.Kaiser J. Gene therapy. Seeking the cause of induced leukemias in X-SCID trial. Science. 2003;299:495. doi: 10.1126/science.299.5606.495. [DOI] [PubMed] [Google Scholar]
- 82.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. Journal of dental research. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panetta NJ, Gupta DM, Slater BJ, Kwan MD, Liu KJ, Longaker MT. Tissue engineering in cleft palate and other congenital malformations. Pediatr Res. 2008;63:545–551. doi: 10.1203/PDR.0b013e31816a743e. [DOI] [PubMed] [Google Scholar]
- 84.Garcia-Godoy F, Murray PE. Status and potential commercial impact of stem cell-based treatments on dental and craniofacial regeneration. Stem Cells Dev. 2006;15:881–887. doi: 10.1089/scd.2006.15.881. [DOI] [PubMed] [Google Scholar]
- 85.Song J, Izumi K, Lanigan T, Feinberg SE. Development and characterization of a canine oral mucosa equivalent in a serum-free environment. J Biomed Mater Res A. 2004;71:143–153. doi: 10.1002/jbm.a.30144. [DOI] [PubMed] [Google Scholar]
- 86.Nakanishi Y, Izumi K, Yoshizawa M, Saito C, Kawano Y, Maeda T. The expression and production of vascular endothelial growth factor in oral mucosa equivalents. International journal of oral and maxillofacial surgery. 2007;36:928–933. doi: 10.1016/j.ijom.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 87.Xu Q, Izumi K, Tobita T, Nakanishi Y, Feinberg SE. Constitutive release of cytokines by human oral keratinocytes in an organotypic culture. J Oral Maxillofac Surg. 2009;67:1256–1264. doi: 10.1016/j.joms.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morelli T, Neiva R, Nevins ML, McGuire MK, Scheyer ET, Oh TJ, Braun TM, Nor JE, Bates D, Giannobile WV. Angiogenic biomarkers and healing of living cellular constructs. Journal of dental research. 2011;90:456–462. doi: 10.1177/0022034510389334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGuire MK, Scheyer ET, Nunn ME, Lavin PT. A pilot study to evaluate a tissue-engineered bilayered cell therapy as an alternative to tissue from the palate. Journal of periodontology. 2008;79:1847–1856. doi: 10.1902/jop.2008.080017. [DOI] [PubMed] [Google Scholar]
- 90.Owen P, Connaghan DG, Holland HK, Steis RG. Bone marrow transplantation: cancer therapy comes of age. Journal of the Medical Association of Georgia. 1998;87:145–146. 148. [PubMed] [Google Scholar]
- 91.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 92.Park SR, Oreffo RO, Triffitt JT. Interconversion potential of cloned human marrow adipocytes in vitro. Bone. 1999;24:549–554. doi: 10.1016/s8756-3282(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 93.Cooper LF, Harris CT, Bruder SP, Kowalski R, Kadiyala S. Incipient analysis of mesenchymal stem-cell-derived osteogenesis. Journal of dental research. 2001;80:314–320. doi: 10.1177/00220345010800010401. [DOI] [PubMed] [Google Scholar]
- 94.Nakade O, Takahashi K, Takuma T, Aoki T, Kaku T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J Bone Miner Metab. 2001;19:13–19. doi: 10.1007/s007740170055. [DOI] [PubMed] [Google Scholar]
- 95.Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral diseases. 2010;16:709–716. doi: 10.1111/j.1601-0825.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- 96.Noth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Advanced drug delivery reviews. 2010;62:765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 97.Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M, Koike C, Tsuji K, Iba H, Kato Y, Kurihara H. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. Journal of periodontology. 2006;77:1003–1007. doi: 10.1902/jop.2006.050341. [DOI] [PubMed] [Google Scholar]
- 98.Levi B, Longaker MT. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem cells (Dayton, Ohio) 2011;29:576–582. doi: 10.1002/stem.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell transplantation. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 100.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. The New England journal of medicine. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 101.Mastrogiacomo M, Muraglia A, Komlev V, Peyrin F, Rustichelli F, Crovace A, Cancedda R. Tissue engineering of bone: search for a better scaffold. Orthodontics & craniofacial research. 2005;8:277–284. doi: 10.1111/j.1601-6343.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 102.Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue engineering. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 103.Cancedda R, Dozin B, Giannoni P, Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003;22:81–91. doi: 10.1016/s0945-053x(03)00012-x. [DOI] [PubMed] [Google Scholar]
- 104.Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62:166–174. [PubMed] [Google Scholar]
- 105.Schaefer D, Martin I, Shastri P, Padera RF, Langer R, Freed LE, Vunjak-Novakovic G. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599–2606. doi: 10.1016/s0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 106.Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue engineering. 2001;7:363–371. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 107.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, Kasper FK, Mikos AG. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30:2741–2752. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–842. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 110.Mitsiadis TA, Feki A, Papaccio G, Caton J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Advances in dental research. 2011;23:275–279. doi: 10.1177/0022034511405386. [DOI] [PubMed] [Google Scholar]
- 111.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, Choung YH, Kim ES, Yang HC, Choung PH. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue engineering. 2007;13:767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 112.Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14:149–156. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Laino G, Graziano A, d'Aquino R, Pirozzi G, Lanza V, Valiante S, De Rosa A, Naro F, Vivarelli E, Papaccio G. An approachable human adult stem cell source for hard-tissue engineering. Journal of cellular physiology. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 114.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of endodontics. 2008;34:962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 116.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 117.Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. Journal of dental research. 2005;84:907–912. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 118.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of dental research. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2007;75:495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 120.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Giannobile WV. Getting to the root of dental implant tissue engineering. Journal of clinical periodontology. 2010;37:747–749. doi: 10.1111/j.1600-051X.2010.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rios HF, Lin Z, Oh B, Park CH, Giannobile WV. Cell- and Gene-Based Therapeutic Strategies for Periodontal Regenerative Medicine. Journal of periodontology. 2011;82:1223–1237. doi: 10.1902/jop.2011.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 125.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 126.Liu SP, Fu RH, Huang YC, Chen SY, Chien YJ, Hsu CY, Tsai CH, Shyu WC, Lin SZ. Induced pluripotent stem (iPS) cell research overview. Cell transplantation. 2011;20:15–19. doi: 10.3727/096368910X532828. [DOI] [PubMed] [Google Scholar]
- 127.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 128.Tamaoki N, Takahashi K, Tanaka T, Ichisaka T, Aoki H, Takeda-Kawaguchi T, Iida K, Kunisada T, Shibata T, Yamanaka S, Tezuka K. Dental Pulp Cells for Induced Pluripotent Stem Cell Banking. Journal of dental research. 2010;89:773–778. doi: 10.1177/0022034510366846. [DOI] [PubMed] [Google Scholar]
- 129.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 130.Teramoto K, Hara Y, Kumashiro Y, Chinzei R, Tanaka Y, Shimizu-Saito K, Asahina K, Teraoka H, Arii S. Teratoma formation and hepatocyte differentiation in mouse liver transplanted with mouse embryonic stem cell-derived embryoid bodies. Transplantation proceedings. 2005;37:285–286. doi: 10.1016/j.transproceed.2004.12.120. [DOI] [PubMed] [Google Scholar]
- 131.Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. The American journal of pathology. 2005;166:1781–1791. doi: 10.1016/S0002-9440(10)62488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma PX. Biomimetic materials for tissue engineering. Advanced drug delivery reviews. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rice MA, Dodson BT, Arthur JA, Anseth KS. Cell-based therapies and tissue engineering. Otolaryngol Clin North Am. 2005;38:199–214. v. doi: 10.1016/j.otc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 135.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 136.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. Journal of biomedical materials research. 2000;52:430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 137.Hay ED. Cell biology of extracellular matrix. 2. Plenum Press; New York: 1991. [Google Scholar]
- 138.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of biomedical materials research Part A. 2003;67:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 140.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 141.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, Kim GS, Somerman MJ, Ma PX. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 142.Dosunmu OO, Chase GG, Kataphinan W, Reneker DH. Electrospinning of polymer nanofibres from multiple jets on a porous tubular surface. Nanotechnology. 2006;17:1123–1127. doi: 10.1088/0957-4484/17/4/046. [DOI] [PubMed] [Google Scholar]
- 143.Jia H, Zhu G, Vugrinovich B, Kataphinan W, Reneker DH, Wang P. Enzyme-carrying polymeric nanofibers prepared via electrospinning for use as unique biocatalysts. Biotechnol Prog. 2002;18:1027–1032. doi: 10.1021/bp020042m. [DOI] [PubMed] [Google Scholar]
- 144.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 145.Moroni L, de Wijn JR, van Blitterswijk CA. Integrating novel technologies to fabricate smart scaffolds. Journal of biomaterials science. 2008;19:543–572. doi: 10.1163/156856208784089571. Polymer edition. [DOI] [PubMed] [Google Scholar]
- 146.Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science. 1991;254:1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 147.Smith IO, Liu XH, Smith LA, Ma PX. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:226–236. doi: 10.1002/wnan.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. Journal of biomedical materials research. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 149.He C, Xiao G, Jin X, Sun C, Ma PX. Electrodeposition on nanofibrous polymer scaffolds: Rapid mineralization, tunable calcium phosphate composition and topography. Adv Funct Mater. 2010;20:3568–3576. doi: 10.1002/adfm.201000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. Journal of biomedical materials research Part A. 2006;78:306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 151.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 152.Boskey AL. Biomineralization: conflicts, challenges, and opportunities. J Cell Biochem Suppl. 1998;30–31:83–91. [PubMed] [Google Scholar]
- 153.Boskey AL. Biomineralization: an overview. Connective tissue research. 2003;44(Suppl 1):5–9. [PubMed] [Google Scholar]
- 154.Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 155.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, Giannobile WV. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS ONE. 2008;3:e1729. doi: 10.1371/journal.pone.0001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gupte MJ, Ma PX. Nanofibrous scaffolds for dental and craniofacial applications. Journal of dental research. 2012;91:227–234. doi: 10.1177/0022034511417441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, Flanagan CL, Hollister SJ, Giannobile WV. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33:137–145. doi: 10.1016/j.biomaterials.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yin D, Wang Z, Gao Q, Sundaresan R, Parrish C, Yang Q, Krebsbach PH, Lichtler AC, Rowe DW, Hock J, Liu P. Determination of the fate and contribution of ex vivo expanded human bone marrow stem and progenitor cells for bone formation by 2.3ColGFP. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1967–1978. doi: 10.1038/mt.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koller MR, Emerson SG, Palsson BO. Large-scale expansion of human stem and progenitor cells from bone marrow mononuclear cells in continuous perfusion cultures. Blood. 1993;82:378–384. [PubMed] [Google Scholar]
- 161.Koller MR, Manchel I, Palsson BO. Importance of parenchymal:stromal cell ratio for the ex vivo reconstitution of human hematopoiesis. Stem cells (Dayton Ohio) 1997;15:305–313. doi: 10.1002/stem.150305. [DOI] [PubMed] [Google Scholar]
- 162.Mandalam RK, Smith AK. Ex vivo expansion of bone marrow and cord blood cells to produce stem and progenitor cells for hematopoietic reconstitution. Military medicine. 2002;167:78–81. [PubMed] [Google Scholar]
- 163.Chabannon C, Blache JL, Sielleur I, Douville J, Faucher C, Gravis G, Arnoulet C, Oziel-Taieb S, Blaise D, Novakovitch G, Camerlo J, Chabbert I, Genre D, Appel M, Armstrong D, Maraninchi D, Viens P. Production of ex vivo expanded hematopoietic cells and progenitors in a closed bioreactor, starting with a small volume marrow collection: A feasibility study in patients with poor-risk breast cancer and receiving high-doses of cyclophosphamide. Int J Oncol. 1999;15:511–518. doi: 10.3892/ijo.15.3.511. [DOI] [PubMed] [Google Scholar]
- 164.Koller MR, Oxender M, Jensen TC, Goltry KL, Smith AK. Direct contact between CD34+lin− cells and stroma induces a soluble activity that specifically increases primitive hematopoietic cell production. Experimental hematology. 1999;27:734–741. doi: 10.1016/s0301-472x(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 165.Bachier CR, Gokmen E, Teale J, Lanzkron S, Childs C, Franklin W, Shpall E, Douville J, Weber S, Muller T, Armstrong D, LeMaistre CF. Ex-vivo expansion of bone marrow progenitor cells for hematopoietic reconstitution following high-dose chemotherapy for breast cancer. Experimental hematology. 1999;27:615–623. doi: 10.1016/s0301-472x(98)00085-x. [DOI] [PubMed] [Google Scholar]
- 166.Lundell BI, Vredenburgh JJ, Tyer C, DeSombre K, Smith AK. Ex vivo expansion of bone marrow from breast cancer patients: reduction in tumor cell content through passive purging. Bone marrow transplantation. 1998;22:153–159. doi: 10.1038/sj.bmt.1701314. [DOI] [PubMed] [Google Scholar]
- 167.Mendonca JJ, Juiz-Lopez P. Regenerative facial reconstruction of terminal stage osteoradionecrosis and other advanced craniofacial diseases with adult cultured stem and progenitor cells. Plastic and reconstructive surgery. 2010;126:1699–1709. doi: 10.1097/PRS.0b013e3181f24164. [DOI] [PubMed] [Google Scholar]
- 168.Dennis JE, Esterly K, Awadallah A, Parrish CR, Poynter GM, Goltry KL. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem cells (Dayton, Ohio) 2007;25:2575–2582. doi: 10.1634/stemcells.2007-0204. [DOI] [PubMed] [Google Scholar]
- 169.Jager M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, Hendrich C, Krauspe R. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29:173–180. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 170.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 171.Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boiret N, Rapatel C, Boisgard S, Charrier S, Tchirkov A, Bresson C, Camilleri L, Berger J, Guillouard L, Guerin JJ, Pigeon P, Chassagne J, Berger MG. CD34+CDw90(Thy-1)+ subset colocated with mesenchymal progenitors in human normal bone marrow hematon units is enriched in colony-forming unit megakaryocytes and long-term culture-initiating cells. Experimental hematology. 2003;31:1275–1283. doi: 10.1016/j.exphem.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 173.Chen ST, Beagle J, Jensen SS, Chiapasco M, Darby I. Consensus statements and recommended clinical procedures regarding surgical techniques. The International journal of oral & maxillofacial implants. 2009;24(Suppl):272–278. [PubMed] [Google Scholar]
- 174.Caunday O, Bensoussan D, Decot V, Bordigoni P, Stoltz JF. Regulatory aspects of cellular therapy product in Europe: JACIE accreditation in a processing facility. Bio-medical materials and engineering. 2009;19:373–379. doi: 10.3233/BME-2009-0602. [DOI] [PubMed] [Google Scholar]