Abstract
Background
The survival of patients with renal cell carcinoma (RCC) has improved in recent years. However, data on the risk of developing a second cancer after a diagnosis of RCC is limited. We used the data available in the Surveillance Epidemiology and End Results (SEER) database to estimate the risk of second metachronous primary cancers in patients diagnosed with RCC between 1973 and 2006. Further, we also investigated the effect of the second primary cancers on the survival of RCC patients.
Results
3,795 cases of second primary cancers (SPCs) were registered in SEER between 1973–2006. The ratio of observed/expected number of SPCs in RCC was 1.18 which was significantly greater than expected. Solid tumors comprised 90% of all second malignancies in RCC patients, with the most second cancers reported in the prostate gland and the digestive and respiratory systems. The overall risk of second primaries was highest in patients aged <30 years at the time of diagnosis. The site-specific risk of second cancers varied with the age at diagnosis, gender, race of the patient, size of the primary renal tumor and history of radiation therapy. Patients with second primaries had a significantly longer overall survival than those without second malignancies. An interval of <1 year between the diagnosis of RCC and the second primary was the strongest predictor of poor OS in RCC patients with a second malignancy.
Conclusion
Patients with RCC are at a significantly higher risk of developing a second malignancy suggesting the need for careful surveillance for their early detection and management.
Keywords: Renal cell carcinoma, SEER, second primary, prognosis
I. INTRODUCTION
Malignancies of the kidney (including the renal pelvis) are the seventh and eighth leading cause of cancer among men and women in the United States respectively. They are also among the top ten causes of cancer related deaths among men in the country 1. Globally, they are more common in developed rather than developing countries and in males rather than females 2. About 85% of all kidney cancers arise from the renal parenchyma (termed as renal cell carcinomas or RCCs) while the remaining arise from the urothelium lining the renal pelvis 3. It is estimated that nearly 58,240 cancers of the kidney and renal pelvis were diagnosed in the United States in 2010, and about 13,040 patients died from these malignancies in the same period. Males accounted for more than 61% of all newly diagnosed cases and about 63% of all deaths from the malignancy 1. An interesting statistic is that there are an estimated 148,840 survivors of kidney and renal pelvis cancers in the United States alone 4. The growing population of cancer survivors (both in case of RCC and other malignancies) has resulted in the emergence of a new area of cancer research, i.e. the study of second primaries arising in patients with an existing malignancy. Understanding the epidemiology of second primary cancers is an important first step in studying the molecular and genetic mechanisms underlying their development.
According to the National Cancer Institute, a second primary cancer (SPC) is defined as a new primary malignancy that occurs in a patient with a prior history of cancer (www.cancer.gov). Metachronous SPCs (i.e. SPCs that were diagnosed after a certain period of time following diagnosis of the first primary cancer) have been reported in association with several malignancies including male breast cancer 5, colon cancer 6, gastric cancer 7, esophageal cancer and other head and neck cancers 8;9. In some of these malignancies, SPCs have also been reported to influence survival. For instance, patients with squamous cell carcinoma of the head and neck who developed a SPC had a 24% lower mortality after 15-years compared to those who did not develop a second tumor 10. Liu and co-workers reported that patients who developed a lung cancer first followed by a second primary cancer had a significantly longer median survival than patients who had a non-lung primary tumor followed by a second primary cancer in the lungs 11. Data on the incidence of SPCs in patients with a prior diagnosis of RCC however is very limited, restricted only to a few European studies. One population-based study in Norway for instance, noted that the incidence of multiple primary malignancies in patients with a prior diagnosis of RCC was nearly 47% 12. Other smaller studies have reported that between 16%–18.5% of RCC patients develop a second primary malignancy 13;14.
Given that newer treatments are improving survival of patients with RCC 15;16, understanding the incidence and prognostic significance of second primary cancers in RCC survivors becomes an important question, both from the perspective of cancer treatment and the quality of life of the patient. Hence, the aim of our present study was to investigate the risk of second malignancies in patients with RCC using data from the Surveillance, Epidemiology and End Results (SEER) database. Further, we also sought to examine the effect of second primary cancers on the survival of RCC patients.
II. METHODS
2.1 Population selection
The SEER database (9 registries limited use, updated: November 2008) was queried for patients with RCC (ICD code: 8312/3) who were diagnosed between January 1973 and December 2006 with RCC and a second primary cancer (SPC). Only cases where RCC was the first of two or more cancers were considered for the analysis. Patients in whom the diagnosis of RCC was made at autopsy or mentioned in the death certificate only were excluded as were any SPCs diagnosed within 1 month following the detection of RCC. De-identified information about individual patients with either RCC alone or RCC with SPC was also obtained from the SEER database (17 registries limited use, updated November 2008, containing information of cases between 1973–2006) and was used to analyze the effect of SPCs on survival of RCC patients and the effect of individual factors on the survival of RCC patients with SPCs.
2.2 Statistical analysis
Statistical analysis was performed using the PASW Statistics 18 software program (Release 18.0, SPSS Inc, IL, USA). The SEER*Stat version 6.5.2 was used to stratify the cases by age, gender, race, radiation therapy, site of SPC and latency period and to obtain the de-identified information about individual cases. The risk of an SPC was calculated by determining the standardized incidence ratio (SIR) which is defined as the ratio of the numbers of observed cases (O) of subsequent primary cancers (i.e. 2nd, 3rd etc.) at a given site (in patients with a pre-existing primary cancer) to the number of expected (E) subsequent cancers at the same site in the general population. Age, size of the primary RCC and the time elapsed between RCC and the second primary were considered as continuous variables, while gender, race, marital status, stage, grade and laterality of RCC, history of radiation therapy and history of radiation sequence with surgery were considered as categorical variables. A two-tailed Student’s t-test was used to compare the mean values for continuous variables. Z-test was applied to compare the proportion of patients between two categories. Kaplan Meier survival analysis was used to examine the effect of individual risk factors on the overall survival (OS) of patients. A Cox proportional hazards test was used for multivariate analysis of factors affecting survival. All tests were conducted with 95% confidence intervals. A p-value <0.05 was considered significant.
III RESULTS
3.1 Study population
3,795 patients with RCC who were subsequently diagnosed with one or more second primary cancers were registered in the multiple primary (MP-SIR) section of the SEER database (comprising information from 9 registries in the United States) between 1973 and 2006. The expected number of second primary cancers in the population during the same period was estimated to be 3,208. Thus, the ratio of observed to expected number of second primaries (i.e. the SIR) was 1.18 (95% C.I. 1.15–1.22), which was significantly higher than expected (p<0.05).
Solid tumors were the most common type of second malignancy in patients with RCC, accounting for over 90% of all SPCs. About 8% of second malignancies arose in the lymphatic and hematopoietic system and in about 2% cases the sites did not fit either classification. The most common sites of occurrence of second malignancies in RCC patients were the male genital system (n=896, 23.6%), the digestive system (n=718, 18.9%) and the respiratory system (n=562, 14.8%) (Table 1).
Table 1.
Effect of age on the risk of second primary cancers in renal cell carcinoma
| Site of second malignancy | Age at diagnosis of RCC | Age attained at diagnosis of second primary cancer | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0–29 yrs. | 30–59 yrs. | ≥60 yrs. | 0–29 yrs. | 30–59 yrs. | ≥60 yrs. | |
|
| ||||||
| O(SIR) | O(SIR) | O(SIR) | O(SIR) | O(SIR) | O(SIR) | |
|
| ||||||
| All sites | 23 (6.6)* | 1,232 (1.3)* | 2,540 (1.13)* | 5 (12.02)* | 574 (1.72)* | 3,216 (1.12)* |
| All solid tumors | 19 (6.4) | 1,143 (1.32) | 2,258 (1.13) | 5 (16.0) | 533 (1.78)* | 2,882 (1.12)* |
| All lymphatic and Hematopoietic diseases | 3 (7.1)* | 72 (1.01) | 223 (1.25)* | 0 (0) | 33 (1.23) | 265 (1.2)* |
| Oral cavity and pharynx | 0 (0) | 22 (0.8) | 32 (0.7)* | 0 (0) | 14 (1.04) | 40 (0.63)* p |
| Digestive system | 0 (0) | 200 (1.2)b | 518 (1.05) | 0 (0) | 81 (1.4)* k | 637 (1.04) q |
| Liver, GB IHBD, EHBD, other biliary | 0 (0) | 22 (1.4)c | 35 (0.93)h | 0 (0) | 12 (2.1)* l | 45 (0.94) r |
| Respiratory system | 0 (0) | 188 (1.14) d | 374 (0.97) | 0 (0) | 74 (1.42)* m | 488 (0.98) |
| Bones and Joints | 0 (0) | 1 (1.0) | 3 (1.9) | 0 (0) | 0 (0) | 4 (1.9) |
| Soft tissue including heart | 0 (0) | 3 90.7) | 12 (1.32) | 0 (0) | 2 (1.0) | 13 (1.13) |
| Skin excluding BCC and SCC | 1 (2.9) | 43 (1.2) | 77 (1.24) | 0 (0) | 24 (1.42) | 97 (1.2) |
| Breast | 1 (1.4) | 79 (0.8) | 172 (1.05) | 0 (0) | 45 (0.95) | 207 (0.96) |
| Female Genital system | 0 (0) | 31 (0.8) | 65 (0.98) | 0 (0) | 16 (0.91) | 80 (0.92) |
| Male Genital system | 1 (3.7) | 273 (1.2)*e | 622 (1.2)*i | 0 (0) | 81 (1.5)*n | 815 (1.17)* s |
| Urinary bladder | 0 (0) | 44 (0.9) | 198 (1.4)* | 0 (0) | 15 (1.2) | 227 (1.3)* |
| Kidney | 14 (170.0)* | 197 (7.6)* | 123 (2.7)* | 5 (1,101)* | 139 (13.6)* | 190 (3.1) |
| Renal pelvis | 0 (0) | 4 (2.3) | 1 (0.2)* | 0 (0) | 2 (4.2) | 3 (0.4) |
| Ureter | 0 (0) | 2 (1.8) | 8 (2.0) | 0 (0) | 1 (4.2) | 9 (1.8) |
| Eye and Orbit | 0 (0) | 3 (2.0) | 2 (0.7) | 0 (0) | 2(3.0) | 3 (0.8) |
| Brain and other Nervous system | 0 (0) | 14 (1.21) | 18 (0.91) | 0 (0) | 9 (1.7) | 23 (0.9) |
| Endocrine system | 2 (8.1) | 37 (3.4)*f | 31 (2.9)*j | 0 (0) | 27 (4.3)* o | 43 (2.8)* t |
| Lymphoma | 3 (9.9)*a | 38 (1.0) | 102 (1.2) | 0 (0) | 18 (1.15) | 125 (1.15) |
| Myeloma | 0 (0) | 11 (0.95) | 39 (1.3) | 0 (0) | 7 (1.8) | 43 (1.1) |
| Leukemia | 0 (0) | 23 (1.1)g | 82 (1.31)* | 0 (0) | 8 (1.1) | 97 (1.3)* |
| Mesothelioma | 0 (0) | 4 (1.7) | 7 (0.93) | 0 (0) | 2 (3.1) | 9 (0.97) |
| Kaposi Sarcoma | 1 (10.3) | 0 (0) | 2(1.3) | 0 (0) | 1 (0.5) | 2 (1.1) |
| Miscellaneous | 0 (0) | 15 (0.8) | 50 (0.8) | 0 (0) | 6 (0.98) | 59 (0.8) |
p<0.05, O-observed number of cases; SIR- Standardized incidence ratio (ratio of observed to expected number of second malignancies);
Significant increase in the risk of non-lymphocytic lymphomas (SIR:15.1) specifically for nodal type of NHL (SIR: 23.2);
Significant increase in the risk of an SPC in the hepatic flexure of the colon (SIR: 2.4);
Significant increase in the risk of liver cancer (SIR: 1.9);
Significant increased risk of developing cancer of the lung and bronchus (SIR: 1.2);
Significant increase in the risk of cancer of the prostate (SIR: 1.2);
Significant increase in the risk of thyroid cancer (SIR: 3.5);
Significantly increased risk of developing Acute Monocytic leukemia (SIR: 7.3);
Significant increase in risk of pancreatic cancer (SIR: 1.34);
Significant increase in risk of prostate cancer (SIR: 1.2);
Significant increase in the risk of thyroid cancer (SIR: 27) and malignant neoplasms of the adrenal gland (SIR: 7.2);
Significant increase in the risk of SPC in the colon (excluding rectum) (SIR: 1.5);
Significant increase in the risk of an SPC in the liver (SIR: 2.63);
Significant increase in the risk of SPC in the lung and bronchus (SIR: 1.54);
Significant increase in risk of prostate cancer (SIR: 1.5);
Significant increase in the risk of SPC in the thyroid gland (SIR: 4.3);
Significant decrease in the risk of an SPC in the pharynx (SIR: 0.4) and hypopharynx (SIR: 0.16);
Significant increase in the risk of SPC in the hepatic flexure of the colon (SIR: 1.66) ;
Significant increase in the risk of pancreatic cancer (SIR: 1.31);
Significant increase in the risk of prostate cancer (SIR: 1.17);
Significant increase in the risk of thyroid cancer (SIR: 2.9) and cancer of the adrenal gland (SIR: 6.6).
3.2 Relationship of patient and tumor related factors on the risk of second primary cancers in renal cell carcinoma
We first sought to investigate whether patient and/or tumor related factors had an effect on the risk of second primary cancers in patients with RCC. For this, the risk of SPCs was estimated from the actual number of second primaries reported during a follow-up period of >20 years (range: 1 month to >20 years) from the time of diagnosis of RCC. Two types of risks were examined: a) risk of a second cancer at all sites combined (termed as “overall risk”) and b) risk of a second cancer at a specific site (termed as “site-specific risk”). The following factors were investigated:
A. Age at diagnosis of renal cell carcinoma
Patients with RCC followed by a second cancer were divided into three groups based on their age at the time of diagnosis of the RCC: <30 years, 30–59 years, and ≥60 years. As shown in Table 1, the overall risk of SPCs was significantly increased in all three age groups with patients younger than 30 years nearly 4 times more likely to develop a second malignancy than their older counterparts.
Analysis of the risk at specific sites revealed that in patients <30 years old, the risk of a SPC was highest in the kidney (SIR:170). Among hematologic malignancies, the risk was highest (23-fold) for Non-Hodgkin lymphoma (NHL). For patients between 30–59 years old, the risk was highest for Acute monocytic leukemia (SIR:7.3) followed by colon and thyroid cancer (SIR: 2.4 and 3.5 respectively). Among patients aged 60 years or more, the risk of a second malignancy was highest in the endocrine glands, particularly in the thyroid (SIR: 27) and adrenal glands (SIR: 7).
B. Age attained at the time of detection of the second primary cancer
We next investigated whether the age attained by the patient at the time of detection of the second cancer had an effect on their risk of developing the second malignancy at a specific site.
Only five RCC patients were younger than 30 years at the time of their second malignancy and all were diagnosed with a second primary in the kidney. This translated into a >1,000 fold higher risk of developing SPCs in the kidney (Table 1).
Among patients aged 30–59 years, the risk of a SPC was highest in the kidney (SIR: 13.6) followed by the thyroid gland and liver (SIR: 4.5 and 2.6 respectively).
Patients 60 years or older at the time of diagnosis of the second primary were at the highest risk of a second malignancy in the endocrine system, specifically in the thyroid and adrenal glands (SIR: 3 and 6.6 respectively)
C. Race
The overall risk of a second cancer was significantly increased in whites and blacks but not in other racial groups (Table 2). The risk was higher in blacks compared to whites (SIR: 1.38 vs. 1.15 respectively). The adrenal gland was the site with the highest risk of a second malignancy among whites (SIR:7.3), while the kidney, thyroid gland, small intestine and colon were at the highest risk of a second primary among blacks (SIR: 9.9, 4.7, 3.4 and 3 respectively). Notably, the risk of a second cancer in the kidney, prostate, urinary bladder and the thyroid gland were increased in all racial groups.
Table 2.
Race and the site-specific risk of second primary cancers in renal cell carcinoma
| Site of second malignancy | Whites (O, SIR) | Blacks (O, SIR) | Others (O, SIR)† |
|---|---|---|---|
|
| |||
| All sites | 3,230 (1.15)* | 382 (1.38)* | 182 (1.7) |
| All solid tumors | 2,897 (1.15)* | 355 (1.4)* | 167 (1.73) |
| All lymphatic and Hematopoietic diseases | 263 (1.17)* | 20 (1.14) | 15 (2.01)* |
| Oral cavity and pharynx | 50 (0.74)*a | 3 (0.44) | 1 (0.4) |
| Digestive system | 605 (1.06)b | 59 (0.96)h | 54 (1.7)*l |
| Liver, GB IHBD, EHBD, other biliary | 51 (1.2)c | 3 (0.6) | 3 (0.55) |
| Respiratory system | 474 (0.99)d | 69 (1.3)i | 19 (1.1)m |
| Bones and Joints | 4 (1.7) | 0 (0) | 0 (0) |
| Soft tissue including heart | 12 (1.0) | 3 (3.0) | 0 (0) |
| Skin excluding BCC and SCC | 120 (1.23)*e | 0 (0) | 1 (1.5) |
| Breast | 216 (0.93) | 0 (0) | 10 (1.4) |
| Female Genital system | 88 (0.96) | 5 (0.6) | 3 (0.98) |
| Male Genital system | 751 (1.16)*f | 102 (1.3)*j | 43 (1.9)*n |
| Urinary bladder | 217 (1.22)* | 15 (2.0)* | 10 (2.53)* |
| Kidney | 251 (4.01)* | 65 (9.9)* | 18 (8.1)* |
| Renal pelvis | 4 (0.6) | 1 (3.1) | 0 (0) |
| Ureter | 10 (2.1) | 0 (0) | 0 (0) |
| Eye and Orbit | 4 (0.91) | 0 (0) | 0 (0) |
| Brain and other Nervous system | 30 (1.03) | 1 (0.7) | 1 (1.4) |
| Endocrine system | 57 (3.1)*g | 6 (3.9)k | 7 (5.3)*o |
| Lymphoma | 125 (1.1) | 10 (1.55) | 8 (1.9) |
| Myeloma | 45 (1.3) | 4 (0.6) | 1 (0.8) |
| Leukemia | 93 (1.21) | 6 (1.2) | 6 (.9)*p |
| Mesothelioma | 11 (1.2) | 0 (0) | 0 (0) |
| Kaposi Sarcoma | 3 (0.93) | 0 (0) | 0 (0) |
| Miscellaneous | 58 (0.84) | 7 (0.98) | 0 (0) |
p<0.05
American Indian, Alaskan Native, Native American, Asian and Pacific Islander
Risk of SPC in the hypopharynx significantly decreased (SIR, 0.16);
Risk of SPC in the hepatic flexure of the colon (SIR: 1.7) significantly increased;
Risk of SPC in the pancreas significantly increased (SIR: 1.33);
Risk of SPC in the larynx significantly decreased (SIR: 0.16);
Risk of melanoma of the skin significantly increased (SIR: 1.22);
Risk of SPC in the prostate significantly increased (SIR: 1.16);
Risk of SPC in the thyroid gland (SIR: 3.01) and adrenal gland (SIR: 7.3) significantly increased;
Risk of SPC in the small intestine and transverse colon significantly increased (SIR: 3.4 and 3.05 respectively);
Risk of SPC in the lung and bronchus significantly increased (SIR: 1.4);
Risk of SPC in the prostate gland significantly increased (SIR: 1.3);
Risk of SPC in the thyroid gland significantly increased (SIR: 4.7);
Risk of SPC in the colon (excluding the rectum, SIR:2.25), specifically in the hepatic (SIR:5.2) and splenic flexures (SIR: 7.7) and the sigmoid colon (SIR:2.3), rectum and rectosigmoid junction (SIR:2.22) is significantly increased;
Risk of SPC in the trachea (SIR: 84.3) significantly increased;
Risk of SPC in the prostate significantly increased (SIR: 1.87);
Risk of SPC in thyroid gland significantly increased (SIR: 6.0);
Risk of ALL (SIR: 17.6) significantly increased;
D. Gender
Although the overall risk of second malignancies was similar in both males and females (SIR: 1.2), it was significantly elevated only in the former. Significantly, males had a statistically higher risk of solid cancers while the risk of hematologic malignancies was significantly elevated in females. In males, the risk of a second primary was highest in the adrenal glands (SIR:6.6) followed by the kidneys and thyroid gland (SIR: 4.4 and 3.4 respectively) (Table 3). In females, the risk of a second malignancy was highest in the kidneys (SIR: 5.6) followed by the thyroid gland (SIR: 3.2). Significantly, the risk of a second cancer in both the sexes was highest in the first 6 months after the diagnosis of RCC but declined thereafter (Supplementary Tables 1 and 2).
Table 3.
Effect of gender, size of primary renal tumor and radiation on the risk of second primary cancers in renal cell carcinoma (RCC)
| Site of second malignancy | Gender | Size of RCC | H/O of radiation? | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Males | Females | ≤5cm | >5 to <10cm | ≥10cm | Yes | No | |
|
| |||||||
| O(SIR) | O(SIR) | O(SIR) | Total (O, SIR) | Total (O, SIR) | Total (O, SIR) | Total (O, SIR) | |
|
| |||||||
| All sites | 2,712 (1.2)* | 1,083 (1.2) | 971 (1.3)* | 914 (1.2)* | 226 (1.05) | 110 (1.11) | 3,666 (1.18)* |
| All solid tumors | 2,458 (1.2)* | 962 (1.2) | 873 (1.3)* | 819 (1.2)* | 207 (1.08) | 99 (1.12) | 3,304 (1.19)* |
| All lymphatic and Hematopoietic diseases | 203 (1.2) | 95 (1.3)* | 77 (1.29)* | 77 (1.3) | 13 (0.77) | 11 (1.5) | 286 (1.18)* |
| Oral cavity and pharynx | 39 (0.6)*a | 15 (0.9) | 9 (0.54) | 13 (0.7) | 6 (1.2) | 1 (0.4) | 53 (0.71)*t |
| Digestive system | 489 (1.1) | 229 (1.12) | 186 (1.23)* g | 152 (0.98) | 38 (0.9)p | 18 (0.9)q | 695 (1.08)u |
| Liver, GB IHBD, EHBD, other biliary | 45 (1.2) | 12 (0.8) | 18 (1.37) h | 16 (1.2) | 3 (0.8) | 0 (0) | 57 (1.1) |
| Respiratory system | 401 (0.96) | 161 (1.2)e | 129 (1.03) | 135 (1.1) | 39 (1.1) | 20 (1.1) | 540 (1.02) |
| Bones and Joints | 3 (1.7) | 1 (1.2) | 1 (1.67) | 1 (1.6) | 0 (0) | 0 (0) | 4 (1.6) |
| Soft tissue including heart | 10 (1.04) | 5 (1.3) | 7 (2.11) | 4 (1.2) | 0 (0) | 0 (0) | 15 (1.140 |
| Skin excluding BCC and SCC | 93 (1.2) | 28 (1.21) | 41 (1.61)* i | 28 (1.1) | 9 (1.2) | 2 (0.7) | 119 (1.23)*v |
| Breast | 6 (1.3) | 246 (0.95) | 80 (1.22) | 48 (0.77)*m | 14 (0.8) | 4 (0.7) | 245 (0.96) |
| Female Genital system | - | 96 (0.92) | 16 (0.64) | 24 (0.98) | 5 (0.8) | 4 (1.7) | 92 (0.91) |
| Male Genital system | 896 (1.2)*b | - | 215 (1.21)* j | 222 (1.2)*n | 54 (1.01) | 33 (1.3)r | 860 (1.9)* |
| Urinary bladder | 203 (1.2)* | 39 (1.5)* | 71 (1.63)* | 60 (1.33) | 12 (0.96) | 5 (0.8) | 236 (1.3)* |
| Kidney | 241 (4.4)* | 93 (5.6)* | 88 (5.0)* | 92 (5.0)* | 29 (5.3)* | 7 (3.2)* | 325 (4.7)* |
| Renal pelvis | 4 (0.7) | 1 (0.5) | 0 (0) | 0 (0) | 2 (4.3) | 0 (0) | 5 (0.7) |
| Ureter | 8 (2.0) | 2 (1.8) | 2 (1.74) | 2 (1.7) | 0 (0) | 0 (0) | 10 (2.0) |
| Eye and Orbit | 4 (1.2) | 1 (0.8) | 2 (1.96) | 2 (1.9) | 0 (0) | 0 (0) | 5 (1.2) |
| Brain and other Nervous system | 22 (0.9) | 10 (1.1) | 4 (0.56) | 12 (1.6) | 1 (0.5) | 1 (1.0) | 30 (0.99) |
| Endocrine system | 36 (3.3)*c | 34 (3.2)*f | 23 (4.07)* k | 22 (3.8)*o | 2 (1.2) | 3 (5.2)* | 67 (3.2)*w |
| Lymphoma | 93 (1.1) | 50 (1.3) | 32 (1.06) | 33 (1.1) | 9 (1.05) | 2 (0.6) | 141 (1.2)x |
| Myeloma | 30 (1.01) | 20 (1.6) | 10 (0.99) | 22 (2.2)* | 1 (0.4) | 1 (0.8) | 49 (1.2) |
| Leukemia | 80 (1.3)*d | 25 (1.1) | 35 (1.80)* l | 2 (1.1) | 3 (0.5) | 8 (3.1)*s | 96 (1.2) |
| Mesothelioma | 10 (1.1) | 1 90.98) | 5 (2.17) | 2 (0.8) | 0 (0) | 1 (3.1) | 10 (1.0) |
| Kaposi Sarcoma | 3 (0.9) | 0 (0) | 1 (1.36) | 1 (1.2) | 0 (0) | 0 (0) | 3 (0.84) |
| Miscellaneous | 39 (0.8) | 26 (0.9) | 14 (0.81) | 16 (0.9) | 0 (0) | 0 (0) | 64 (0.84) |
p<0.05, O-observed number of cases; SIR- Standardized incidence ratio (ratio of observed to expected number of second malignancies);
Significant decrease in the risk of SPC in the pharynx (SIR: 0.3) and the hypopharynx (SIR: 0.15);
Significantly increased risk of prostate cancer (SIR: 1.2);
Significantly increased risk of thyroid (SIR: 3.4) and adrenal gland cancer (SIR: 6.6);
Significantly increased risk of non-lymphocytic leukemias (SIR: 1.4);
Significant increase in the risk of SPC in the lung and bronchus (SIR: 1.22);
Significant increase in the risk of thyroid cancer (SIR: 3.2);
Significant increase (p<0.05) in the risk of cancer of the hepatic flexure of the colon (SIR: 2.68);
Significantly increased risk of pancreatic cancer (SIR: 1.84);
Significantly increased risk of cutaneous melanoma (SIR: 1.5) and other non-epithelial skin cancers (SIR: 2.7);
All the cases comprise cancer of the prostate whose risk is significantly increased (SIR:1.22);
Significantly (p<0.05) increased risk of cancer of the thyroid gland (SIR: 3.9) and adrenal gland (SIR: 9.6);
Significantly increased risk of chronic lymphocytic leukemia (CLL, SIR: 1.94), Non-lymphocytic leukemias (SIR: 1.81) and Myeloid and Monocytic leukemia (SIR:2.0);
Significantly decreased risk of cancer in the female breast (SIR: 0.73);
Significantly increased risk of prostate cancer (SIR: 1.2);
Significantly increased risk of thyroid cancer (SIR: 3.84);
Significant increase in the risk of cancer of the hepatic flexure of the colon (SIR: 3.9);
Significantly increased risk of second cancer in the descending colon (SIR: 6.5);
Significantly increased risk of penile cancer (SIR: 17.6);
Significantly increased risk of other leukemias (excluding lymphocytic leukemias and MAML) was significantly increased (SIR: 9.8);
Significant reduction in the risk of cancer of the pharynx (SIR: 0.41), specifically the hypopharynx (SIR:0.14);
Significant increase in risk of cancer of the hepatic flexure of the colon (SIR: 1.81);
Significantly increased risk of melanoma of the skin;
Significant increase in the risk of thyroid cancer (SIR: 3.3) and cancer of the adrenal glands (SIR: 5.6);
Significant increase in the risk of NHL.
E. Size of the kidney tumor
We next examined if there was a relationship between the size of the first primary cancer (i.e. RCC) and the risk of a second malignancy at a specific site. For this, the RCC patients were divided into three groups based on the maximum diameter of the renal tumor: <5cm, between 5 and 10cm and ≥10cm.
As shown in Table 3, the risk of a second malignancy was significant in cases for renal tumors less than 10cm in diameter, but not for larger tumors. For tumors <10cm in size, the risk of a second malignancy was highest in the kidney (SIR:5) and endocrine glands (SIR:3.8–4.1), particularly in the thyroid gland and adrenals. Interestingly, the risk of a second malignancy in the urinary bladder and prostate was strongly increased within the first 6 months after diagnosis of RCC, with a decline thereafter (Supplementary Table 3 and 4). Among hematologic malignancies, the risk of lymphocytic leukemias, specifically chronic lymphocytic leukemia was significantly elevated (SIR: 1.94) only for tumors <5cm in diameter but not for larger tumors. The only site with a significant risk of a second malignancy for tumors ≥10cm in size was the kidney (SIR:5.3).
G. History of radiation therapy
Therapeutic radiation is well known to increase the risk of cancers in general 17–19. We sought to investigate whether RCC patients who received radiation therapy had a difference in the risk of a second malignancy compared to those who had not received any form of radiation (including radioisotopes) therapy.
We found that the overall risk of a second malignancy was slightly higher in those patients who had not received radiation therapy compared to those who did (SIR: 1.18 and 1.11 respectively). The increased risk extended to both solid and hematologic malignancies in the former as summarized in Table 3. Among patients who received radiation therapy, the sites with the highest risk of a second malignancy were the adrenal glands and the thyroid (SIR: 201 and 32 respectively), with the risk being significant only within 6months and 1 year of diagnosis of RCC respectively (Supplementary Table 5). A similar increase in risk of thyroid cancer, although of a much lower magnitude (about 2-fold lower) was observed in patients who did not receive any radiation therapy (Supplementary Table 6). The risk of leukemias was specifically increased (by about 3-fold) in those who received radiation (SIR:3.1). Conversely, the sites with an increased risk of a second malignancy specific to those who did not receive radiation were the skin and urinary bladder (SIR: 1.2 each).
3.3 Prognostic significance of Second primary cancers in Renal cell carcinoma
Having determined the sites with a differential risk of a second malignancy, we next sought to investigate the effect of second malignancies on the survival of RCC patients.
For this, we retrieved de-identified information on all RCC patients registered with the SEER database. A total of 58,174 patients with RCC were registered in 17 registries of the SEER database. Of these, 42,314 (72.7%) had RCC as the only primary tumor. This group was designated as “Only primary cancer” or OPC. In 5,769 cases (9.9%) RCC was the first of two or more primary malignancies. This group was designated as “second primary cancer” or “SPC”. In the remaining cases, RCC was diagnosed as the third or later of multiple primary cancers. Comparison of demographic characteristics of patients belonging to the OPC or SPC group (summarized in Supplementary Table 7) revealed that SPC patients were significantly older at the time of diagnosis and were more likely to be male, black and married, and less likely to have received radiation compared to the OPC group.
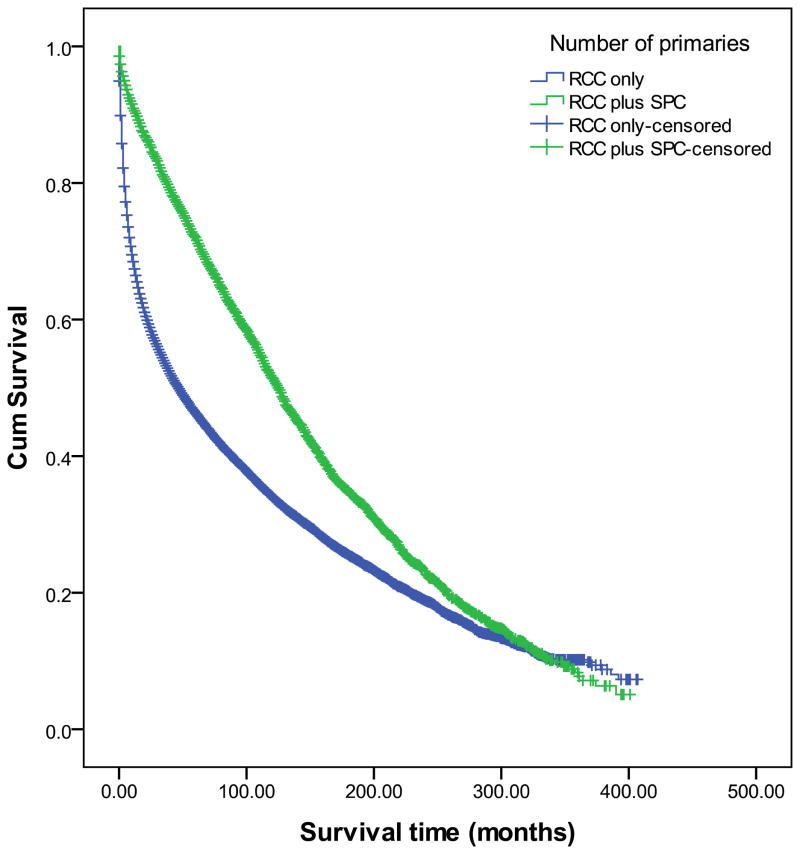
To determine the prognostic significance of SPCs in RCC, we first analyzed the effect of patient and tumor-related factors (including presence of second primaries) on the overall survival (OS) of patients (with RCC±SPC) by the Kaplan Meier method. As shown in Figure 1 and summarized in Table 4, patients in the SPC group had a significantly better survival than those in the OPC group. The other factors that predicted a longer OS in RCC patients included age≤60 years at the time of diagnosis of RCC, non-white non-black race, female gender, marital status (married/single), a well-differentiated, localized, unilaterlal renal tumor ≤5cm in diameter, and the absence of radiation therapy (with/without subsequent surgery) (Supplementary Figure 1).
Figure 1. Kaplan Meier analysis to analyze the effect on second primary cancers (SPCs) on overall survival in patients with renal cell carcinoma (RCC).
Univariate analysis revealed that the survival of patients with RCC and one/more second primary cancers was significantly greater (151±2 months) than that of patients where RCC was the only primary cancer (111±1 months, p<0.001 by Log Rank test)
Table 4.
Kaplan Meier analysis of factors affecting overall survival in patients with renal cell carcinoma
| Factor tested | Patient group | Total N | Mean survival (±SE) | 95% C.I. | χ2 | Log Rank test (Mantel-Cox) |
|---|---|---|---|---|---|---|
|
| ||||||
| Number of primary tumors | OPC | 42,314 | 111±1 months | 109–113 months | 691.4 | P<0.001 |
| SPC | 5,769 | 151±2 months | 147–155 months | |||
| Age at diagnosis of RCC | <60 years | 20,243 | 173±1.7 months | 170–176 months | 4,007.7 | P<0.001 |
| ≥60 years | 37,931 | 76±0.6 months | 74.6–77 months | |||
| Race | White | 49,317 | 110±0.8 months | 108–111 months | 18.3 | P<0.001 |
| Black | 5,903 | 106±2.5 months | 101–111 months | |||
| Others | 2,761 | 115±3.5 months | 108.5–122 months | |||
| Gender | Male | 36,630 | 104±1 months | 102–106 months | 75.7 | P<0.001 |
| Female | 21,544 | 119.5±1.4 months | 117–122 months | |||
| Marital status at time of diagnosis of RCC | Married | 36,410 | 121±1 months | 119–123 months | 1562.0 | P<0.001 |
| Widowed | 8,754 | 62±1.2 months | 60–64 months | |||
| Divorced | 4,307 | 103±2.6 months | 118–130 months | |||
| Single | 5,955 | 124±3 months | 118–129.5 months | |||
| Separated | 590 | 91±5.6 months | 80–102 months | |||
| Summary stage (2004+) | Localized | 15,112 | 81±0.4 months | 80.5–82 months | 14,845.0 | P<0.001 |
| Regional spread | 3,966 | 58.5±0.8 months | 57–55 months | |||
| Distant spread | 6,328 | 13±0.3 months | 12–14 months | |||
| Grade of RCC | Well differentiated | 4,911 | 179±3.5 months | 172–186 months | 2385.0 | P<0.001 |
| Moderately differentiated | 10,631 | 154±2.9 months | 148–159 months | |||
| Poorly differentiated | 5,882 | 98.5±3 months | 93–104 months | |||
| Undifferentiated | 1,591 | 67±3.8 months | 59.5–74 months | |||
| Size of tumor | ≤5cm | 12,948 | 124±1 months | 122–126 months | 1955.3 | P<0.001 |
| >5cm-≤10cm | 13,410 | 97±0.9 months | 96–99 months | |||
| >10cm | 5,561 | 66±1.2 months | 63–68 months | |||
| Unilateral or Bilateral RCC | Unilateral | 56,308 | 113±0.8 months | 111–114 months | 3052.3 | P<0.001 |
| Bilateral | 1,866 | 22±1.6 months | 19–25 months | |||
| Radiation therapy given? | Yesc | 4,898 | 26±1 months | 24–27 months | 5057.4 | P<0.001 |
| No | 52,214 | 119±0.9 months | 117–121 months | |||
| Radiation sequence with surgery | Received both | 2,092 | 45±1.8 months | 41.5–49 months | 727.3 | P<0.001 |
| Received neither | 56,078 | 113±0.8 months | 111–114 months | |||
Number of patients (N), only primary cancer (OPC), Secondary primary cancer (SPC), Well differentiated (WD), moderately differentiated (MD), poorly differentiated (PD),
Black, American Indian, Alaskan Native, Asian, Pacific islander
Regional or distant spread of tumor
Received either radiation or radioisotope therapy
Upon multivariate analysis, age <60 years at the time of diagnosis of RCC, female gender, married status, localized stage, well or moderate degree of differentiation (of RCC), tumor size ≤5cm, absence of radiation therapy and an unilateral RCC were significant independent predictors of a longer OS (Table 5). Presence of second primary cancers and race however, were not retained as significant predictors of OS in the multivariate analysis.
Table 5.
Multivariate analysis of factors that influence overall survival in patients with renal cell carcinoma
| Variable | Categories | N | H.R. | 95% CI for H.R.
|
p-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
|
| ||||||
| Age at diagnosis of RCC | <60 | 3,799 | 0.54 | 0.49 | 0.58 | <0.001 |
| ≥60 yrs | 3,156 | - | ||||
| Gender | Male | 2,593 | 1.22 | 1.12 | 1.33 | <0.001 |
| Female | 4,362 | 1.00 | ||||
| Race | Others | 5,858 | 1.23 | 1.10 | 1.37 | <0.001 |
| Whites | 1,097 | 1.00 | ||||
| Marital status | Othersa | 4,710 | 1.34 | 1.23 | 1.46 | <0.001 |
| Married | 2,245 | 1.00 | ||||
| Summary stage | Regional or Distant | 4,739 | 2.94 | 2.68 | 3.22 | <0.001 |
| Localized | 2,216 | 1.00 | ||||
| Grade | PD or UD | 4,592 | 1.83 | 1.68 | 1.99 | <0.001 |
| WD or MD | 6,718 | - | ||||
| Size of tumor | ≤5cm | 3,586 | 0.69 | 0.63 | 0.75 | <0.001 |
| >5cm | 3,369 | - | ||||
| Radiation therapy | No | 302 | 0.39 | 0.34 | 0.45 | <0.001 |
| Yes | 6,653 | - | ||||
| Laterality | Unilateral | 6,952 | 0.31 | 0.14 | 0.71 | 0.001 |
| Bilateral | 6 | |||||
Hazards ratio (H.R.), confidence interval (CI), only primary cancer (OPC), second primary cancer (SPC), well differentiated (WD), moderately differentiated (MD), poorly differentiated (PD)
Variables not in the final model: Sequence of RCC (OPC vs. SPC).
3.4 Factors influencing prognosis of RCC patients with one or more second primary cancers
Patients with multiple primary malignancies may be affected by prognostic factors unique from those with single primary cancers. Hence, we also investigated the factors that influence the OS of patients with RCC and one/more second cancers.
As summarized in Table 6 and illustrated in Supplementary Figure 2, age <60 years at the time of diagnosis of either RCC or the second malignancy, white or non-white non-black race, married status, a localized, well-differentiated, right sided renal tumor, lack of a history of radiation and an interval of ≥60 months between diagnosis of RCC and the second malignancy were associated with longer OS in patients with RCC and one/more second malignancies. Gender of the patient and size of the primary renal tumor however did not affect the OS significantly.
Upon multivariate analysis (Table 7), only age <60 years at the time of diagnosis of the second primary cancer, lack of a history of radiation therapy and an interval of ≥1 year between the diagnosis of RCC and the second malignancy were significant predictors of longer survival in RCC patients with second malignancies.
Table 7.
Multivariate analysis of factors influencing overall survival in renal cell carcinoma patients with second primary cancers
| Variable | Categories | H.R. | 95% CI for H.R.
|
p-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
|
| |||||
| Age at diagnosis of SPC | <60 years | 0.46 | 0.27 | 0.82 | 0.006 |
| ≥60 years | - | ||||
| Received radiation | No | 0.16 | 0.07 | 0.38 | 0.006 |
| Yes | - | ||||
| Months elapsed between FPC and SPC | 0–11 months | 2.10 | 1.40 | 3.20 | <0.001 |
| ≥12 months | - | ||||
Hazards ratio (H.R.), confidence interval (CI), only primary cancer (OPC), second primary cancer (SPC), well differentiated (WD), moderately differentiated (MD), poorly differentiated (PD) and undifferentiated (UD).
Including divorced, widowed, separated and never married (single) individuals Variables not included in the model: Age at diagnosis of SPC (<60 years vs. ≥60 years), Race (Whites vs. others), grade of tumor (well and moderately differentiated vs. undifferentiated), size of tumor (≤5cm vs. >5cm), Radiation sequence with surgery (given vs. not given), gender (male vs. female), radiation or radioisotope therapy (given vs. none) and laterality (right vs. left origin of the primary renal tumor).
IV DISCUSSION
The present study represents the first comprehensive analysis of the risk of second primary cancers (SPCs) in patients diagnosed with renal cell carcinoma from data available in the SEER database. We observed that about 10% of all patients with RCC had one or more new primary tumors. The largest number of second cancers arose in the male genital system (particularly the prostate gland) and in the digestive and respiratory system. The overall risk of a second malignancy was highest in patients <30 years old at the time of diagnosis of either RCC or the second primary malignancy. Further, patients with a second cancer had a significantly longer overall survival than those without a second malignancy. The incidence of second cancers among RCC patients reported in our study is much lower than that reported in other non-US populations. For instance, a study by Beisland and co-workers in Norway had reported that the incidence of second malignancies in RCC was 47% 12. Other studies have reported an incidence between 16%–18.5% 13;14. A possible explanation for the lower incidence of second malignancies in the registries registered with SEER could be international difference in registration procedures used by various cancer registries 20. The completeness of data in a cancer registry is determined by several factors particularly the guidelines for screening and diagnosis of cancer. Differences in these guidelines could potentially influence the reported incidence of cancer in a given population 21. Further, genetic differences (including polymorphisms) and differences in the environment (e.g. food habits, exposure to environmental carcinogens and lifestyle) might contribute to a higher or lower risk of second primary cancers in a given population.
In terms of the total number, the sites with the most second primary tumors in the SEER database were in the male genital system (24% of total), digestive system (19% of total) and respiratory system (15% of total). These three systems together accounted for 58% of all second malignancies. Specifically, the prostate gland, colon and the lung and bronchus were the sites in these systems with a consistent increase in risk of second malignancies in nearly all patient and treatment groups. This finding is similar to a report by Thompson et al., who observed that the risk of a second malignancy in RCC patients was highest in the prostate and colon 14. Rabbani et al. also reported that the papillary type of RCC is associated with a significantly increased risk of second cancers in the prostate and bladder 13. Other smaller studies have reported that the most common sites for second primary cancers in RCC are the prostate, colon and urinary bladder 22;23. While second cancers in the bladder accounted for 6.4% of all SPCs in our study (Table 1), the risk was significant only in patients aged 60 years or more (at time of diagnosis of either RCC or the SPC), with RCCs ≤5cm in size and in patients without a history of radiation therapy (Table 3). There was no difference in incidence of second cancers in the bladder with either gender or race of the patient.
Among hematological malignancies, lymphomas were the most common second malignancies in RCC patients with 143 (3.8% of total) observed in the SEER population. Specifically, the risk of Non-Hodgkin lymphoma was increased, being significantly elevated in those < 30 years old at the time of diagnosis of RCC and within 6 months after the diagnosis of RCC in both genders (Supplementary Tables 1 and 2). Beisland et al. had also reported in their aforementioned study that NHL (together with melanoma) was the most common second malignancy in patients with an existing RCC 12.
The occurrence of two primary cancers in the same patient is, however, by itself not sufficiently strong to support the hypothesis that a common underlying mechanism may be driving both malignancies. However, if the risk of development of one malignancy in presence of the second is reciprocal (i.e. presence of either malignancy as the first increases the risk of the second in the same patient), the chance that the two are driven by a common mechanism would be significantly increased. Using this principle of reciprocal risk, we noted that solid tumors with a mutually increased risk of occurrence (with RCC) were the skin (excluding basal and squamous cell carcinomas), urinary bladder, prostate, thyroid and adrenal glands (Supplementary Table 8). Among the lymphatic and hematopoietic malignancies, the risk of leukemias was reciprocally increased in RCC patients. Several other studies have also reported (using the principle of reciprocal risk), that significant reciprocal associations exist between RCC and malignancy at another site. For instance, a study using data from the New South Wales Central Cancer Registry (Australia) reported that cancer of the renal parenchyma was associated with a significant increase in the risk of prostate cancer, and that the reciprocal was also true. A similar reciprocal risk relationship was reported by McCredie and co-workers for RCC and cancer of the urinary bladder. However, the enhanced risk was confined only to women 24. An analysis of the Danish Cancer Registry (1943–1980) revealed that patients with a primary malignancy in the kidney were at a significantly elevated risk of a second primary in the bladder (SIR: 7.1, 95% C.I. 6.0–8.4) and the reciprocal was true for patients with a primary malignancy of the bladder (SIR for SPC in the kidney being 3.2, 95% C.I. 2.7–3.8) 25. Although the exact mechanism for this reciprocal risk phenomenon is not known, a few possibilities can be suggested. These include a common environmental (e.g. cigarette smoking and/or alcohol are linked to cancers of the urinary system, respiratory system, oral cavity, pharynx, esophagus, pancreas and cervix, and benzidene for RCC and bladder cancer) or genetic risk factor (co-occurrence of familial papillary carcinoma of thyroid and papillary carcinoma of the kidney linked to the chromosome locus 1q21) 25–27. Familial RCC has also been shown to increase the risk of a second primary cancer in the urinary bladder and endocrine glands by nearly 3-fold 28. The exact mechanism by which these factors induce oncogenic transformation at two separate anatomic sites however remains to be elucidated.
A significant observation in our analysis was a prominent increase in the risk of endocrine cancers, specifically thyroid and adrenal gland malignancies in patients with RCC. While the exact mechanism underlying the co-occurrence of RCC with thyroid cancer is unknown, one possibility is the occurrence of common genetic alterations that drive the development of tumors at the two sites. Missense mutations in CHEK2, a gene encoding the human analogue of the yeast checkpoint kinases Cds1 and Rad53 have, for instance, been associated with a significant increase in the risk of thyroid, prostate, colon breast and kidney cancer 29. On the other hand, the best known condition associated with an inherited risk of clear cell carcinoma of the kidney together with cancers in the adrenal glands, is the von Hippel-Lindau (VHL) syndrome, an autosomal dominant disease characterized by hemangioblastomas in the brain, spinal cord and retina and tumors in the kidney, adrenal glands and lymphatics 30. Sporadic cases of RCC with adrenal malignancies are however quite rare, limited to a handful of case reports 31. Future studies in patients with these inherited syndromes could help elucidate the molecular mechanism underlying the development of multiple primaries including RCC.
An important focus of oncologic research from the standpoint of public health is the impact of cancers (in this case second primary cancers) on the survival of patients. Once the predictors of survival are identified, these could be utilized to guide therapeutic interventions and surveillance strategies to improve survival. We observed that patients who had both an RCC and a second primary cancer had a significantly longer survival than those with RCC alone (Table 4). However, this difference did not remain significant on multivariate analysis suggesting that, while the presence of second primaries may improve survival, they were not an independent predictor of overall survival in these patients. A similar observation (i.e. better survival in patients with one/more second primary cancers) has previously been reported in a study of 80 patients with cancers of the renal pelvis. Median survival was significantly better in those patients who had a history of a prior urothelial malignancy (42 months) compared to those in whom it was the only malignancy (19 months) 32. Notably, RCC patients who received radiation therapy had a dramatic reduction in OS (26±1 months vs. 119±0.9 months in those who did not receive radiation). This concurs with previous reports in literature that suggested that radiation therapy does not improve survival in patients with RCC 33. Recent clinical trials have also not found any significant benefit with radiation therapy in RCC except in patients with brain metastasis, and even in them it has not been shown to produce a significant improvement in the progression free interval 34;35. It is possible that the lower survival in patients who received therapeutic radiation was due to the adverse effects of radiation itself including bone marrow depression and the resulting morbidity. Our finding of a longer overall survival in female RCC patients agrees with a recent study by Aron et al. which found that females presented with smaller tumors which were of a lower histologic grade compared to males who most often presented with regional or distant metastasis 36. While it is not clear how being married improved survival in RCC patients, one possible explanation is that these patients were more likely to receive emotional and psychological support that might have contributed to their longer survival.
When we examined the factors that affect survival in RCC patients with one/more second primaries (SPCs), we noted that they were very similar to those that predicted longer survival in patients with RCC alone. Thus, age <60 years at the time of diagnosis of RCC (or the second primary), white or non-black race, married status, localized and well-differentiated primary renal tumors arising from the left side and absence of a history of therapeutic radiation were predictors of a longer survival on univariate analysis (Table 6). An additional factor that specifically predicted a better survival in patients with second primaries was a longer interval between diagnosis of RCC and the second primary (patients diagnosed with a second primary 5 years or later after the diagnosis of RCC had the longest OS). Upon multivariate analysis, time elapsed between the diagnosis of RCC and the second primary was an independent predictor of survival with, patients diagnosed with a second malignancy less than 1 year after being diagnosed with RCC having a significantly poorer OS compared to those diagnosed >1 year later (Hazards ratio 2.1, p<0.001, Table 7). In order to validate the results of our multivariate survival analysis in patients with second primaries, we searched the literature for actual case studies on survival in patients with RCC and a second malignancy. Studies wherein a second primary was diagnosed <1month after diagnosis of the RCC were excluded. We identified a study by Choueiri et al., wherein they had reported a series of four patients with RCC who subsequently developed multiple myeloma (MM) (summarized in Supplementary Table 9) 37. Of the four patients, the shortest survival time (9 months from the time of diagnosis of RCC) was noted for a 66yr old male patient (patient #3) who was diagnosed with MM 1month after being diagnosed with RCC, while the longest survival (11 yrs) was observed for a 61 yr old male diagnosed with MM 108 months after being diagnosed with RCC (patient #1). Based on our multivariate analysis, patient #3 would be expected to have the shortest survival as he had two adverse prognostic factors- age>60 years at presentation with RCC and time elapsed between the diagnosis of RCC and the second primary <1month. Patient#1 with one adverse factor (age>60 years) would be predicted to have a longer survival. A comparison of the predicted and actual survival for the four patients in this series is presented in Supplementary Table 9.
Table 6.
Kaplan Meier analysis of factors influencing overall survival in renal cell carcinoma patients with second primary cancers
| Factor tested | Patient group | N | Mean survival (±SE) | 95% C.I. | χ2 | Log Rank test (Mantel-Cox) |
|---|---|---|---|---|---|---|
|
| ||||||
| Age at diagnosis of RCC | <60 years | 1,119 | 222±4.5 months | 213.5–221 months | 356.7 | P<0.001 |
| ≥60 years | 2,253 | 130±2.0 months | 126–134 months | |||
| Age at occurrence of SPC | <60 years | 538 | 198.6±8 months | 184–213.5 months | 26.8 | P<0.001 |
| ≥60 years | 2,834 | 158±2.4 months | 153–162 months | |||
| Race | White | 2,871 | 164±2.5 months | 159.5–169 months | 8.7 | P=0.013 |
| Black | 340 | 141±6.3 months | 129–154 months | |||
| Othersa | 160 | 164±9.5 months | 145.6–183 months | |||
| Gender | Male | 2,395 | 162±4 months | 154–170 months | 0.005 | P=0.95 |
| Female | 977 | 163±2.8 months | 157–168 months | |||
| Marital status at time of diagnosis of RCC | Married | 2,463 | 171±2.8 months | 166–178.5 months | 65.4 | P<0.001 |
| Widowed | 358 | 119±5 months | 109–129 months | |||
| Divorced | 188 | 150±8 months | 133–166 months | |||
| Separated | 244 | 155±8.5 months | 1390172 months | |||
| Single (Never married) | 25 | 155±19 months | 118.5–192 months | |||
| Summary stage (2004+) | Localized | 404 | 79.5±1.8 months | 76–83 months | 84.5 | P<0.001 |
| Regional | 82 | 74±4 months | 66–82 months | |||
| Distant | 34 | 30.5±1.7 months | 19.1–42 months | |||
| Grade of RCC | Well differentiated | 350 | 184±8 months | 168.6–200 months | 15.5 | 0.001 |
| Moderately differentiated | 610 | 143.5±4.5 months | 134.6–152 months | |||
| Poorly differentiated | 194 | 149±10 months | 129–169.5 months | |||
| Undifferentiated | 31 | 143±23 months | 99–188 months | |||
| Size of primary renal tumor | ≤5cm | 989 | 131±2.5 months | 126–136 months | 0.78 | 0.67 |
| >5cm-≤10cm | 702 | 129±3.0 months | 123–135 months | |||
| >10cm | 157 | 124±6.5 months | 112–137 months | |||
| Laterality | Right origin of primary | 1,712 | 158±3 months | 152–164 months | 3.9 | 0.048 |
| Left origin of primary | 1,632 | 168±3.5 months | 161.5–175 months | |||
| Radiation therapy given? | Yesc | 99 | 119±11 months | 98–141 months | 15.3 | P<0.001 |
| No | 3,255 | 164±2.4 months | 159.5–169 months | |||
| Radiation sequence with surgery | Received both | 80 | 131±12 months | 108–154 months | 6.7 | 0.01 |
| Received neither | 3,291 | 163.6±2.4 months | 159–168 months | |||
| Months between diagnosis of FPC and SPC | 0–11 months | 613 | 92.6±4.7 months | 83.5–102 months | 756.1 | P<0.001 |
| 12–59 months | 1,093 | 120±3.6 months | 113–127 months | |||
| ≥ 60 months | 1,666 | 210±2.3 months | 204–216 months | |||
Number of patients (N), only primary cancer (OPC), Secondary primary cancer (SPC), Well differentiated (WD), moderately differentiated (MD), poorly differentiated (PD),
Black, American Indian, Alaskan Native, Asian, Pacific islander
Regional or distant spread of tumor
Received either radiation or radioisotope therapy
A key observation in our analysis was the significantly higher risk of developing a SPC (nearly 5-fold) in younger patients (age <30 years at the time of diagnosis of RCC) than in older patients with an antecedent RCC (Table 1). Hartley et al. had reported in a series of 218 patients diagnosed with renal tumors in childhood that the excess risk of developing a second malignancy was significantly higher than expected (N=8, SIR: 14.7)38. The authors also suggested that development of bony exostoses might predict the development of a subsequent malignancy in patients with childhood renal tumors. It is possible that the elevated risk of SPCs in younger patients observed in our study could be at least partly due to an inherited predisposition to develop multiple tumors. However, this would need to be confirmed by detailed genetic analysis in a separate study.
In conclusion, we have conducted the first comprehensive analysis of SPCs in patients with RCC as the first primary malignancy using data from the SEER database. We have also examined the prognostic significance of factors influencing OS of patients with RCC with/without SPCs. Our results suggest that several patient, tumor and treatment related factors can affect the risk of SPCs at specific sites and influence the prognosis of RCC patients. By comparing our findings with published studies, we have demonstrated the correlation of database based studies with incidence and prognosis of RCC patients in actual hospital based settings. The results from the study have important implications in investigating the mechanisms underlying the occurrence of multiple primary tumors and in planning surveillance strategies for patients with newly diagnosed renal cell carcinoma.
Supplementary Material
Kaplan-Meier curves to illustrate the effect of specific factors that significantly impact survival in patients with Renal cell carcinoma (irrespective of the presence or absence of a second primary malignancy). The numbers of patients at risk in each group are summarized in Table 4.
Site specific risk of second primary cancers in males with renal cell carcinoma
Site specific risk of second primary cancers in females with renal cell carcinoma
Kaplan-Meier curves to illustrate the effect of specific factors on the overall survival of patients with renal cell carcinoma and a second primary malignancy. The numbers of patients at risk in each group are summarized in Table 6.
Site specific risk of second primary cancers in renal cell carcinoma with primary tumor ≤ 5cm in size
Site specific risk of second primary cancers in renal cell carcinomas with primary tumor 5cm to <10 cm in size
Site specific risk of second primary cancers in RCC patients who received radiation therapy
Site specific risk of second primary cancers in renal cell carcinomas patients who did not receive radiation therapy
Characteristics of patients with either RCC only (OPC) or RCC plus one or more second primary cancers (SPC) in the SEER database (17 registries limited use, updated November 2008, containing information of cases between 1973–2006)
Comparison of the risk for reciprocal occurrence of second malignancies in patients with renal cell carcinoma
Validation of the results of multivariate analysis (in Table 7) using case series published by Choueri et al. 37
Acknowledgments
Surinder K Batra and Subhankar Chakraborty are supported in part by grants from the US Department of Defense (BC074631, BC083295 and PC074289) and the National Institute of Health (RO1 CA78590, UO1 CA111294, RO1 CA131944, RO1 CA133774, RO1 CA 138791 and P50 CA127297).
Abbreviations
- FPC
First primary cancer
- OS
Overall survival
- RCC
renal cell carcinoma
- SPC
Second primary cancer
Footnotes
DISCLOSURE
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The authors declare no conflicts of interest.No writing assistance was utilized in the production of this manuscript.
Reference List
- 1.American Cancer Society. Cancer Facts & Figures 2010. 2010. [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Lipworth L, Tarone RE, McLaughlin JK. Renal cell cancer among African Americans: an epidemiologic review. BMC Cancer. 2011;11:133. doi: 10.1186/1471-2407-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Satram-Hoang S, Ziogas A, nton-Culver H. Risk of second primary cancer in men with breast cancer. Breast Cancer Res. 2007;9:R10. doi: 10.1186/bcr1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noura S, Ohue M, Seki Y, et al. Second primary cancer in patients with colorectal cancer after a curative resection. Dig Surg. 2009;26:400–405. doi: 10.1159/000229991. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto N, Morio S, Inoue R, et al. The risk of a second primary cancer occurring in five-year survivors of an initial cancer. Jpn J Clin Oncol. 1987;17:205–213. [PubMed] [Google Scholar]
- 8.Chuang SC, Scelo G, Tonita JM, et al. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer. 2008;123:2390–2396. doi: 10.1002/ijc.23798. [DOI] [PubMed] [Google Scholar]
- 9.Chuang SC, Hashibe M, Scelo G, et al. Risk of second primary cancer among esophageal cancer patients: a pooled analysis of 13 cancer registries. Cancer Epidemiol Biomarkers Prev. 2008;17:1543–1549. doi: 10.1158/1055-9965.EPI-07-2876. [DOI] [PubMed] [Google Scholar]
- 10.Jones AS, Morar P, Phillips DE, et al. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995;75:1343–1353. doi: 10.1002/1097-0142(19950315)75:6<1343::aid-cncr2820750617>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Liu YY, Chen YM, Yen SH, et al. Multiple primary malignancies involving lung cancer-clinical characteristics and prognosis. Lung Cancer. 2002;35:189–194. doi: 10.1016/s0169-5002(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 12.Beisland C, Talleraas O, Bakke A, et al. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97:698–702. doi: 10.1111/j.1464-410X.2006.06004.x. [DOI] [PubMed] [Google Scholar]
- 13.Rabbani F, Reuter VE, Katz J, et al. Second primary malignancies associated with renal cell carcinoma: influence of histologic type. Urology. 2000;56:399–403. doi: 10.1016/s0090-4295(00)00682-8. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH, Leibovich BC, Cheville JC, et al. Second primary malignancies associated with renal cell carcinoma histological subtypes. J Urol. 2006;176:900–903. doi: 10.1016/j.juro.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 15.Rixe O, Rini B. Renal cell carcinoma: ten years of significant advances. Target Oncol. 2010;5:73–74. doi: 10.1007/s11523-010-0150-9. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Campbell SC, Rathmell WK. Renal cell carcinoma. Curr Opin Oncol. 2006;18:289–296. doi: 10.1097/01.cco.0000219260.60714.c4. [DOI] [PubMed] [Google Scholar]
- 17.Tucker MA, Jones PH, Boice JD, Jr, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. The Late Effects Study Group. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 18.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 19.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Harris RE, Gao YT, et al. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991;20:76–81. doi: 10.1093/ije/20.1.76. [DOI] [PubMed] [Google Scholar]
- 21.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Koyama K, Furukawa Y, Tanaka H. Multiple primary malignant neoplasms in urologic patients. Scand J Urol Nephrol. 1995;29:483–490. doi: 10.3109/00365599509180031. [DOI] [PubMed] [Google Scholar]
- 23.Turkic M, Znaor A, Novosel I, et al. Second primary malignant tumors in patients with primary renal cell carcinoma. Acta Med Croatica. 2005;59:91–95. [PubMed] [Google Scholar]
- 24.McCredie M, Macfarlane GJ, Stewart J, et al. Second primary cancers following cancers of the kidney and prostate in New South Wales (Australia), 1972–91. Cancer Causes Control. 1996;7:337–344. doi: 10.1007/BF00052939. [DOI] [PubMed] [Google Scholar]
- 25.Storm HH, Lynge E, Osterlind A, et al. Multiple primary cancers in Denmark 1943–80; influence of possible underreporting and suggested risk factors. Yale J Biol Med. 1986;59:547–559. [PMC free article] [PubMed] [Google Scholar]
- 26.Malchoff CD, Sarfarazi M, Tendler B, et al. Papillary thyroid carcinoma associated with papillary renal neoplasia: genetic linkage analysis of a distinct heritable tumor syndrome. J Clin Endocrinol Metab. 2000;85:1758–1764. doi: 10.1210/jcem.85.5.6557. [DOI] [PubMed] [Google Scholar]
- 27.Morikawa Y, Shiomi K, Ishihara Y, et al. Triple primary cancers involving kidney, urinary bladder, and liver in a dye worker. Am J Ind Med. 1997;31:44–49. doi: 10.1002/(sici)1097-0274(199701)31:1<44::aid-ajim7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Czene K, Hemminki K. Kidney cancer in the Swedish Family Cancer Database: familial risks and second primary malignancies. Kidney Int. 2002;61:1806–1813. doi: 10.1046/j.1523-1755.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 29.Cybulski C, Gorski B, Huzarski T, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75:1131–1135. doi: 10.1086/426403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linehan WM, Vasselli J, Srinivasan R, et al. Genetic basis of cancer of the kidney: disease-specific approaches to therapy. Clin Cancer Res. 2004;10:6282S–6289S. doi: 10.1158/1078-0432.CCR-050013. [DOI] [PubMed] [Google Scholar]
- 31.Valeri A, Borrelli A, Presenti L, et al. Adrenal masses in neoplastic patients: the role of laparoscopic procedure. Surg Endosc. 2001;15:90–93. doi: 10.1007/s004640000245. [DOI] [PubMed] [Google Scholar]
- 32.Raabe NK, Fossa SD, Bjerkehagen B. Carcinoma of the renal pelvis. Experience of 80 cases. Scand J Urol Nephrol. 1992;26:357–361. doi: 10.3109/00365599209181226. [DOI] [PubMed] [Google Scholar]
- 33.Patel NP, Lavengood RW. Renal cell carcinoma: natural history and results of treatment. J Urol. 1978;119:722–726. doi: 10.1016/s0022-5347(17)57611-9. [DOI] [PubMed] [Google Scholar]
- 34.Manon R, O’Neill A, Knisely J, et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397) J Clin Oncol. 2005;23:8870–8876. doi: 10.1200/JCO.2005.01.8747. [DOI] [PubMed] [Google Scholar]
- 35.Brouwers AH, Mulders PF, de Mulder PH, et al. Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J Clin Oncol. 2005;23:6540–6548. doi: 10.1200/JCO.2005.07.732. [DOI] [PubMed] [Google Scholar]
- 36.Aron M, Nguyen MM, Stein RJ, et al. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133–140. doi: 10.1016/j.eururo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Choueiri TK, Baz RC, McFadden CM, et al. An association between renal cell carcinoma and multiple myeloma: a case series and clinical implications. BJU Int. 2008;101:712–715. doi: 10.1111/j.1464-410X.2007.07268.x. [DOI] [PubMed] [Google Scholar]
- 38.Hartley AL, Birch JM, Blair V, et al. Second primary neoplasms in a population-based series of patients diagnosed with renal tumours in childhood. Med Pediatr Oncol. 1994;22:318–324. doi: 10.1002/mpo.2950220504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier curves to illustrate the effect of specific factors that significantly impact survival in patients with Renal cell carcinoma (irrespective of the presence or absence of a second primary malignancy). The numbers of patients at risk in each group are summarized in Table 4.
Site specific risk of second primary cancers in males with renal cell carcinoma
Site specific risk of second primary cancers in females with renal cell carcinoma
Kaplan-Meier curves to illustrate the effect of specific factors on the overall survival of patients with renal cell carcinoma and a second primary malignancy. The numbers of patients at risk in each group are summarized in Table 6.
Site specific risk of second primary cancers in renal cell carcinoma with primary tumor ≤ 5cm in size
Site specific risk of second primary cancers in renal cell carcinomas with primary tumor 5cm to <10 cm in size
Site specific risk of second primary cancers in RCC patients who received radiation therapy
Site specific risk of second primary cancers in renal cell carcinomas patients who did not receive radiation therapy
Characteristics of patients with either RCC only (OPC) or RCC plus one or more second primary cancers (SPC) in the SEER database (17 registries limited use, updated November 2008, containing information of cases between 1973–2006)
Comparison of the risk for reciprocal occurrence of second malignancies in patients with renal cell carcinoma
Validation of the results of multivariate analysis (in Table 7) using case series published by Choueri et al. 37