Abstract
We tested the hypothesis that the assessment of lesion morphology helped to detect acute coronary syndrome (ACS) during index hospitalization among patients with acute chest pain who had a significant stenosis on coronary computed tomography angiography (CTA). Patients who presented to the emergency department with chest pain but no objective signs of myocardial ischemia (non-diagnostic ECG and negative initial biomarkers) underwent CTA. CTA was analyzed for the degree and length of stenosis, plaque area and volume, remodeling index, CT attenuation of plaque, and spotty calcium in all patients with a significant stenosis (>50% in diameter) in CTA. ACS during the index hospitalization was determined by the panel of 2 physicians blinded to results of CTA. For lesion characteristics associated with ACS, we determined cutpoints optimized for diagnostic accuracy and created lesion scores. For each score, we determined odds ratio and discriminatory capacity for the prediction of ACS. Of the overall population of 368 patients, 34 had significant stenosis and among those 21 had ACS. Score A (remodeling index+spotty calcium: OR 3.5, 95%CI 1.2–10.1, AUC 0.734), B (remodeling index+spotty calcium+stenosis length: OR 4.6, 95%CI 1.6–13.7, AUC 0.824) and C (remodeling index+spotty calcium+stenosis length+volume of <90HU plaque: OR 3.4, 95%CI 1.5–7.9, AUC 0.833) were significantly associated with ACS. In conclusion, among patients presenting with acute chest pain and with a stenosis on coronary CTA, a CT-based score incorporating morphologic characteristics of coronary lesions had a good discriminatory value for the detection ACS during index hospitalization.
Keywords: cardiac computed tomography, coronary computed tomography angiography, acute coronary syndrome, coronary atherosclerotic plaque
INTRODUCTION
In previous studies coronary computed tomography angiography (CTA) imaging of patients with acute coronary syndrome (ACS) and with stable angina demonstrated differences in the morphology of segments with significant stenosis. Morphologic characteristics such as positive remodeling, spotty calcium, larger plaque area and volume, presence of low CT (computed tomography) attenuation plaque, and appearance of peripheral contrast rim were more frequent in patients with ACS.1–5 We studied whether the detection of previously identified characteristics of culprit lesions in patients with acute chest pain who have a significant stenosis on coronary CTA in the absence of objective evidence of myocardial ischemia or necrosis at the initial evaluation would permit discrimination of those with and without ACS during index hospitalization.
METHODS
A description of the patient population in the present study was reported recently.6 A convenience sample of low- to intermediate-risk patients presenting to the emergency department with a chief complaint of chest discomfort and clinical suspicion for ACS, but who had normal initial troponin and an initial ECG without evidence of myocardial ischemia, was enrolled. ACS was defined as either an acute myocardial infarction or unstable angina pectoris during index hospitalization according to the AHA/ACC/ESC guidelines.7,8 Unstable angina pectoris was defined as clinical symptoms suggestive of ACS (typical chest discomfort or equivalent with an unstable pattern of chest pain, i.e. at rest, new onset, or crescendo angina) in the absence of elevated troponin, coinciding with appropriate objective evidence of myocardial ischemia in stress perfusion imaging or coronary angiography demonstrating a >50% coronary stenosis. To establish the diagnosis of ACS, an outcome panel of 2 experienced physicians blinded to CT findings reviewed patient data. Disagreement was resolved by consensus, which included an additional senior cardiologist. We collected information on cardiovascular risk profile and Thrombolysis in Myocardial Infarction (TIMI) risk score. For the purposes of the present analysis, we included all patients with a definitive significant stenosis (>50% in diameter) on coronary CTA by qualitative assessment. The Institutional Review Board approved the study.
All patients provided written informed consent. All patients were scanned using a 64-row CT scanner (Sensation 64; Siemens Medical Solutions, Forchheim, Germany). Intravenous beta-blocker (metoprolol, 5–20 mg) was administered in all patients with a heart rate >60 beats per minute. All patients received 0.6 mg of sublingual nitroglycerin. Contrast agent (Iodhexodol 320 g/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ, USA) was injected at a rate of 5 mL/s. The scan parameters were: 64×0.6 mm detector configuration, 330 ms gantry rotation time, 120 kV tube potential, 850 mAs effective tube current-time product, and ECG-based tube current modulation. Axial images were reconstructed with a slice thickness of 0.75 mm using a retrospectively ECG-gated reconstruction.
Coronary CTA datasets were transferred to an offline workstation (Vitrea, Vital Images, Minnetonka, MN). An independent reader (>3 years cardiac CT experience) reviewed all studies and selected the series in cardiac phase with the best image quality in each subject for further analysis. The reader determined the culprit lesion in each patient with ACS using previously described rules.1 In patients with ACS and 1 significant lesion, this lesion was considered the culprit lesion. In patients with ACS and multiple significant lesions, we used information from diagnostic testing (ECG, nuclear perfusion imaging, invasive coronary angiography) to determine the culprit vessel. If there were >1 significant lesions in this vessel, the lesion with the most severe stenosis was analyzed. In patients without ACS, the lesion with the most severe stenosis by CTA was analyzed.
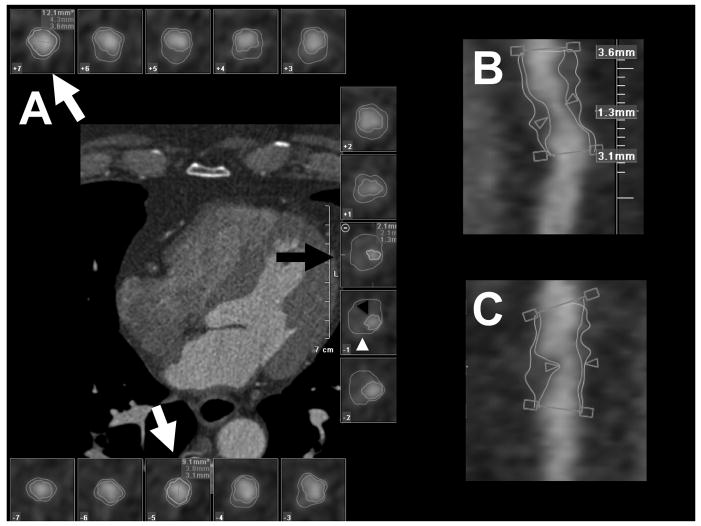
Two independent readers blinded to the demographic information as well as the presence of ACS evaluated the CT data set for characteristics of significant lesions. Each vessel containing a significant stenosis was analyzed in curved multiplanar reformat images in long axis and cross-sectional views. For each lesion, the proximal and distal reference were determined (Figure 1). We used a semi-automated software (Vessel Probe, Vitrea, Vital Images, Minnetonka, MN) to segment lumen and outer vessel boundaries in all coronary artery cross-sections between the proximal and distal reference (Figure 1). If necessary, the boundaries were manually adjusted. The lumen boundary was defined as the transition between high attenuation coronary lumen filled with the contrast agent and the intermediate attenuation of the non-calcified atherosclerotic plaque or the high attenuation of calcified plaque. The outer vessel boundary was defined as the transition between the intermediate attenuation on the non-calcified plaque or the high attenuation of the calcified plaque and the low attenuation of the perivascular fat.
Figure 1.
Vessel Analysis. Panel A shows a series of vessel cross-sections created from a curved multiplanar reformatted image. Lumen (b lack arrowhead) and outer vessel (white arrowhead) boundaries were created with a semi-automated software. Lumen and outer vessel diameters were measured at the site of stenosis (black arrow) and at the proximal and distal references (white arrows). Panels B and C show curved multiplanar reformatted images in long axis with the depiction of the stenosis, proximal and distal reference.
The diameters at the site of the maximum stenosis and at the proximal and distal references were measured (Figure 1). The degree of stenosis was calculated as the ratio of the difference between the diameter at the maximum stenosis and the mean of the diameters at the proximal and distal references divided by the mean of the diameters at the proximal and distal references and expressed as percentage. The remodeling index was calculated as the outer vessel area at the site of the maximum stenosis divided by the mean of the outer vessel areas at the proximal and distal references. Positive remodeling was defined as a remodeling index of >1.05.1,9,10 The plaque length was measured as the length of the coronary segment between the proximal and distal reference in the curved multiplanar reformat images. The stenosis length was measured as the length of the visually significantly narrowed segment in the curved multiplanar reformat images.
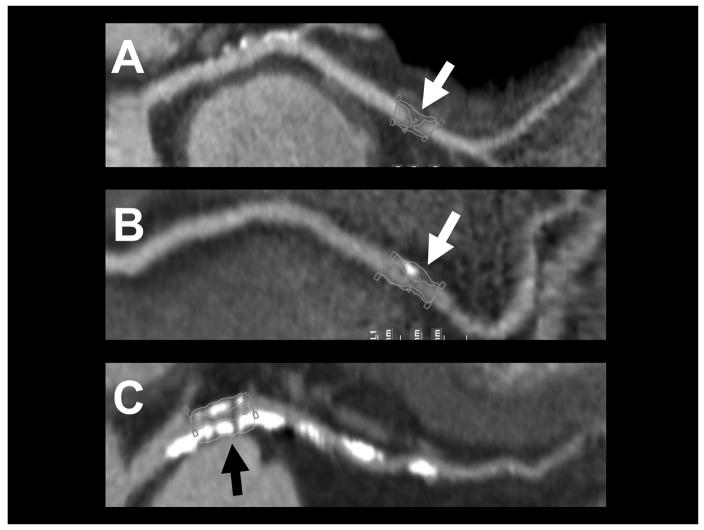
The plaque volume was automatically calculated as the volume of all voxels segmented between the luminal and outer vessel boundaries in curved multiplanar reformatted images. The proximal and distal references were used as the proximal and distal ends of the plaques. We reported the total volume of plaque and the volumes of plaques in the range of <30 HU, <90 HU, 90–150 HU, and >150 HU.2,4,11–13 The composition of the plaque was assessed visually and categorized as calcified and non-calcified plaques as described previously (Figure 2).14 In the presence of coronary calcium, calcified plaques were further characterized as spotty calcium (discrete calcified nodules clearly surrounded by non-calcified plaque, <3 mm in diameter) and heavy calcium (confluent calcium occupying most of the vessel wall) (Figure 2).2
Figure 2.
Plaque Composition. Coronary plaques were classified as non-calcified (Panel A), with spotty calcium (Panel B) and heavy calcium (Panel C)
Continuous variables are reported as mean (standard deviation) or median (interquartile range) and categorical variables as percentage (counts). Wilcoxon signed-rank test, and Fishers exact test were used to assess for differences within continuous and categorical variables. We calculated Pearson’s correlation coefficient to assess the agreement of the measurements between 2 observers.
In univariate analysis, characteristics of the stenosis and plaque were individually evaluated for association with ACS. We generated 3 scores (Score A, B and C) based on the characteristics of the stenosis and plaque. Score A consisted of the 2 features previously shown to be associated with culprit lesions in ACS: positive remodeling index and spotty calcium.1–3 For additional lesion scores continuous variables associated with the presence of the ACS at a significance level of <0.10 in univariate analysis were added according to their AUC (area under the receiver-operating characteristics curve) values in univariate analysis in the descending order. The variables were dichotomized by using the cut-points with the best discriminatory capacity (highest AUC) for ACS. The presence of each plaque feature increases the score by 1 point.
The discriminatory capacity of the scores for the prediction of ACS was assessed using c-statistics.15 The asymptotic 95% confidence intervals (95%CI) for the AUC were estimated using a nonparametric approach which is closely related to the jackknife technique.16 Logistic regression analysis was performed to calculate odds ratios with 95%CI for the diagnosis of ACS by each score. To assess the internal validity of ACS prediction using the model scores and to adjust for over-fitting/optimism, bootstrap resampling procedures were used. One hundred bootstrap samples were drawn with replacement from the original data set. The difference of AUC from the original dataset and from the bootstrap sample (mean AUC) represented an estimate of the optimism in the apparent performance. The optimism was subtracted from the apparent performance to estimate the internally validated performance and adjust for over-fitting/optimism.
Finally, we used 2×2 tables to calculate the diagnostic accuracy (sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of single score values for the presence of ACS and provided binomial 95%CI. Pre-test probability of ACS was defined as the prevalence of ACS within the cohort (21/34). The post-test probability of ACS for all lesion scores was determined using Baye’s theorem (pretest odds×positive likelihood ratio=post-test odds of ACS). All performed tests were 2-sided and a p-value <0.05 was considered as statistically significant. All analyses were performed with SAS (version 9.2, SAS Institute Inc., Cary, NC USA).
RESULTS
The mean age of the entire ROMICAT trial population was 53 (12) years; 223 (61%) were male. Of the overall study population, 31 (8%) were judged to have ACS. Overall, 34/368 patients (9%) had a definite coronary stenosis (>50% in diameter) by coronary CTA. Of those 62% (n=21/34) had ACS (acute myocardial infarction n=5, unstable angina pectoris n=16). In the group of patients with ACS, 13 patients underwent cardiac catheterization, 12 patients underwent percutaneous coronary intervention and 1 patient underwent coronary artery by-pass surgery. Baseline demographics were similar in patients with and without ACS except for lower body mass index in patients with ACS (Table 1). There was also a trend towards less diabetes mellitus in ACS group. TIMI score was similar in patients with and without ACS.
Table 1.
Baseline demographics and TIMI risk score of patients with a significant coronary stenosis, separated by presence of acute coronary syndrome during the index hospitalization
| Variable | Acute Coronary Syndrome
|
||
|---|---|---|---|
| Yes (n=21) | No (n=13) | p-value | |
| Age (years) | 61 (51–67) | 58 (49–65) | 0.97 |
| Men | 18 (86%) | 11 (85%) | 1.00 |
| Body mass index (kg/m2) | 27 (25–30) | 31 (28–33) | 0.05 |
| Hypertension (blood pressure ≥140/90 mmHg or being on antihypertensive treatment) | 14 (67%) | 11 (85%) | 0.43 |
| Hyperlipidemia (total cholesterol ≥200 mg/dL or being on lipid lowering treatment) | 11 (52%) | 8 (62%) | 0.73 |
| Diabetes mellitus | 3 (14%) | 6 (46%) | 0.06 |
| TIMI score | 2 (1–2) | 1 (1–2) | 0.51 |
TIMI – Thrombolysis in Myocardial Infaction
Among 21 patients with ACS, 13 had a single stenosis and 9 had >1 significant stenosis. Among 13 patients without ACS, 10 had a single stenosis and 3 had >1 significant stenosis. The distribution of analyzed lesions was as follows: left anterior descending coronary artery 11 vs. 7, left circumflex coronary artery 3 vs. 1, right coronary artery 7 vs. 5 for ACS vs. no ACS; respectively.
Table 2 describes the characteristics of the coronary lumen and plaque. The degree of stenosis was similar in patients with and without ACS, but the length of the stenosis was significantly greater among those with ACS. The length of the coronary plaque and plaque cross-sectional area at the site of the stenosis were not different between patients with and without ACS. The volume of plaque with CT attenuation <90 HU was larger in patients with ACS. There was also a trend toward larger volume of plaque with CT attenuation <30 HU. Positive remodeling (remodeling index>1.05) was more frequent in patients with ACS. There was a trend toward higher frequency of spotty calcium in patients with ACS. There was a good correlation for the quantification of stenosis (R2=0.71) and plaque volumes (total: R2=0.83, <90 HU: R2=0.77, 90–150 HU: R2=0.91, >150 HU: R2=0.85) between 2 observers.
Table 2.
Computed tomography characteristics of lesions in patients with acute coronary syndrome vs. patients without acute coronary syndrome
| Computed tomography characteristics of stenosis and plaque | Acute Coronary Syndrome
|
||
|---|---|---|---|
| Yes (n=21) | No (n=13) | p-value | |
|
Stenosis
| |||
| Degree of the Stenosis (%) | 73 (65–83) | 66 (56–85) | 0.64 |
| Length of the Stenosis (mm) | 5.4 (3.5–7.7) | 4.2 (3.0–4.5) | 0.02 |
|
Plaque
| |||
| Length of Plaque (mm) | 15.2 (11.0–21.6) | 13.8 (11.9–18.7) | 0.67 |
| Plaque Area (mm2) | 13.7 (10.5–17.1) | 10.0 (6.5–18.1) | 0.11 |
| Positive Remodeling Index (> 1.05) | 13 (62%) | 2 (15%) | 0.04 |
| Total Plaque volume (mm3) | 212 (126–264) | 171 (78–223) | 0.24 |
| Plaque Volume by attenuation (mm3) | |||
| <30 HU | 21.3 (12.9–29.4) | 12.8 (9.3–15.0) | 0.07 |
| <90 HU | 90.6 (51.3–108.6) | 48.7 (40.3–74.5) | 0.03 |
| 90–150 HU | 44.0 (29.1–59.3) | 36.4 (22.1–41.7) | 0.11 |
| >150 HU | 38.8 (23.0–111.2) | 57.1 (7.8–139.7) | 0.87 |
| Calcium | |||
| None | 5 (24%) | 5 (38%) | 0.45 |
| Spotty | 10 (48%) | 2 (15%) | 0.08 |
| Heavy | 6 (29%) | 6 (46%) | 0.29 |
HU – Hounsfield units
We generated 3 lesions scores. Score A contained positive remodeling and spotty calcium. Score B was generated by adding the variable with the highest AUC in the univariate analysis (stenosis length, cut-point 4.5 mm; AUC 0.800) to score A. Score C was generated by adding the variable with the second highest AUC in univariate analysis (low density (<90HU) plaque volume, cut-point 80 mm3; AUC 0.709) to Score B. The odds ratio per lesion score unit for predicting ACS ranged from 3.4 (Score C) to 4.6 (Score B) and all scores were significantly associated with ACS (p<0.05; Table 3).
Table 3.
The odds for predicting acute coronary syndrome for 3 lesion scores
| Variable | Score A | Score B | Score C |
|---|---|---|---|
| Spotty Calcium | X | X | X |
| Positive Remodeling Index | X | X | X |
| Length of the Stenosis, >4.5mm | X | X | |
| Low density (<90HU) Plaque Volume, >80mm3 | X | ||
|
| |||
| Odds Ratio per Score Unit for Predicting ACS | 3.5 | 4.6 | 3.4 |
| 95% Confidence Interval | 1.2 – 10.1 | 1.6 – 13.7 | 1.5 – 7.9 |
| p-value | 0.02 | 0.005 | 0.004 |
HU – Hounsfield units
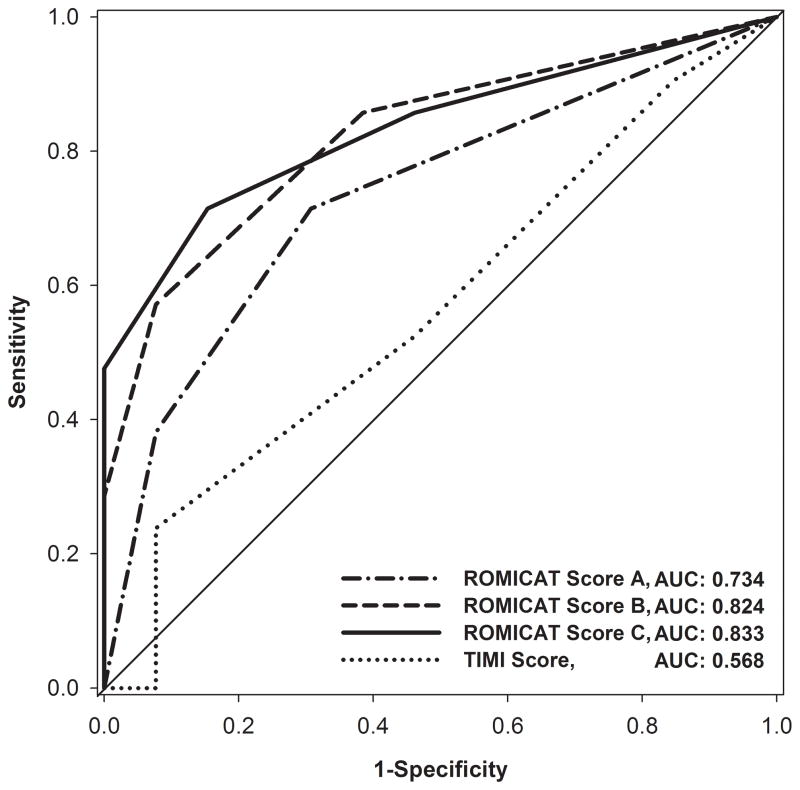
Figure 3 depicts AUC for the 3 lesion scores. Score A had the lowest discriminatory value (AUC 0.734, 95% CI 0.575–0.894). The addition of stenosis length and low CT attenuation plaque volume resulted in an improvement of the discriminatory value for ACS (Score B [AUC 0.824, 95% CI 0.692–0.956] vs. Score A, p = 0.03; Score C [AUC 0.833, 95% CI 0.704–0.963] vs. Score A, p=0.05). Assessing internal validity of the models, we derived low values for optimism (means range from 0.003 to 0.014 and standard deviations range from 0.062 to 0.077) and calculated the optimist corrected AUC of 0.737, 0.811 and 0.829 for Scores A, B and C, respectively.
Figure 3.
The prognostic discriminatory capacity of lesion scores A, B, and C as well as of TIMI score for the prediction of acute coronary syndrome.
AUC – area under the curve
TIMI score did not have a good predictive value for ACS (AUC 0.568, 95% CI 0.374–0.761). Score A was not better than TIMI score for the prediction of ACS (p=0.26). The addition of further lesion characteristics in Score B (p=0.04) and C (p=0.03) resulted in significantly better prediction of ACS as compared to TIMI score. The degree of stenosis was not a good predictor of ACS (AUC 0.551, 95% CI 0.330–0.772). Score B (p=0.02) and C (p=0.01) were better predictors of ACS as compared to stenosis.
The diagnostic performance of the lesion scores for the prediction of ACS is summarized in Table 4. Assuming a pre-test probability of 62%, the post-test probability of ACS using Score A is 89% if both spotty calcium and positive remodeling are present. Post-test probability of ACS is 85% if 2 characteristics and 100% if all 3 characteristics are present in Score B. Similarly, post-test probability of ACS is 100% if ≥3 characteristics are present in Score C. If <3 characteristics are present in Score C, post-test probability of ACS is decreased to 41%. The post-test probability of ACS is further decreased to 31% if <2 characteristics are present in Score C.
Table 4.
The summary of diagnostic accuracies (sensitivity, specificity, positive predictive value and negative predictive value) with 95% confidence interval of lesion scores for the prediction of acute coronary syndrome at various cutpoints.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Score A | ||||
| ≥ 1 | 71% [48–89] | 69% [29–91] | 79% [54–94] | 60% [32–84] |
| = 2 | 38% [18–62] | 92% [64–100] | 89% [52–100] | 48% [25–69] |
| Score B | ||||
| ≥ 1 | 86% [64–97] | 62% [32–86] | 78% [56–96] | 73% [39–94] |
| ≥ 2 | 57% [34–78] | 92% [64–100] | 92% [64–100] | 57% [34–78] |
| = 3 | 29% [11–52] | 100% [75–100] | 100% [54–100] | 46% [28–66] |
| Score C | ||||
| ≥ 1 | 86% [64–97] | 54% [25–81] | 75% [53–90] | 70% [35–93] |
| ≥ 2 | 71% [48–89] | 85% [55–98] | 88% [64–99] | 65% [38–86] |
| ≥ 3 | 48% [26–70] | 100% [75–100] | 100% [69–100] | 54% [33–74] |
| = 4 | 24% [8–47] | 100% [75–100] | 100% [48–100] | 45% [26–64] |
NPV – negative predictive value
PPV – positive predictive value
DISCUSSION
We demonstrated that various CT-based morphologic features of plaque (positive remodeling, spotty calcium, stenosis length, low attenuation plaque volume) were associated with the presence of ACS in patients with acute chest pain and a significant coronary stenosis on CT, but without objective signs of myocardial ischemia or necrosis at the time of CT performed before the hospital admission. We established cut-points for 2 new quantitative measurements: lesion length (4.5 mm) and volume of low attenuation plaque <90HU (80 mm3) optimized for the detection of ACS. Further, we demonstrated the discriminatory value of 3 scores incorporating previously reported features of lesions (spotty calcium and positive remodeling, AUC: 0.734) and new quantitative features of lesion morphology (length of stenosis and volume of low HU attenuation (<90HU), AUC 0.824 to 0.833). Overall, these data suggest that detailed assessment of plaque characteristics may permit guidance of further management in patients with significant stenosis, but no objective signs of myocardial ischemia. A major strength of this study is that, although small, this was a unique data set as the caregivers were blinded to the CT data and so the natural history of these lesions could be assessed. Given the clinical use of CT nowadays such data may not be acquired again.
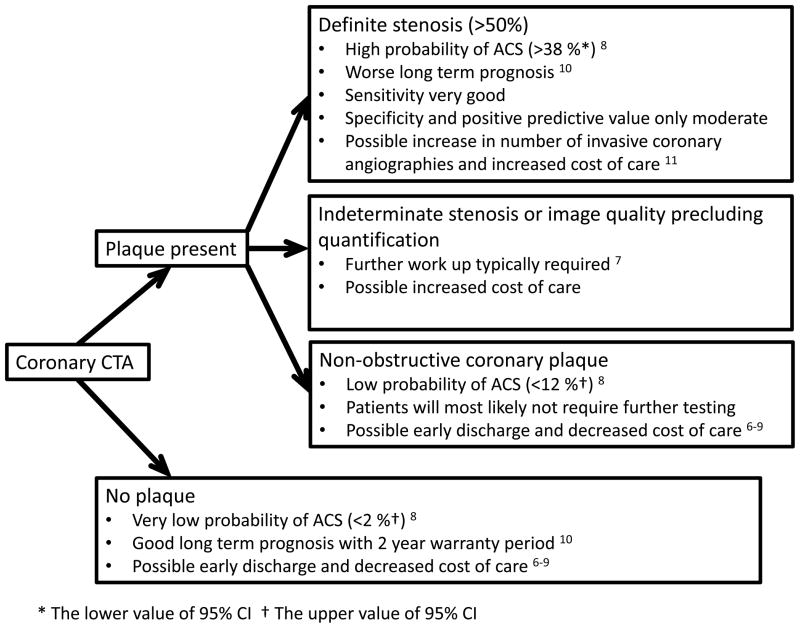
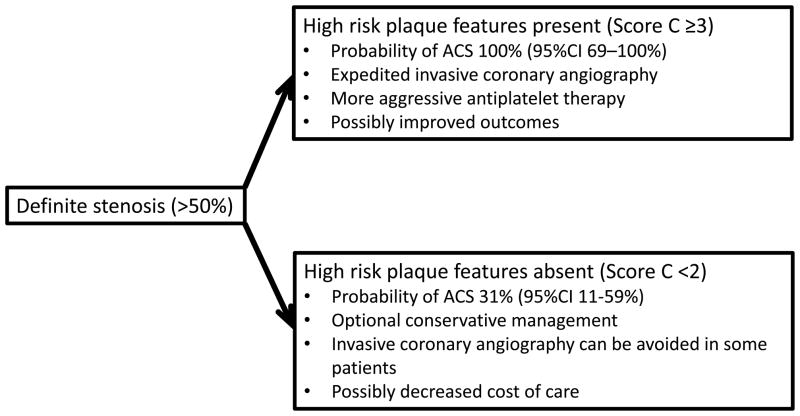
A non-invasive alternative to identify culprit lesions in ACS before objective signs of myocardial ischemia become evident could significantly improve patient management. CT is a non-invasive imaging modality, which can provide information about the severity of coronary stenosis as well as morphologic features and composition of coronary plaques. Observational studies established the feasibility of CT-based differentiation of culprit lesions of ACS from non-culprit lesions in ACS and from stable angina lesions.1–5,13,17 ACS patients had a higher number of non-calcified plaques, larger plaque area, higher remodeling indices, larger plaque volume, lower CT attenuation and peripheral contrast rim.2,3,10 Further, the presence of both positive remodeling and low CT attenuation plaques at the baseline was associated with a 22-times increased relative risk of ACS during an average follow-up of 27 months.13 In our study, we prospectively validated previously described lesion characteristics (positive remodeling and spotty calcium) in prediction of ACS in patients with chest pain and significant stenosis by CT. We further extended the knowledge by 1) assessing new features of plaque such as stenosis length, 2) proposing a scoring system that increases diagnostic accuracy of individual morphological features, and 3) applying the findings to enhance patient management in a clinically challenging situation: when a significant stenosis is detected in a patient with acute chest pain, but there is no objective evidence of ischemia or necrosis (ischemic ECG changes, cardiac biomarker) at the time of coronary CTA. This situation mandates a careful consideration of next steps and whether to treat this patient as an ACS despite the absence of ischemia/necrosis or as no ACS despite the presence of significant stenosis (Figure 4). We demonstrated that lesion characteristics are not only associated with ACS (i.e. the presence of both spotty calcium and positive remodeling increases the likelihood of ACS by a factor of 7), but also can be used to prospectively differentiate between patients with and without ACS in a population of patients with a significant stenosis on coronary CTA. Therefore, patients with a relatively low risk of ACS as determined by clinical characteristics (electrocardiogram, cardiac biomarkers and TIMI score), but with high risk features by CT may warrant aggressive treatment, including dual antiplatelet therapy and early cardiac catheterization (Figure 5). On the other hand, patients with a stenosis, but low risk plaque features can be observed and treated less aggressively, and possibly with a decreased cost of care (Figure 5).
Figure 4.
Findings on coronary CTA and the probability of acute coronary syndrome from ROMICAT trial with possible effects for the further management of patients with acute chest pain
ACS – acute coronary syndrome
CTA – computed tomography angiography
Figure 5.
The change in the probability of acute coronary syndrome based on the results of CT-based lesion score in ROMICAT trial
ACS – acute coronary syndrome
The addition of the stenosis length and low CT attenuation volume to the lesion scores resulted in improved discriminatory capacity of the scores as determined by higher AUC. The combination of multiple plaque features improved significantly diagnostic accuracy for ACS. Motoyama et al. showed the association of low attenuation within the plaque (<30 HU) along with positive remodeling and spotty calcium with ACS.2 We replicated these results and extended the observation to the volume of low attenuation plaque (<30 HU). We found that the volume of plaque with attenuation <90 HU was a stronger predictor of the presence of ACS in our population. CT number measurements within non-calcified plaque are affected by luminal contrast enhancement, image noise, reconstruction and image artifacts.18,19 Plaque volume below a certain threshold rather than the measurement of CT number in a small region of interest may serve as a more robust measurement across various populations.12 Future studies will determine the most accurate definition of “low attenuation plaque” with respect to ACS and culprit lesions.
Stenosis length has not been previously reported in CT literature as a predictor of ACS. In a small study, Kristensen et al. demonstrated that lesion length by CT correlated with a physiologic assessment of stenosis severity by fractional flow reserve.20 Further, lesion length measurement improved correlation between anatomic stenosis severity and functional assessment by fractional flow reserve in stenoses deemed intermediate (50%-70%) by invasive coronary angiography.21 These findings suggest that lesion length has a impact on the physiological significance of intermediate stenoses and may provide an explanation for our observation of association between stenosis length and ACS.
Our study is limited by the small sample size and thus our results warrant further validation in a larger population. Nevertheless, the good internal validity of ACS detection using the model scores was confirmed by the bootstrap resampling procedure. Patients with ACS had lower BMI and a trend toward less diabetes. There were only 5 patients with non-ST elevation myocardial infarction. The sample size calculation was performed for the ROMICAT trail and there was no sample size calculation for our study. The gold standard for the determination of culprit lesions (invasive coronary angiography) was available in only 38% of patients. Further, ACS was diagnosed in 8 patients based on nuclear imaging. This may be a source of bias as nuclear perfusion imaging may have false positive results. The results of our study are not applicable to the minority of patients with ACS that do not have a significant coronary stenosis.
Acknowledgments
This work was supported by the National Institutes of Health (RO1 HL080053) and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Drs. Ferencik, Rogers, Truong and Ghoshhajra were supported by the National Institutes of Health grant (T32 HL076136). Dr. Hoffmann has received research grants from Siemens Medical Solutions and General Electrics Healthcare. Dr. Nagurney is funded by Biosite for a biomarker research study.
Footnotes
There are no other conflicts of interest with regard to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, Dohi Y, Kunita E, Utsunomiya H, Kohno N, Kihara Y. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2:153–160. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Pflederer T, Marwan M, Schepis T, Ropers D, Seltmann M, Muschiol G, Daniel WG, Achenbach S. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis. 2010;211:437–444. doi: 10.1016/j.atherosclerosis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Madder RD, Chinnaiyan KM, Marandici AM, Goldstein JA. Features of disrupted plaques by coronary computed tomographic angiography: correlates with invasively proven complex lesions. Circ Cardiovasc Imaging. 2011;4:105–113. doi: 10.1161/CIRCIMAGING.110.957282. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC., Jr ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 8.Gibler WB, Cannon CP, Blomkalns AL, Char DM, Drew BJ, Hollander JE, Jaffe AS, Jesse RL, Newby LK, Ohman EM, Peterson ED, Pollack CV. Practical implementation of the Guidelines for Unstable Angina/Non-ST-Segment Elevation Myocardial Infarction in the emergency department. Ann Emerg Med. 2005;46:185–197. doi: 10.1016/j.annemergmed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Schmid M, Achenbach S, Ropers D, Komatsu S, Ropers U, Daniel WG, Pflederer T. Assessment of changes in non-calcified atherosclerotic plaque volume in the left main and left anterior descending coronary arteries over time by 64-slice computed tomography. Am J Cardiol. 2008;101:579–584. doi: 10.1016/j.amjcard.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Kataiwa H, Komukai K, Tanimoto T, Takemoto K, Takarada S, Kubo T, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009;2:1412–1419. doi: 10.1016/j.jcmg.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Pohle K, Achenbach S, Macneill B, Ropers D, Ferencik M, Moselewski F, Hoffmann U, Brady TJ, Jang IK, Daniel WG. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: Comparison to IVUS. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhbäck A, Ropers D, Daniel WG, Achenbach S. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011;215:110–115. doi: 10.1016/j.atherosclerosis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 14.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill BD, Pohle K, Baum U, Anders K, Jang IK, Daniel WG, Brady TJ. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography. A segment-based comparison to intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 15.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Leber AW, Knez A, White CW, Becker A, von Ziegler F, Muehling O, Becker C, Reiser M, Steinbeck G, Boekstegers P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol. 2003;91:714–718. doi: 10.1016/s0002-9149(02)03411-2. [DOI] [PubMed] [Google Scholar]
- 18.Cademartiri F, Maffei E, Palumbo AA, Malago R, La Grutta L, Meiijboom WB, Aldrovandi A, Fusaro M, Vignali L, Menozzi A, Brambilla V, Coruzzi P, Midiri M, Kirchin MA, Mollet NR, Krestin GP. Influence of intra-coronary enhancement on diagnostic accuracy with 64-slice CT coronary angiography. Eur Radiol. 2008;18:576–583. doi: 10.1007/s00330-007-0773-0. [DOI] [PubMed] [Google Scholar]
- 19.Achenbach S, Boehmer K, Pflederer T, Ropers D, Seltmann M, Lell M, Anders K, Kuettner A, Uder M, Daniel WG, Marwan M. Influence of slice thickness and reconstruction kernel on the computed tomographic attenuation of coronary atherosclerotic plaque. J Cardiovasc Comput Tomogr. 2010;4:110–115. doi: 10.1016/j.jcct.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen TS, Engstrom T, Kelbaek H, von der Recke P, Nielsen MB, Kofoed KF. Correlation between coronary computed tomographic angiography and fractional flow reserve. Int J Cardiol. 2010;144:200–205. doi: 10.1016/j.ijcard.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Brosh D, Higano ST, Lennon RJ, Holmes DR, Jr, Lerman A. Effect of lesion length on fractional flow reserve in intermediate coronary lesions. Am Heart J. 2005;150:338–343. doi: 10.1016/j.ahj.2004.09.007. [DOI] [PubMed] [Google Scholar]