Abstract
Cell based tissue engineering is limited by the size of cell-containing constructs that can be successfully cultured in vitro. This limit is largely a result of the slow diffusion of molecules such as oxygen into the interior of three dimensional scaffolds in static culture. Bioreactor culture has been shown to overcome these limits. In this study we utilize a tubular perfusion system (TPS) bioreactor for the three dimensional dynamic culture of human mesenchymal stem cells (hMSCs) in spherical alginate bead scaffolds. The goal of this study is to examine the effect of shear stress in the system and then quantify the proliferation and differentiation of hMSCs in different radial annuli of the scaffold. Shear stress was shown to have a temporal effect on hMSC osteoblastic differentiation with a strong correlation of shear stress, osteopontin and bone morphogenic protein-2 occurring on day 21, and weaker correlation occurring at early timepoints. Further results revealed an approximate 2.5 fold increase in cell number in the inner annulus of TPS cultured constructs as compared to statically cultured constructs after 21 days. This result demonstrated a nutrient transfer limitation in static culture which can be mitigated by dynamic culture. A significant increase (p < 0.05) in mineralization in the inner and outer annuli of bioreactor cultured 4 mm scaffolds occurred on day 21 with 79 ± 29% and 53 ± 25% mineralization area respectively compared to 6 ± 4% and 19 ± 6% mineralization area respectively in inner and outer annuli of 4 mm statically cultured scaffolds. Surprising lower mineralization area was observed in 2 mm bioreactor cultured beads which had the highest levels of proliferation. These results may demonstrate a relationship between scaffold position and stem cell fate. In addition the decreased proliferation and matrix production in statically cultured scaffolds compared to bioreactor cultured constructs demonstrate the need for bioreactor systems and the effectiveness of the TPS bioreactor in promoting hMSC proliferation and differentiation in three-dimensional scaffolds.
Keywords: Bioreactor, Tissue Engineering, Signal Expression, Bone, Cell Signaling
Introduction
Tissue engineering exists as a promising treatment for bone injuries that fail to heal through endogenous repair mechanisms however in vitro culture of three dimensional tissue engineering constructs remains a challenge. Bioreactor systems are an important technology to improve this in vitro culture and increase the feasibility of cell based tissue engineering strategies. Many different bioreactors systems have been previously studied for bone tissue engineering including spinner flasks (Kim et al. 2007; Meinel et al. 2004; Sikavitsas et al. 2002; Wang et al. 2009), rotating wall bioreactors (Botchwey et al. 2001; Sikavitsas et al. 2002), and perfusion systems (Bancroft et al. 2003; Grayson et al. 2010; Sikavitsas et al. 2003). In this study we utilize a tubular perfusion system (TPS) bioreactor, a simple, modular bioreactor system, which has previously been demonstrated to enhance proliferation and late osteoblastic differentiation of human mesenchymal stem cells (hMSCs) (Yeatts and Fisher 2011b).
In traditional static culture of three dimensional scaffolds nutrient gradients arise leading to non homogenous cell growth and differentiation (Volkmer et al. 2008). Bioreactor systems enhance cell culture through the creation of a dynamic environment that delivers oxygen and nutrients to cells while exposing them to shear stress. Advantages to using bioreactors for the culture of three dimensional scaffolds are two fold. First through increased nutrient transfer bioreactor systems can support increased cell growth. Cell death can be observed on the interior of scaffolds as little as 200 µm from the scaffold surface (Gomes et al. 2003; Ishaug et al. 1997; Martin et al. 2004; Volkmer et al. 2008; Yu et al. 2004). This cell death occurs as oxygen and other nutrients do not diffuse into scaffolds quickly enough to replace those being used in cell respiration. Lack of oxygen (hypoxia) and lack of nutrients limit both the ability of cells to survive and to differentiate, which leads to an nonhomogeneous cell and matrix distribution (Malda et al. 2007). Bioreactor culture has been used to overcome these limitations (Rodrigues et al. 2011; Yeatts and Fisher 2011a). Second, bioreactors typically expose cells to fluid shear stress, an important stimulus for hMSC osteoblastic cell differentiation (Gomes et al. 2003; Holtorf et al. 2005; Janssen et al. 2010; Kapur et al. 2003; Kreke et al. 2005; Li et al. 2009; McCoy and O'Brien 2010; Sikavitsas et al. 2005; Stolberg and McCloskey 2009; Yeatts and Fisher 2011a).
While bioreactors can regulate shear stress and oxygen concentration to some extent, it is possible exposure varies between cells in different regions of the scaffold. Differing oxygen and shear levels could influence stem cell proliferation and differentiation. While shear is commonly thought to stimulate osteoblastic differentiation, the role of oxygen is less clear. Low oxygen and nutrient levels lead to significant cell death (Potier et al. 2007b) however hMSCs cultured at 2% oxygen levels had increased proliferation and differentiation levels compared to hMSCs cultured at 20% oxygen (Grayson et al. 2007). Other studies found low oxygen levels to have inhibitory effects on osteoblastic differentiation (Iida et al. 2010; Malda et al. 2007; Potier et al. 2007a; Utting et al. 2006).
Based on the influence of oxygen and shear on stem cell development we investigate in this study how spatial positioning of hMSCs within a scaffold influences cell fate. To this end the study first aims to evaluate the effect of shear stress on osteoblastic differentiation in the TPS bioreactor. Second the study aims to evaluate the effect of dynamic culture on the proliferation of human mesenchymal stem cells as a function of radial distance in the scaffold. The final aim of the study is then to compare osteoblastic differentiation of cells present on the exterior annulus of the scaffold to cells on the interior annulus.
Materials and Methods
Human Mesenchymal Stem Cell Culture
Human mesenchymal stem cells (Lonza, Walkersville MD) were expanded prior to the study in control media consisting of DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco), 1.0 % v/v penicillin/ streptomycin (Gibco), 0.1 mM non essential amino acids (Gibco), and 4 mM L-glutamine (Gibco) using protocols set forth by the manufacture and previously described (Betz et al. 2010; Chen et al. 2010; Yeatts and Fisher 2011b; Yeatts et al. 2011). hMSCs were expanded on tissue culture polystyrene flasks with media changes every three days according to the manufacture’s specifications. Cells were stored in a cell culture incubator at 37°C and 5% CO2 and passaged every 6–7 days using trypsin/EDTA (Lonza). Osteogenic media was formulated as described in the literature by supplementing control media with 100 nM dexamethasone (Sigma), 10mM β-glycerophosphate, and 173 µM ascorbic acid (Sigma) (Betz et al. 2010; Chen et al. 2010).
Alginate Bead Fabrication and Cell Seeding
Alginate beads were fabricated as described previously in the literature (Yoon and Fisher 2008; Yoon et al. 2007). Alginate solution (Sigma, St. Louis MO) was sterilized via sterile filtration then mixed with a cell pellet containing hMSCs. Beads were seeded at a concentration of 4×106 cells per mL. This solution was added dropwise by syringe to a 0.10 M calcium chloride solution, in which the alginate was ionically crosslinked into beads. A 20 gauge syringe was used to create large beads and beads for shear stress study while a 30 gauge syringe was used to create small control beads. The solution was stirred for 15 minutes. The calcium chloride solution was then removed and the beads rinsed in control media. Beads were then transferred to six well plates for control groups or TPS bioreactor growth chambers for experimental groups.
Identification of Discrete Bead Layers
In order to develop a calibration curve for annuli isolation beads were immersed in 3 mL of 0.025 M EDTA. Cross sectional areas were measured at five minute time points using Image J software (NIH, Bethesda MD) of a bead based on a image taken with an Axiovert 40 CFL with filter set 23, (Zeiss, Thornwood, NY) equipped with a digital camera (Diagnostic Instruments 11.2 Color Mosaic, Sterling Heights, MI). A calibration curve was constructed in order to determine the dissolution time to divide the scaffold into two annuli equal in radius. The calibration curve had an R2 value of 0.98. Based on the calibration curve 18 minutes was determined as the dissolution time to achieve two equal radii as the dissolution value was 49.4 ± 4.9 % at 18 minutes.
hMSC Isolation from Discrete Bead Layers
In order to isolate beads from specific annuli beads were placed into a twelve-well plate and 1.0 mL of 0.025 M EDTA was added. Beads were dissolved for 18 min as determined by the calibration curve. EDTA, now with suspended cells, was removed and placed into a centrifuge tube (Figure 1). The well was washed with 1.0 mL of phosphate buffered saline (PBS). Beads were moved to a new well containing 1.0 mL of 0.025 M EDTA for the next dissolution. Centrifuge tubes were spun at 5000 xg for 5 minutes to isolate the cell pellet. To isolate cells from entire scaffold 0.025 M EDTA was added and beads were entirely dissolved and pellets isolated as previously described.
Figure 1.
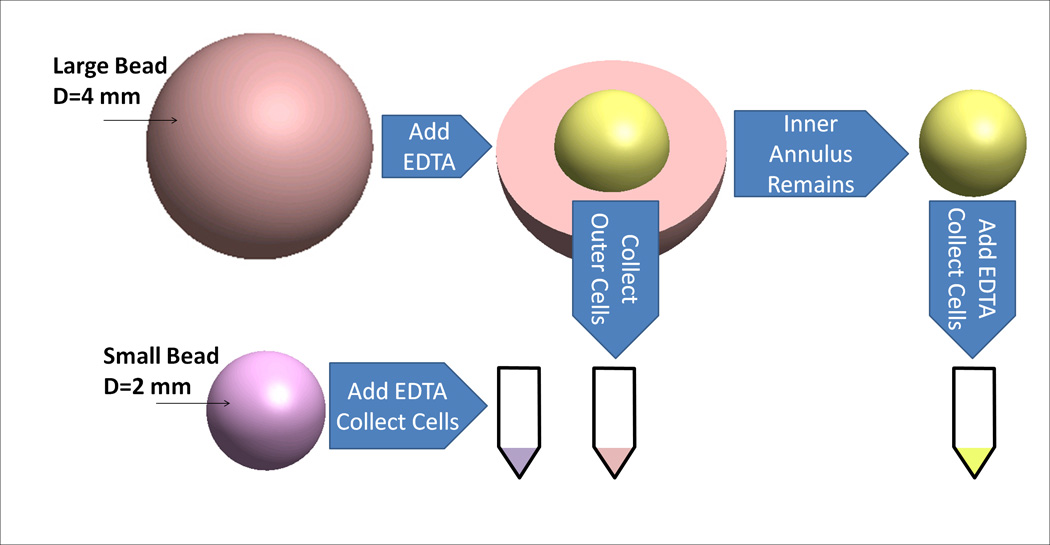
Schematic of cell removal from annuli of alginate beads. Diameters of small beads and inner annulus are equal.
Bioreactor Design
Bioreactor culture was completed in the TPS bioreactor as described previously (Yeatts and Fisher 2011b; Yeatts et al. 2011). Briefly a tubing circuit comprised primarily of platinum-cured silicone tubing (Cole Parmer, Vernon Hills, IL) and PharMed BPT tubing (Cole Parmer) for the section that passes through the pump connected a growth chamber to a media reservoir (Figure 2). The entire tubing circuit was sterilized via autoclave. The growth chamber was composed of platinum-cured silicone (ID of 1/4") and contained the tightly packed alginate scaffolds. Media was pumped through the recirculating system using a peristaltic pump (Cole Parmer) at 1.0 mL/min for annuli studies and 3.0 mL/min for shear stress studies. The entire system was placed in an incubator at 37°C for the duration of the study. Forty mL of osteogenic media was loaded into separate 125 mL Erlenmeyer flasks reservoirs for each growth chamber topped with rubber stoppers. Media was withdrawn and replaced from the reservoir through two tubes that penetrate the stopper and changed every three days.
Figure 2.
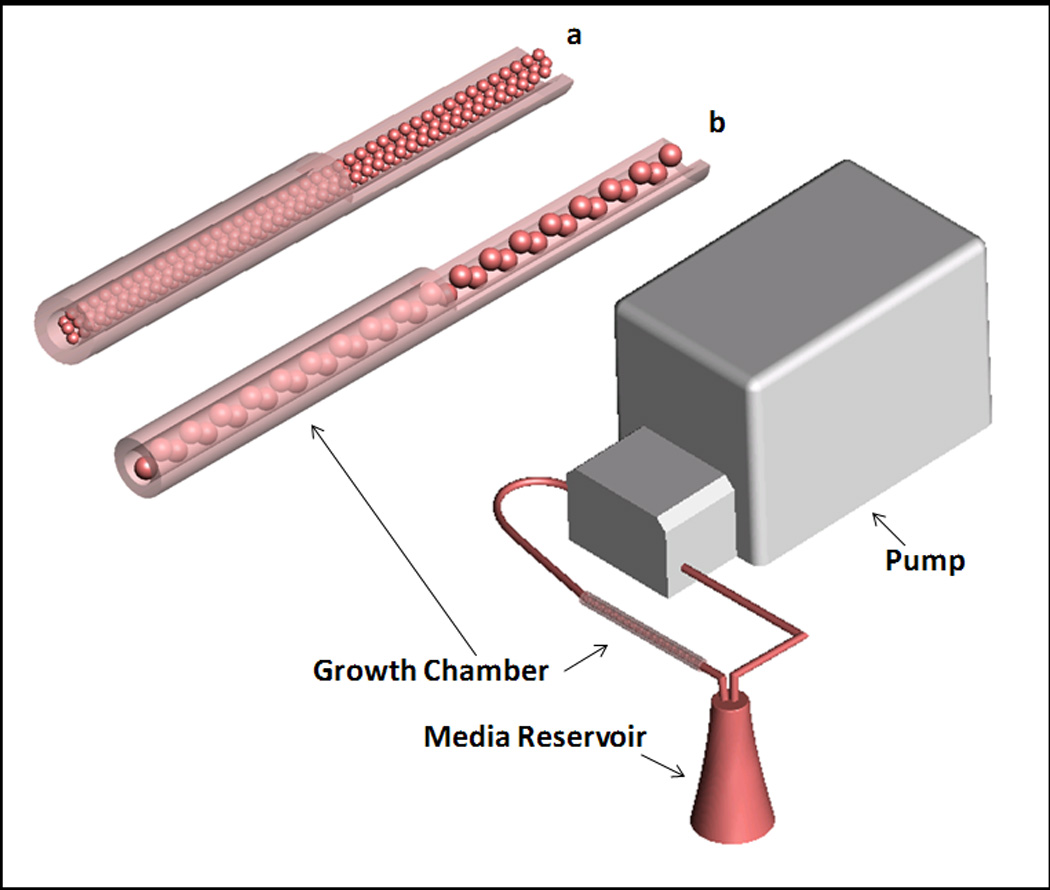
Schematic of TPS bioreactor. Bioreactor consists of media reservoir, growth chamber, and pump. Enlargement of this growth chamber can be seen with (a) small beads (2 mm diameter) and (b) large beads (4 mm diameter).
Modification of Shear Stress
In order to analyze shear stress in the system 70,000 MW dextran (Sigma) was added to the media. Dextran was chosen as a thickening agent as it has previously been shown to not affect hMSC proliferation and differentiation (Li et al. 2008; Li et al. 2009). The shear stress study consisted of three groups all cultured in osteogenic media. The first group with zero shear was cultured in static culture. The second group was cultured in the TPS bioreactor at 3 mL/min in media supplemented with 3% dextran yielding a media viscosity of 1.66 ± 0.05 mPA · S. The third group was cultured in the TPS bioreactor at 3 mL/min in media supplemented with 9% dextran yielding a media viscosity of 4.21 ± 0.03 mPA · S. Surface shear stress was then calculated using a two-dimensional steady state Navier-Stokes model of the tubular perfusion system developed using COMSOL Multiphysics Version 3.5 (COMSOL, Burlington MA) (Yeatts and Fisher 2011b).
Experimental Setup
In order to determine the effect of shear stress on hMSC osteoblastic differentiation a shear stress study was completed using the entire 4 mm bead. This study consisted of 3 groups, a static group and two bioreactor groups with dextran added as a thickening agent to change shear while not affecting nutrient transfer. The samples were analyzed for late osteoblastic marker osteopontin (OPN) at days 14 and 21 and osteogenic signaling protein bone morphogenetic protein-2 (BMP-2) on days 1, 4, 8, 14, and 21.
In order to determine hMSC growth and osteoblastic differentiation in relation to radial position the cells were isolated from the outer 2 mm radius (outer) and inner 2 mm radius (inner) of alginate beads. These beads were cultured in the TPS bioreactor and compared to a static control. In addition small beads equal to the inner bead size were cultured in the TPS bioreactor and static culture as a control. All samples were cultured in osteogenic media. These samples were analyzed at days 1, 7, 14, and 21 for ALP and mineralization and at days 7, 14, and 21 for proliferation and late osteoblastic differentiation using marker osteopontin.
Live/Dead Assay
Five beads each cultured in control media were degraded for 18 min as determined previously in order to isolate cells from discrete layers. After each time point, beads were immediately removed from the EDTA solution and immersed in live dead solution containing 2 µm ethidium homodimer and 4 µm calcein AM (Invitrogen, Carlsbad, CA) for thirty minutes. Fluorescent images were then taken using a fluorescent microscope (Axiovert 40 CFL with filter set 23, Zeiss, Thornwood, NY) equipped with a digital camera (Diagnostic Instruments 11.2 Color Mosaic, Sterling Heights, MI) for the live/dead assay, as described previously (Yeatts and Fisher 2011b; Yeatts et al. 2011).
DNA Quantification
DNA was extracted at each time point and quantified using pico green as previously described (Yeatts and Fisher 2011b). Cell pellets were isolated from the two annuli as well as from small beads cultured as a control. Isolated cell pellets were resuspended in PBS and DNA isolated using a DNeasy Tissue Kit (Qiagen, Valencia CA) following standard protocols. Double stranded DNA was then quantified using Quant-iT PicoGreen dsDNA reagent (Molecular Probes, Carlsbad, CA), incubated for five minutes in the dark and fluorescence measured using an M5 SpectraMax plate reader (Molecular Devices, Sunnyvale, CA) with excitation/emission of 480/520 nm. All samples were performed in triplicate (n=3).
Histomorphometric analysis
At each timepoint beads were collected and fixed in 4% paraformaldehyde (Sigma-Aldrich) and 0.1 M sodium cacodylate (Sigma-Aldrich) buffer containing 10mM CaCl2 at pH 7.4 for 4 hours at room temperature. Following fixation, the beads were placed in cassettes and washed with 0.1 M sodium cacodylate buffer and 10 mM CaCl2 at pH 7.4 at room temperature for 24 hours. The beads were then dehydrated for histological processing by ethanol washes followed by two citrisolv (Fisher) washes. The samples were then embedded in paraffin (Fisher) and sectioned to 5 µm thickness sections and placed on glass slides. Sections were taken from the same position in each sample for histomorphometric analysis. Sections were oven dried at 64°C for 2 hours, deparaffinized in citrisolv and rehydrated in ethanol. Von Kossa staining was performed to visualize mineralization using a Nuclear Fast Red (Poly Scientific, Bay Shore, NY) counterstain using standard protocols.
For histomorphometric analysis images the entire sample were taken using the Axiovert 40 CFL microscope equipped with a digital camera. Images were divided into inner and outer annuli for large beads using diameter measurements. Using Image J 1.44p (NIH, Bethesda MD) images were converted to binary where the dark Von Kossa stain represented mineralized area. Black area was calculated as percent of total area to represent mineralization percent.
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
Cell Pellets were isolated as described previously. RNA was extracted using an RNeasy mini plus kit (Qiagen) following standard protocols. The isolated RNA was then reverse transcribed to cDNA using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The expression of bone morphogenetic protein-2 (BMP-2, Taqman Assay ID: Hs00154192_m1) (only shear study), osteopontin (OPN, Hs00960641_m1) (all studies) and alkaline phosphatase (ALP, Hs00758162_m1) (only for annuli study) was analyzed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs00960641_m1) as an endogenous control gene for all samples. The sequences are propietary. Gene expression assays (Applied Biosystems) were combined with the cDNA to be analyzed and Taqman PCR master mix (Applied Biosystems). The reaction was performed on a 7900HT real time PCR System (Applied Biosystems) using thermal conditions of 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The relative gene expression level of each target gene was then normalized to the mean of the GAPDH in each group. Fold change was calculated using the ΔΔCT relative comparative method as described previously (Kim et al. 2009; Yoon et al. 2009). Samples were evaluated in triplicate and standard deviations are reported (n=3).
Statistical Analyses
All samples were completed in triplicate (n=3). Data was analyzed using single factor ANOVA followed by Tukey’s Multiple Comparison Test assuming normal data distribution with a confidence of 95% (p < 0.05). Mean values of triplicates and standard deviation error bars are reported on each figure as well as relevant statistical relationships.
Results
Effect of Shear Stress on Late Osteoblastic Differentiation and BMP-2 Expression
An investigation was completed to determine the effect of shear on osteoblastic differentiation. Osteogenic signaling protein BMP-2 was analyzed at days 1, 4, 8, 14 and 21 (Figure 3a). On days 1, 4, and 8 a weak correlation was observed between shear stress and BMP-2 expression. Specifically on day 4 BMP-2 fold change increased an average of 0.19 per dyne/cm2. On day 8 the fold change was 0.44 per dyne/cm2. By day 14 a strong correlation began to emerge between shear stress and BMP-2 expression. Specifically BMP-2 fold change increased 0.95 per dyne/cm2. By day 21 this correlation was stronger with an average fold change of 1.64 per dyne/cm2. Following this analysis late osteoblastic marker osteopontin was evaluated to determine the effect of shear on late osteoblastic differentiation. As OPN expression was not observed at early timepoints, it was analyzed at day 14 and 21. By day 14 the fold change of OPN increased 0.46 per dyne/cm2 (Figure 3b). This trend strengthened by day 21 when a fold change of 1.26 per dyne/cm2 was observed. These results demonstrate a strong time dependent effect of shear stress. Following this study, an investigation was completed to analyze the effect of hMSC radial position in scaffolds on hMSC osteoblastic differentiation and proliferation.
Figure 3.
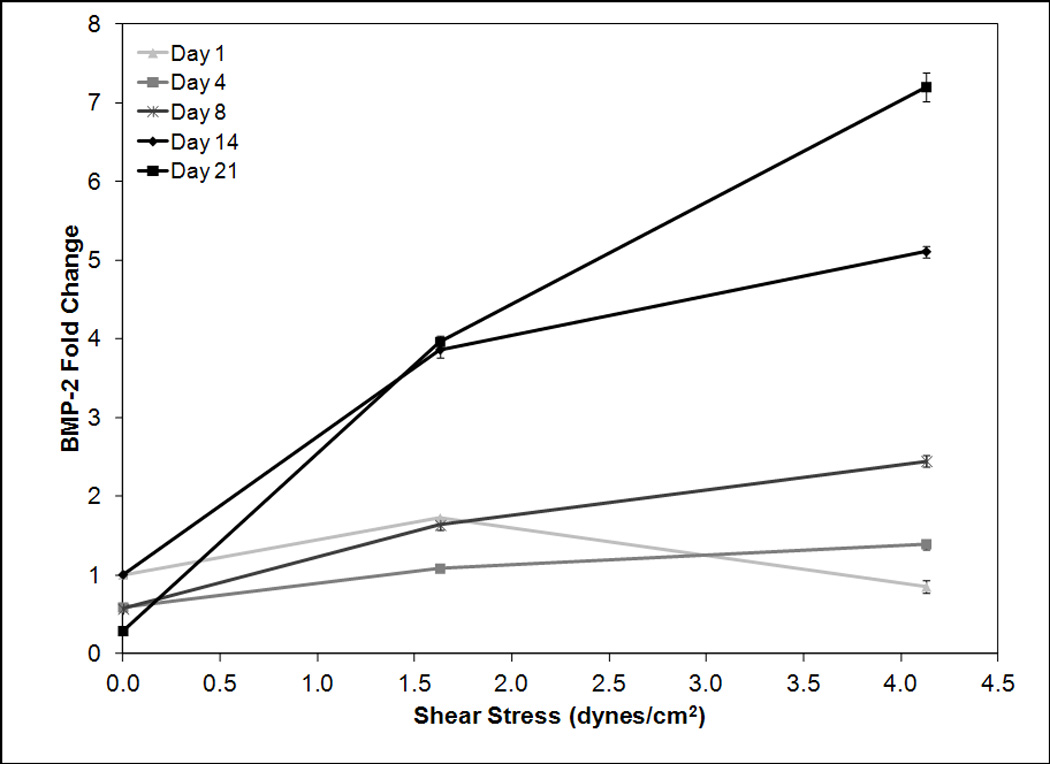
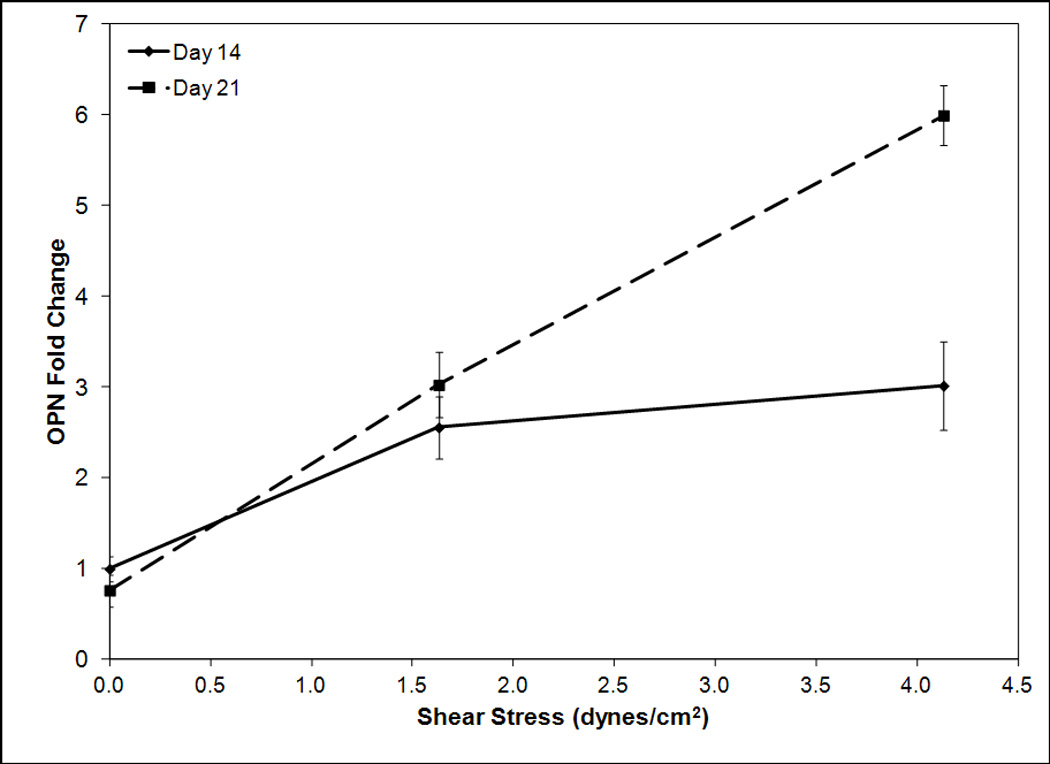
RT-PCR analysis for BMP-2 (a) and osteopontin (b) mRNA expression versus shear stress for timepoints 1, 4, 8, 14, and 21 (a) and 14 and 21 (b). Day 1, 4, and 8 BMP-2 data is normalized to day 1 zero shear, day 14 and 21 BMP-2 and OPN data is normalized to day 14 zero shear. Expression levels of BMP-2 and osteopontin are dependent on shear stress with higher shear stresses correlating to greater expression levels. The magnitude of this increase becomes stronger at later timepoints.
hMSC Proliferation in Discrete Layers
Based on live dead staining of entire bead, inner annuli, and small bead nearly all cells were viable throughout the alginate beads after 1 day of culture. Dead cells were not observed (Figure 4). On day 7 proliferation remained comparable in all groups except for bioreactor cultured small beads which had significantly higher (p < 0.05) levels of DNA (Figure 5). By day 14 a sharp decrease in proliferation could be observed in the inner annulus of control beads while the highest proliferation continues to be observed in the bioreactor small beads. On day 21 significantly (p < 0.05) higher proliferation was observed in bioreactor cultured beads as compared to control beads of the same size. DNA concentration in the outer and inner annuli of bioreactor cultured beads was 3.82 ± 0.31 and 3.33 ± 0.58 ng/mm3 respectively. This was significantly higher (p < 0.05) compared to the outer and inner annuli of statically cultured beads which were 1.59 ± 0.34 and 1.24 ± 0.22 ng/mm3 respectively. Bioreactor small beads had the highest DNA amount at 5.55 ± 0.82 ng/mm3. Statically cultured small beads had a lower DNA amount at 3.15 ± 0.64 ng/mm3. This amount is lower but statistically similar (p > 0.05) to bioreactor cultured large beads.
Figure 4.
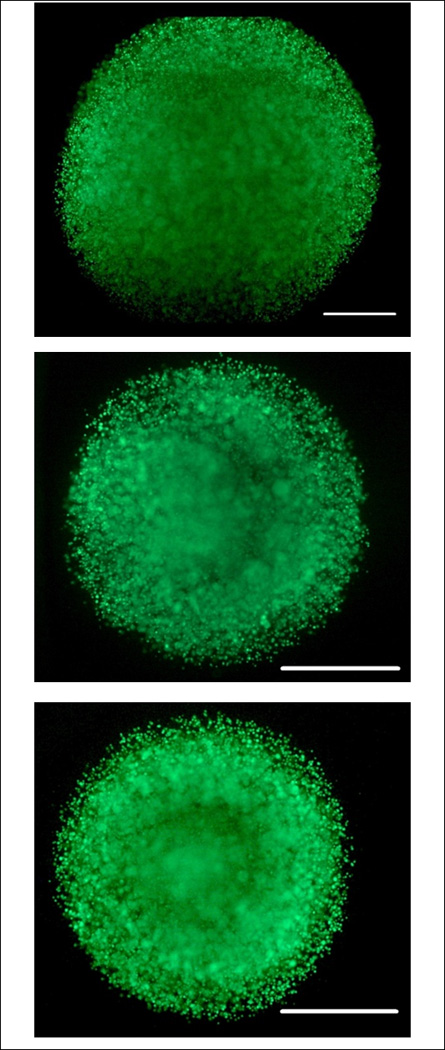
Live Dead Images of entire bead (top), inner annuli (middle), and small bead (bottom) after one day of bioreactor culture. Scale bar represents 1000 µm. Note small bead and inner annuli are approximately the same size and half that of the entire bead. Cells appear viable in all groups throughout the bead.
Figure 5.
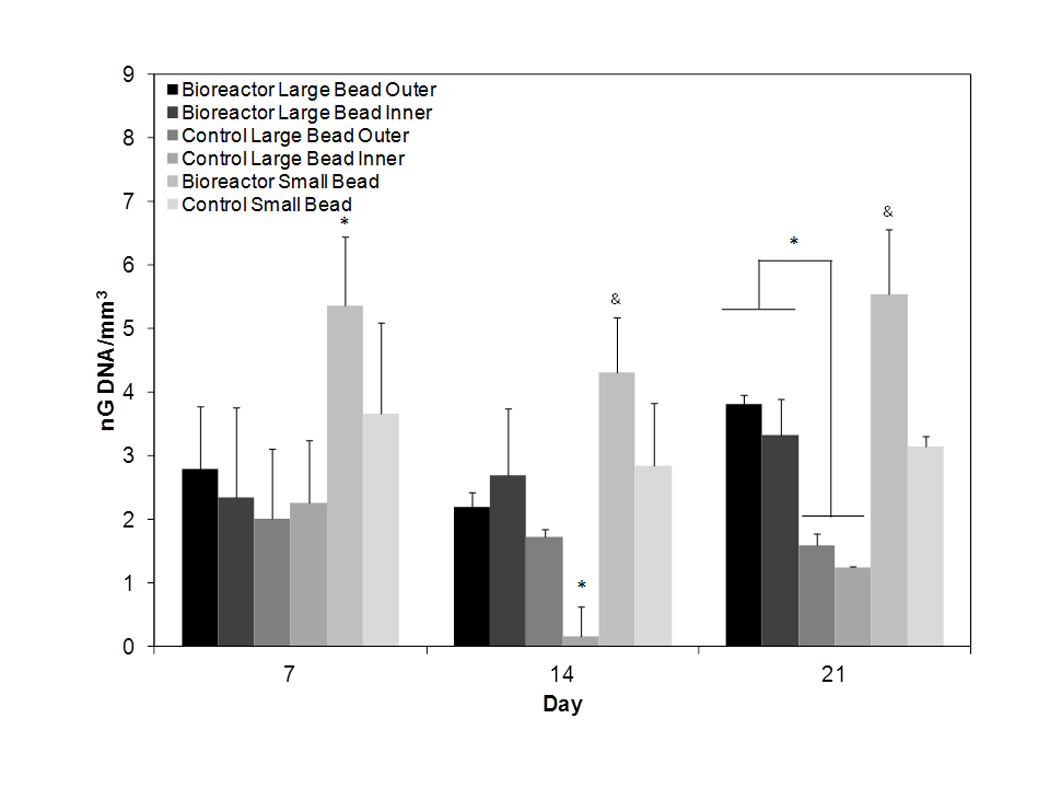
DNA amount normalized to scaffold volume for days 7, 14, and 21. On day seven similar proliferation is observed in all groups except bioreactor small bead. By day 14 control inner exhibits decreased proliferation. On day 21 note significantly higher DNA amounts in bioreactor cultured large beads as compared to static cultured large beads. The symbols *, & represent statistical significance within a timepoint (p < 0.05). Groups with symbol * or & are statistically different from all groups except those groups with the same symbol. Groups with the same symbol are statistically similar to each other.
hMSC Differentiation and Matrix Production in Discrete Layers
ALP was used as a marker of early osteoblastic differentiation. The earliest peaks in ALP expression were observed in the control beads indicating they may be differentiating more rapidly than bioreactor cultured beads (Figure 6). By day 21 the highest expression levels were observed in the inner annuli of bioreactor cultured beads with a fold change of 5.85 ± 0.41 as compared to day one control inner. The inner annuli of control beads also exhibited high expression levels with a 3.54 ± 0.08 fold change compared to the same group. Interestingly low expression was observed in the bioreactor outer and bioreactor small groups with expression levels of 0.34 ± 0.04 and 0.82 ± 0.06 respectively.
Figure 6.
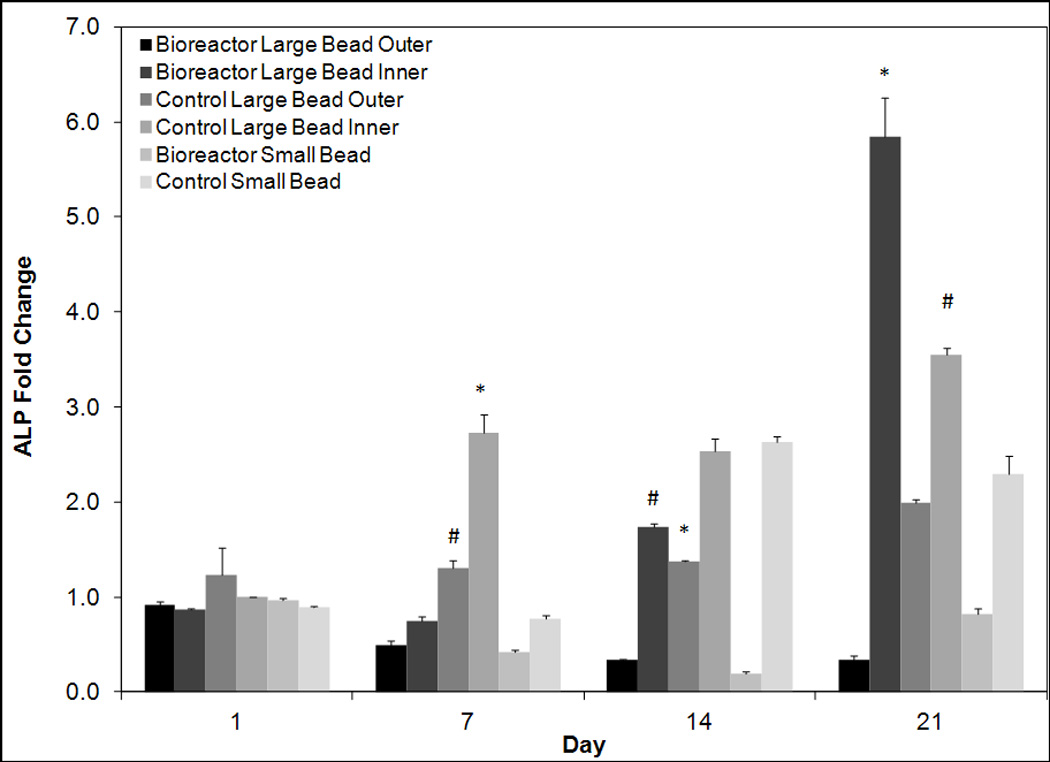
Quantitative reverse transcriptase polymerase chain analysis for days 1, 7, 14, and 21 for early osteoblastic marker ALP. Values are normalized to day one control large bead inner. All groups are cultured in osteogenic media. On day 21 ALP expression levels are significantly higher in bioreactor large bead inner cells than all other groups. The symbols (*, #) indicate statistical significance from all other groups within a timepoint (p < 0.05). Groups with symbol * or # are statistically different from all groups except those groups with the same symbol. Groups with the same symbol are statistically similar to each other.
Following this an analysis of osteoblastic differentiation marker OPN was completed. As OPN is a late osteoblastic marker low levels were observed on days 7 and 14 (Figure 7). By day 21 expression of OPN began to increase in some groups with the highest levels observed in the inner annuli of bioreactor cultured beads. This group exhibited a fold change of 12.31 ± 1.47 as compared to day 7 control inner. The outer annuli of the bioreactor cultured beads exhibited a significantly (p < 0.05) lower fold change of 4.55 ± 0.32. Control groups exhibited low day 21 levels of 1.45 ± 0.41 and 1.37 ± 0.54 for the outer and inner annuli respectively. The control small group was slightly but statistically significantly higher (p < 0.05) at a fold change of 1.65 ± 0.33. The bioreactor small group was significantly higher (p < 0.05) at a fold change of 7.21 ± 1.24.
Figure 7.
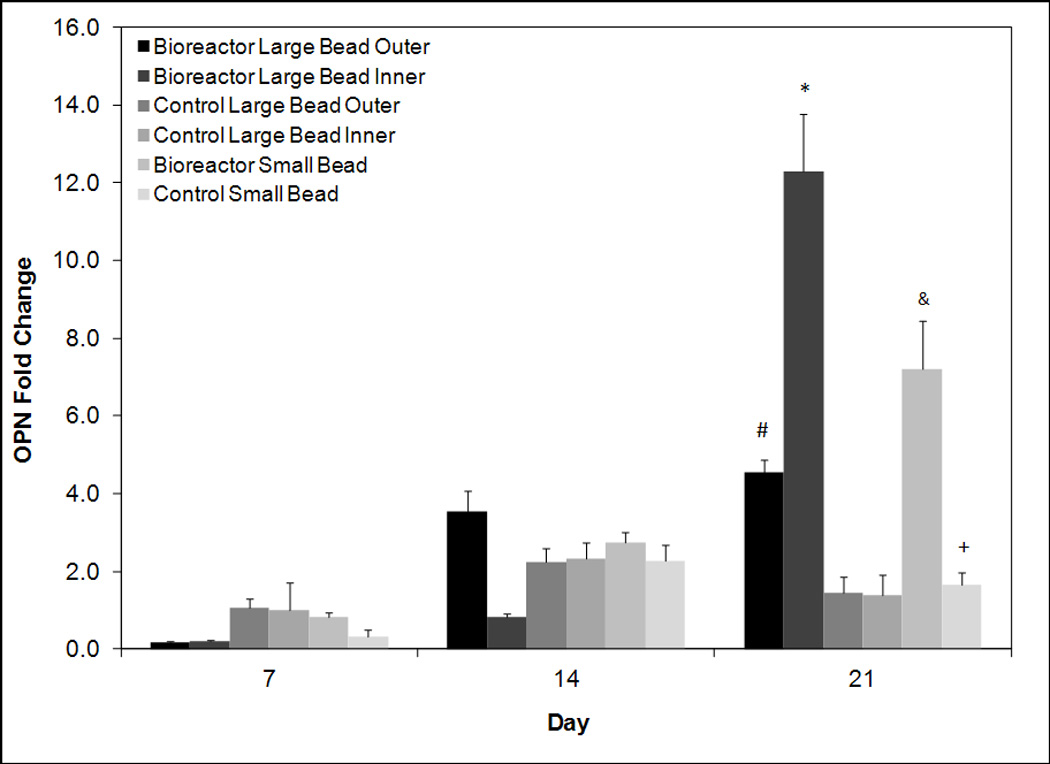
RT-PCR analysis for days 7, 14, and 21 for late osteoblastic marker osteopontin. Values are normalized to day seven control large bead inner. All groups are cultured in osteogenic media. On day 21 expression levels are significantly greater in bioreactor inner than all other groups. The symbols (*, #, &, +) indicate statistical significance from all other groups within a timepoint (p < 0.05). Groups with symbol *, #, & or & are statistically different from all groups except those groups with the same symbol. Groups with the same symbol are statistically similar to each other.
To analyze mineralized matrix production, another marker of late osteoblastic differentiation Von Kossa staining was quantified using a histomorphometric analysis (Figure 8). Surprisingly higher levels of staining were observed in the control small group on days 7 and 14 while other groups do not show large increases in mineralization until day 21. On day 21 significantly higher (p < 0.05) amounts of mineralization were observed in the inner annuli of bioreactor cultured beads with 79 ± 29% percent mineralization. High levels of mineralization were also observed in the outer annuli of bioreactor cultured beads with 53 ± 25% mineralization. The control small exhibited similar levels of mineralization at 53 ± 22% mineralization. Low levels of mineralization was observed in the large control beads with only 19 ± 6% and 6 ± 4% mineralization area for the outer and inner sections respectively. Surprisingly the bioreactor small beads also had low levels of mineralization with 11 ± 9% mineralization. Representative images in Figure 9 illustrate these trends.
Figure 8.
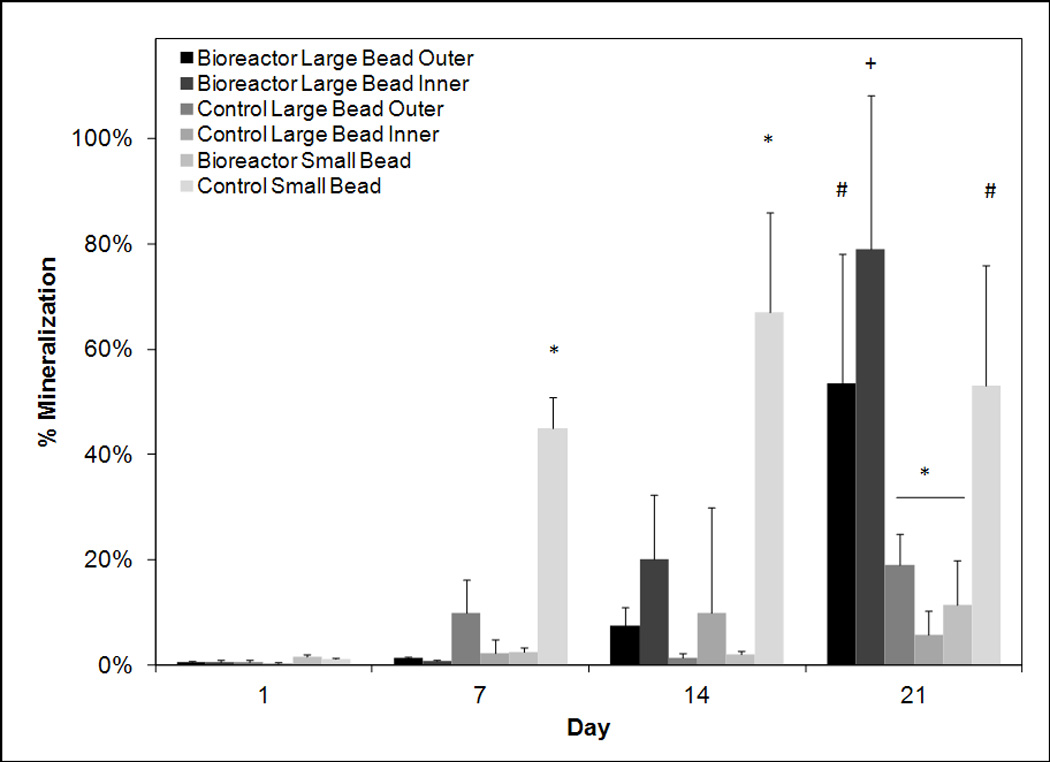
Percent mineralization based on histomorphometric analysis of Von Kossa stain of beads on days 1, 7, 14, and 21. Highest mineralization can be observed on day 21 in the inner annuli of bioreactor cultured beads. The outer annuli of bioreactor cultured beads and statically cultured small beads almost exhibit high mineralization on day 21. The symbols (*, #, +) indicate statistical significance within a timepoint (p < 0.05). Groups with symbol *, #, or + are statistically different from all groups except those groups with the same symbol. Groups with the same symbol are statistically similar to each other.
Figure 9.
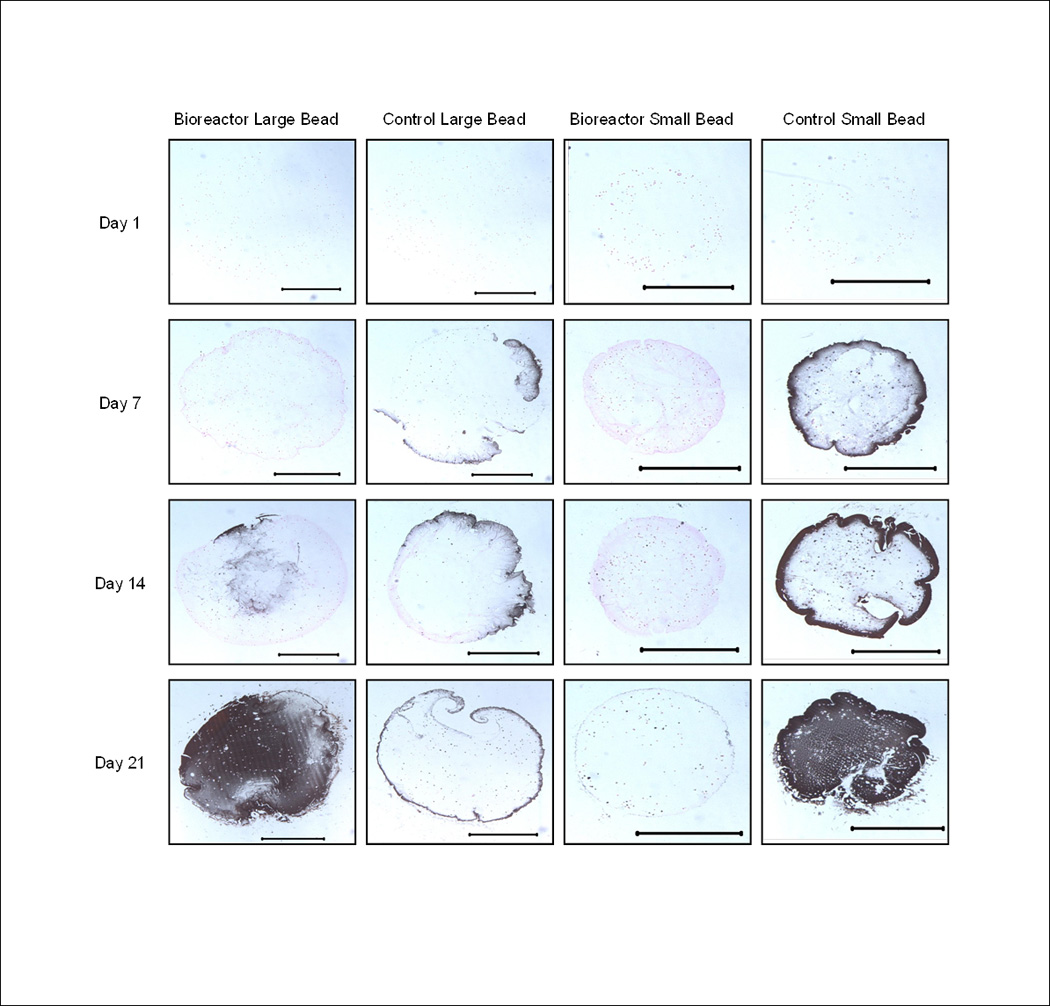
Representative images of bioreactor cultured large beads, statically cultured large beads, bioreactor cultured small beads, and statically cultured small beads. Scale bar represents 1000 µm. Note increased calcium staining on day 21 and the highest overall percentage of calcified area in the bioreactor large bead on day 21.
Discussion
The overall goal of this study was to determine how external culture conditions including shear stress and nutrient transfer affect hMSCs proliferating and differentiating in the TPS bioreactor as compared to static controls. The first part of the study focused on the role of shear stress in the osteoblastic differentiation of bioreactor cultured beads. Shear stress is commonly thought to induce osteoblastic differentiation in perfusion systems (Gomes et al. 2003; Holtorf et al. 2005; Janssen et al. 2010; Kapur et al. 2003; Kreke et al. 2005; Li et al. 2009; McCoy and O'Brien 2010; Sikavitsas et al. 2005; Stolberg and McCloskey 2009; Yeatts and Fisher 2011a). Shear can be isolated from mass transport in a perfusion system using a thickening agent to change media viscosity. In this manner increasing shear has been demonstrated to increase mineralization in rat BMSCs (Sikavitsas et al. 2003) and upregulate osteoblastic differentiation in hMSCs (Li et al. 2009). Though increasing flow rate was previously shown to upregulate osteoblastic markers in the TPS bioreactor, shear stress was not isolated and specifically investigated (Yeatts and Fisher 2011b). In addition the TPS bioreactor utilizes bulk hydrogels rather than porous scaffolds used in previous bioreactor shear studies. Thus the direct effect of surface shear stress on encapsulated cells was previously unknown. It was discovered that increasing shear in the system led to an increase in late osteoblastic marker osteopontin and osteogenic signaling protein BMP-2 (Figure 3). Interestingly the effect of shear on osteopontin expression was temporal in nature with a greater correlation occurring at day 21 than on day 14. Though shear has previously been observed to upregulate osteoblastic differentiation of hMSCs, to our knowledge this is the first observation of a temporal correlation of this effect in a bioreactor system. This result was also observed when analyzing BMP-2 expression as minimal correlation is observed at days 1, 4, and 8, but a strong correlation is observed on day 21. Following previous work, shear stresses were calculated at the surface of the bead equaling 1.63 ± 0.13 dynes/cm2 for the 3% dextran media and 4.13 ± 0.34 dynes/cm2 for the 9% dextran media (Yeatts and Fisher 2011b). Flow rate through the bead can be related to the permeability of alginate (1.2 ± 0.1×10−12) (Hwang et al. 2010) using Darcy’s law in permeability (Hwang et al. 2010; Truskey 2004) where Permeability = (QuL)/(A(ΔP)) where Q is flow rate, u is dynamic viscosity of the media, L is the diffusion length, A is the surface area of the bead, and ΔP is change in pressure across the bead. Using the pressures in the system calculated from the COMSOL model (Yeatts and Fisher 2011b) and assuming bead homogeneity, flow rate through a 4 mm cross section of the large bead was calculated to be approximately 3×10−7 ml/min. As shear is proportional to the change in velocity and the flow rate in the bead (3×10−7 mL/min) is 107 lower than the flow rate at the surface (3 mL/min), we can conclude that shear stresses will be reduced by similar amounts on the interior portions of the bead assuming a no slip condition at the surface. Therefore we speculate the effects of shear stress likely result from cells in the inner core of the bead responding to paracrine signals released from cells on the surface of the bead, however prolonged exposure of cells in the core of the bead to these small shear stresses could also impact cellular responses.
In the second part of the study the role of the cellular position in a scaffold as it relates to cell proliferation was investigated. The objective of this part of the study was to determine if nutrient transfer limitations were occurring and what role the TPS bioreactor had on these limitations. Nutrient and oxygen levels must be kept at sufficient levels throughout a three dimensional construct in order for cells to remain viable. The effect of oxygen concentration on hMSC proliferation has been previously investigated (Potier et al. 2007b). It was reported that hMSCs can survive low oxygen levels for up to 48 hours, but if the low oxygen levels are combined with nutrient deprivation, significant cell death occurs. Based on proliferation data in this study it can be concluded that hMSCs in large (4 mm diameter) static cultured scaffolds are proliferating at lower rates than cells in bioreactor culture (Figure 5). This was likely caused by a deprivation of nutrients and oxygen to cells in these scaffolds. Day 21 DNA amount is over twice as high in bioreactor groups as compared to static control groups. The decrease in proliferation was most significant in the inner annuli of statically cultured large beads in which nearly a 50% drop in DNA amount is observed from day 7 to 21. These results are further supported when the small bead control is taken into account. Just as dynamic culture mitigates diffusion limitations, increasing the surface area to volume ratio of scaffolds through decreased size also mitigates this limitation. Thus small (2 mm diameter) static control beads had increased proliferation as compared to large statically cultured beads. Bioreactor cultured small beads had the highest levels of proliferation. These results highlight the need for bioreactor systems for the culture of tissue engineering constructs to avoid cell death resulting from hypoxia.
Finally, in vitro osteoblastic differentiation was analyzed as another key aspect of a cell based tissue engineering strategy. The objective of this final part of the study was to determine if scaffold radial position influenced hMSC osteoblastic differentiation. It is hypothesized that radially dependent differentiation will be influenced by two factors, shear stress (as discussed earlier) and oxygen content. The effect of oxygen content on stem cell osteoblastic differentiation is conflicted in the literature as increased proliferation and differentiation of hMSCs have been reported upon exposure to low (2% oxygen) conditions (Grayson et al. 2007). A similar result has been observed in rat MSCs as cells cultured under 5% oxygen conditions increased in proliferation and differentiation as compared to 20% oxygen conditions. However it has been demonstrated previously that low oxygen concentrations can inhibit bone formation and in vitro osteoblastic differentiation (Iida et al. 2010; Malda et al. 2007; Potier et al. 2007a; Utting et al. 2006). In hMSCs it was observed that even short term (48 hr) hypoxia caused a down regulation in several osteoblastic factors and markers (Potier et al. 2007a). In a study with rat osteoblasts sufficient oxygen was found as a requirement for bone growth as hypoxic conditions (2%) oxygen resulted in a downregulation of ALP and osteocalcin as well as an 11 fold decrease in mineralized bone (Utting et al. 2006). Knowledge of the culture environment is critical as divergent cellular pathways of MSC differentiation and proliferation have previously been reported in the literature (Augello and De Bari 2010; Bianco et al. 1993; Kolf et al. 2007). MSCs may directed down a specific pathway by physical factors in their environment (Augello and De Bari 2010). Wnts have been demonstrated to be crucial and the modulation of these pathways (De Boer et al. 2004; Kolf et al. 2007) and may be regulated via other signaling pathways including BMP-2 (Boland et al. 2004). BMP-2 has been shown to enhance to stem cell differentiation and promote osteogenesis (Bessa et al. 2008a; Bessa et al. 2008b; Betz et al. 2008) and was measured in the first part of the study because of its strong association with bone formation.
This work supports findings that static culture of large constructs leads to reduced osteoblastic differentiation as large bead static control groups failed to express elevated levels of late osteoblastic marker osteopontin (Figure 7). These groups also produced low levels of mineralization further indicating an inhibition of osteoblastic differentiation in these groups. It is likely the observed decrease in proliferation in large control groups also results in less mineralization. These findings demonstrate the critical need for sufficient oxygen and nutrient transport during the three dimensional culture of hMSCs.
Interestingly low levels of mineralization were observed in the bioreactor cultured small beads. These beads exhibited the highest levels of proliferation, but mineralization levels were similar to that of large control beads. In addition osteopontin levels and mineralization percent were higher on the interior annulus of bioreactor cultured beads than the exterior annulus. The combination of these results with the observed increases in proliferation of cells in the exterior annulus of large beads and small beads of bioreactor cultured scaffolds could indicate the hMSCs being directed toward a proliferative pathway when more directly exposed to shear stress at the scaffold surface. In this manner the scaffold position could be altering the time course of differentiation by promoting a longer proliferation stage rather than differentiation and subsequent mineralization. Though the bioreactor smalls bead are not significantly mineralized in 21 days, osteopontin expression begins to rise on day 21 indicating these cells are undergoing late osteoblastic differentiation. This effect could result from two factors or a combination thereof. First, oxygen levels and shear vary throughout the scaffold (Yeatts and Fisher 2011b) and the oxygen content and shear levels could be optimal for proliferation closer to the surface of the scaffold and differentiation closer to the center. Second the higher level of mineralization observed on the interior of bioreactor cultured large beads could result from signaling molecules released by cells on the exterior portions of scaffolds. We speculate exposure to shear stress may be inducing these cells to release factors that signal cells on the interior portion of the scaffolds to differentiate, while the cells directly exposed to shear and higher nutrient concentration follow a proliferative pathway. These results demonstrate the ability of the TPS bioreactor to both promote an osteoblastic differentiation pathway or a proliferative pathway prior to osteoblastic differentiation depending on culture conditions.
This study demonstrates the critical need for bioreactor systems in three dimensional cell culture. Reduced proliferation and differentiation were observed in 4 mm statically cultured constructs and the ability of the TPS bioreactor to mitigate these results in three dimensional scaffolds was demonstrated. Osteoblastic differentiation and shear results demonstrate shear stress to induce a temporal and potentially indirect effect on hMSC osteoblastic differentiation. Bioreactor systems provide a complex environment and when evaluated on a cellular level can deliver an array of culture conditions to cells cultured in the same macro environment. This can lead to a multitude of outcomes on the individual cell level. Thus while bioreactor systems are vital to provide sufficient nutrients to prevent cell death they must be thoroughly evaluated to identify optimal culture conditions for the desired differentiation level prior to implantation.
Conclusions
This study demonstrated shear stress as a potent and temporal stimulus of hMSC osteoblastic differentiation within bulk scaffolds. Mineralization and proliferation levels were decreased in statically cultured constructs highlighting a need for bioreactor systems. In addition it was discovered that hMSC spatial position within scaffolds had an effect on both the osteoblastic differentiation and proliferation of these cells. These results could be used to tailor a flow and shear regime for either expansion or differentiation of a stem cell population to dictate the desired outcome of in vitro culture
Acknowledgements
This research was supported by the National Institutes of Health (R01 AR061460), Howard Hughes Medical Institute (University of Maryland Undergraduate Research Fellowship), and the National Science Foundation’s Research Experiences for Undergraduates Site (EEC 1005123).
References
- Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21(10):1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Engineering. 2003;9(3):549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008a;2(2–3):81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008b;2(1):1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- Betz MW, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP. Tissue response and orbital floor regeneration using cyclic acetal hydrogels. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32131. [DOI] [PubMed] [Google Scholar]
- Betz MW, Yeatts AB, Richbourg WJ, Caccamese JF, Coletti DP, Falco EE, Fisher JP. Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules. 2010;11(5):1160–1168. doi: 10.1021/bm100061z. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Bonucci E, Termine JD, Robey PG. Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J Histochem Cytochem. 1993;41(2):183–191. doi: 10.1177/41.2.8419458. [DOI] [PubMed] [Google Scholar]
- Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- Botchwey EA, Pollack SR, Levine EM, Laurencin CT. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. Journal of Biomedical Materials Research. 2001;55(2):242–253. doi: 10.1002/1097-4636(200105)55:2<242::aid-jbm1011>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Betz MW, Fisher JP, Paek A, Chen Y. Macroporous Hydrogel Scaffolds and Their Characterization By Optical Coherence Tomography. Tissue Eng Part C Methods. 2010 doi: 10.1089/ten.TEC.2010.0072. [DOI] [PubMed] [Google Scholar]
- De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10(3–4):393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. Journal of Biomedical Materials Research Part A . 2003;67A(1):87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3):948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. Journal of Biomedical Materials Research Part A. 2005;72A(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- Hwang CM, Sant S, Masaeli M, Kachouie NN, Zamanian B, Lee SH, Khademhosseini A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication. 2010;2(3):035003. doi: 10.1088/1758-5082/2/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Takeda-Kawaguchi T, Tezuka Y, Kunisada T, Shibata T, Tezuka K. Hypoxia enhances colony formation and proliferation but inhibits differentiation of human dental pulp cells. Arch Oral Biol. 2010;55(9):648–654. doi: 10.1016/j.archoralbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. Journal of Biomedical Materials Research. 1997;36(1):17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Janssen FW, van Dijkhuizen-Radersma R, Van Oorschot A, Oostra J, de Bruijn JD, Van Blitterswijk CA. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. Journal of Tissue Engineering and Regenerative Medicine. 2010;4(1):12–24. doi: 10.1002/term.197. [DOI] [PubMed] [Google Scholar]
- Kapur S, Baylink DJ, Lau KHW. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32(3):241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim UJ, Leisk GG, Bayan C, Georgakoudi I, Kaplan DL. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromolecular Bioscience. 2007;7(5):643–655. doi: 10.1002/mabi.200700030. [DOI] [PubMed] [Google Scholar]
- Kim K, Dean D, Mikos AG, Fisher JP. Effect of Initial Cell Seeding Density on Early Osteogenic Signal Expression of Rat Bone Marrow Stromal Cells Cultured on Cross-Linked Poly(propylene fumarate) Disks. Biomacromolecules. 2009;10(7):1810–1817. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6):1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Li D, Dai K, Tang T. Effects of dextran on proliferation and osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2008;10(6):587–596. doi: 10.1080/14653240802238330. [DOI] [PubMed] [Google Scholar]
- Li D, Tang T, Lu J, Dai K. Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Eng Part A. 2009;15(10):2773–2783. doi: 10.1089/ten.TEA.2008.0540. [DOI] [PubMed] [Google Scholar]
- Malda J, Klein TJ, Upton Z. The roles of hypoxia in the In vitro engineering of tissues. Tissue Engineering. 2007;13(9):2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends in Biotechnology. 2004;22(2):80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- McCoy RJ, O'Brien FJ. Influence of Shear Stress in Perfusion Bioreactor Cultures for the Development of Three-Dimensional Bone Tissue Constructs: A Review. Tissue Eng Part B Rev. 2010 doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Annals of Biomedical Engineering. 2004;32(1):112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007a;40(4):1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007b;13(6):1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- Rodrigues CA, Fernandes TG, Diogo MM, da Silva CL, Cabral JM. Stem cell cultivation in bioreactors. Biotechnol Adv. 2011;29(6):815–829. doi: 10.1016/j.biotechadv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MAK, Dauner M, Mikos AG. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Annals of Biomedical Engineering. 2005;33(1):63–70. doi: 10.1007/s10439-005-8963-x. [DOI] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. Journal of Biomedical Materials Research. 2002;62(1):136–148. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- Stolberg S, McCloskey KE. Can Shear Stress Direct Stem Cell Fate? Biotechnology Progress. 2009;25(1):10–19. doi: 10.1002/btpr.124. [DOI] [PubMed] [Google Scholar]
- Truskey GA, Yuan F, Katz DF. Transport Phenomena in Biological Systems. Upper Saddle River: Pearson Prentice Hall; 2004. [Google Scholar]
- Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Experimental Cell Research. 2006;312(10):1693–1702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Volkmer E, Drosse I, Otto S, Stangelmayer A, Stengele M, Kallukalam BC, Mutschler W, Schieker M. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Engineering Part A. 2008;14(8):1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- Wang TW, Wu HC, Wang HY, Lin FH, Sun JS. Regulation of adult human mesenchymal stem cells into osteogenic and chondrogenic lineages by different bioreactor systems. Journal of Biomedical Materials Research Part A. 2009;88A(4):935–946. doi: 10.1002/jbm.a.31914. [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011a;48(2):171–81. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Fisher JP. Tubular perfusion system for the long-term dynamic culture of human mesenchymal stem cells. Tissue Eng Part C Methods. 2011b;17(3):337–348. doi: 10.1089/ten.TEC.2010.0172. [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Gordon CN, Fisher JP. Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng Part C Methods. 2011;17(12):1171–1178. doi: 10.1089/ten.tec.2011.0263. [DOI] [PubMed] [Google Scholar]
- Yoon DM, Curtiss S, Reddi AH, Fisher JP. Addition of Hyaluronic Acid to Alginate Embedded Chondrocytes Interferes with Insulin-like Growth Factor-1 Signaling In Vitro and In Vivo. Tissue Engineering Part A. 2009;15(11):3449–3459. doi: 10.1089/ten.TEA.2009.0069. [DOI] [PubMed] [Google Scholar]
- Yoon DM, Fisher JP. Effects of exogenous IGF-1 delivery on the early expression of IGF-1 signaling molecules by alginate embedded chondrocytes. Tissue Eng Part A. 2008;14(7):1263–1273. doi: 10.1089/ten.tea.2007.0172. [DOI] [PubMed] [Google Scholar]
- Yoon DM, Hawkins EC, Francke-Carroll S, Fisher JP. Effect of construct properties on encapsulated chondrocyte expression of insulin-like growth factor-1. Biomaterials. 2007;28(2):299–306. doi: 10.1016/j.biomaterials.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: The influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11203–11208. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]


